Effect of Mechanical Activation on the Kinetics of Copper Leaching from Copper Sulfide (CuS)
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Leaching
3. Results and Discussion
3.1. Mechanical Activation (MA) Treatment of CuS
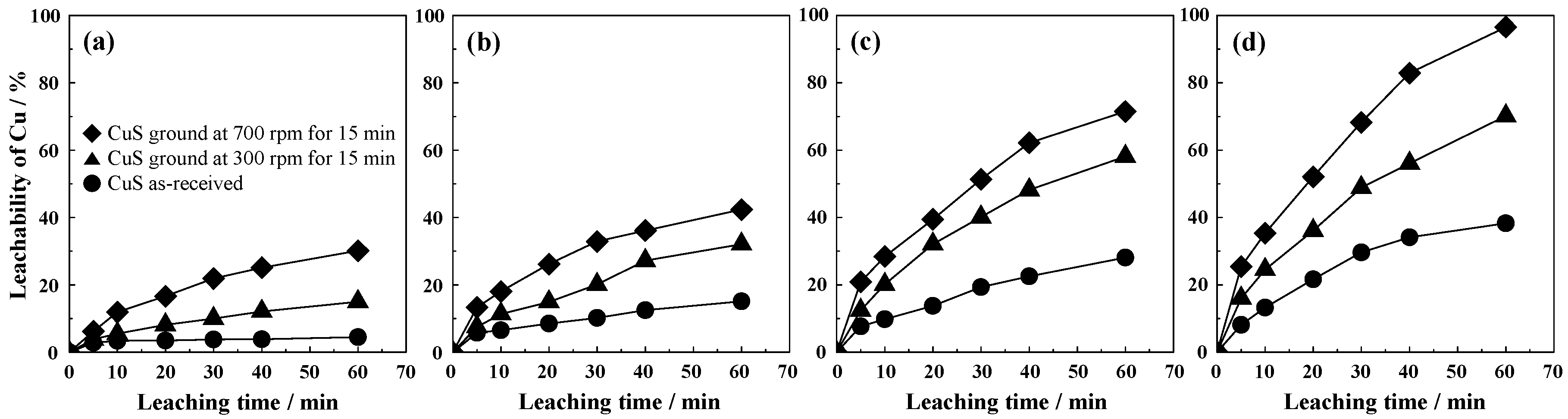
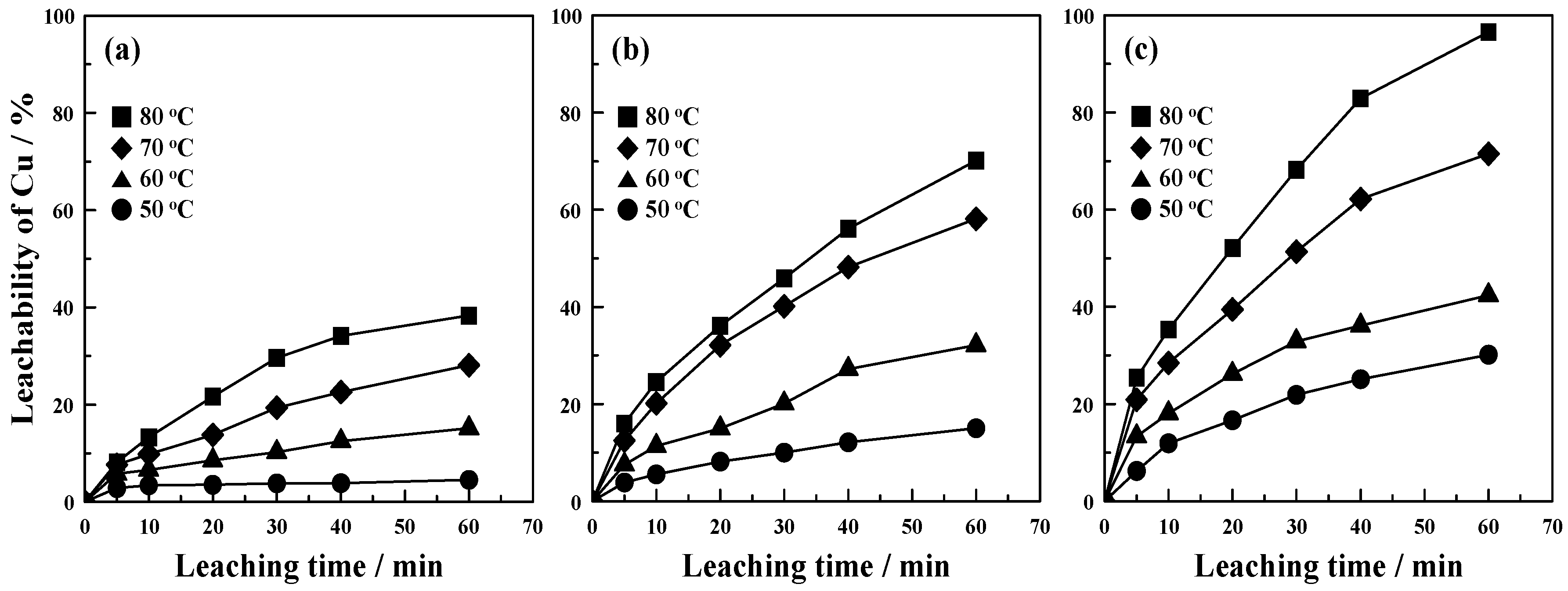
3.2. Copper Leaching of the Mechanically Activated CuS
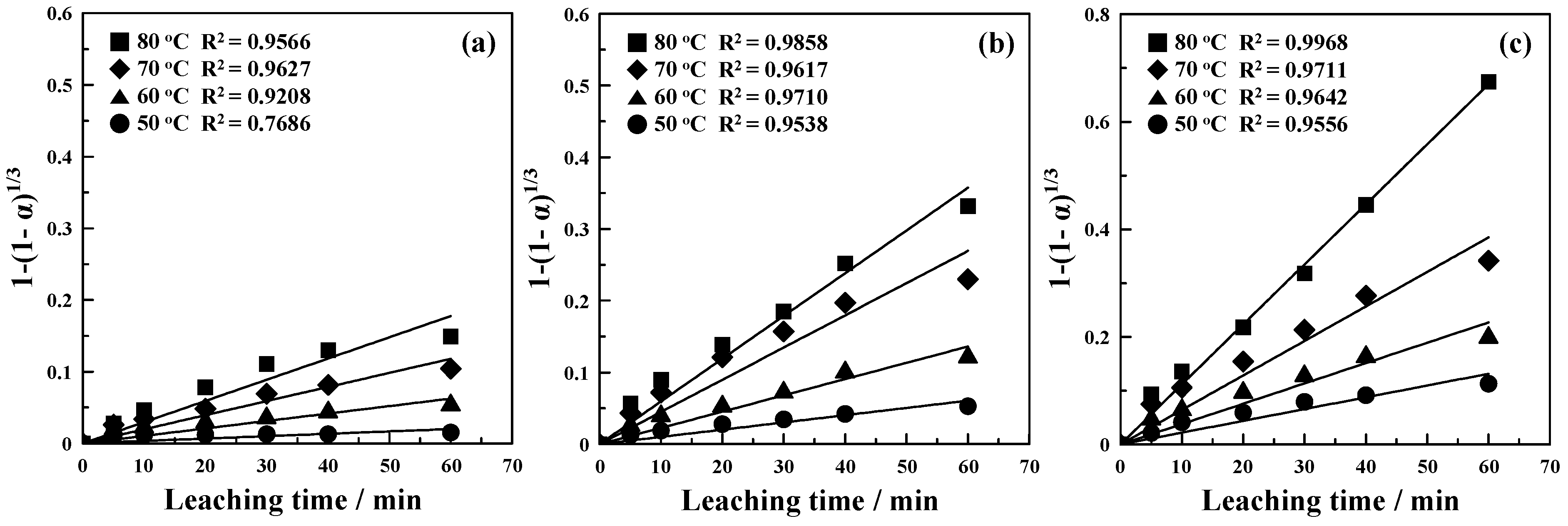
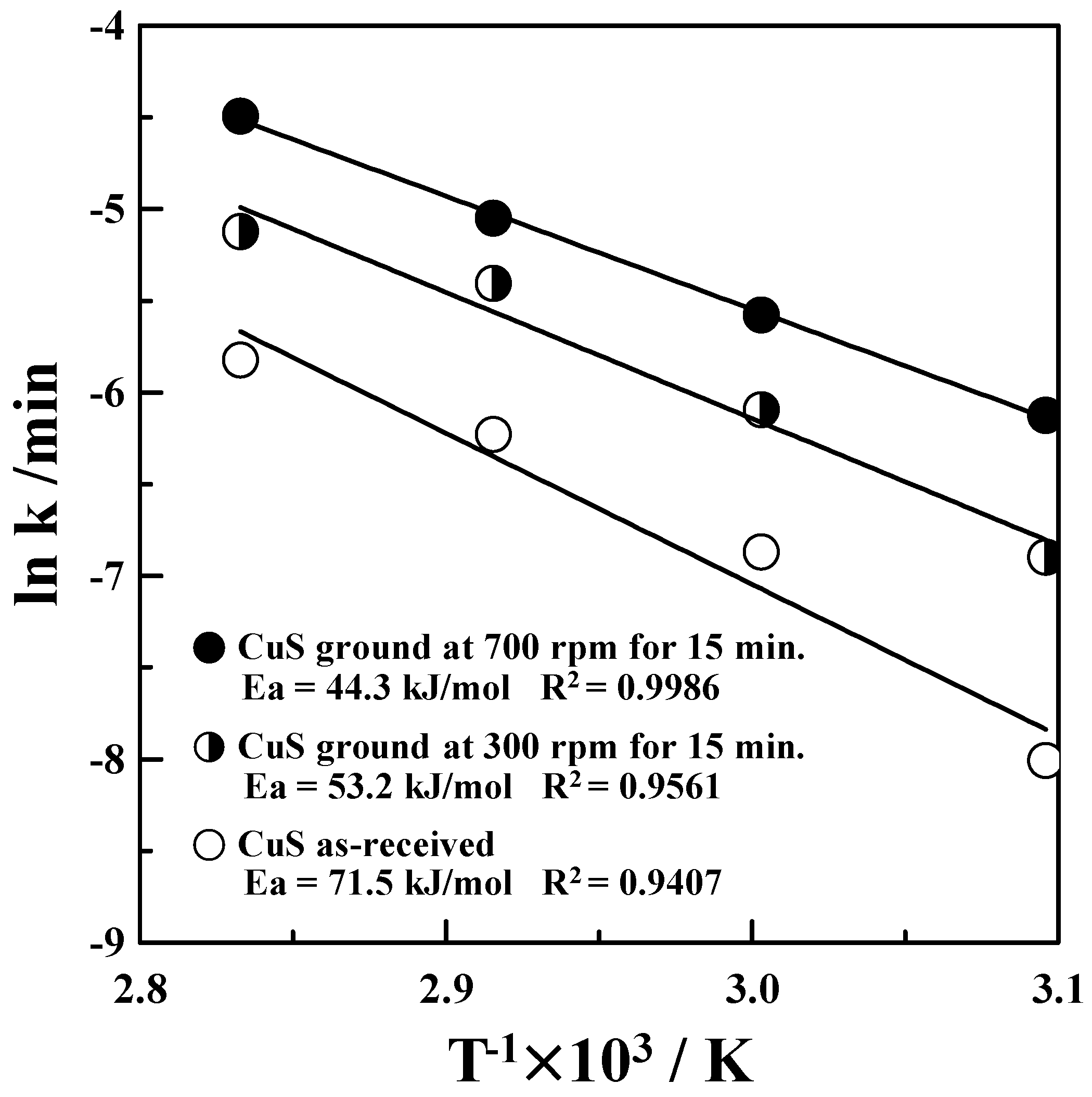
3.3. Kinetics of Copper Leaching for the Mechanically Activated CuS
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Biswas, A.K.; Davenport, W.G.; Hopkins, D.W. Extractive Metallurgy of Copper, 2nd ed.; Pergaman Press: New York, NY, USA, 1974; pp. 64–69. [Google Scholar]
- Burkin, A.R. Topics in Non-Ferrous Extractive Metallurgy; Blackwell Scientific Publications: Oxford, MS, USA, 1980; pp. 1–41. [Google Scholar]
- Li, M. Non-Ferrous Metals Metallurgy Process; Chemical Industry Press: Beijing, China, 2010. [Google Scholar]
- The World Copper Factbook. Available online: http://www.icsg.org/ (accessed on 1 November 2017).
- Mudd, G.M. The Environmental sustainability of mining in Australia: Key mega-trends and looming constraints. Resour. Policy 2010, 35, 98–115. [Google Scholar] [CrossRef]
- Wang, M.; Chen, W.; Li, X. Substance flow analysis of copper in production stage in the U.S. from 1974 to 2012. Resour. Conserv. Recycl. 2015, 105, 36–48. [Google Scholar] [CrossRef]
- Yin, Q.; Vaughan, D.J.; England, K.E.R.; Kelsall, G.H. Electrochemical Oxidation of Covellite (CuS) in Alkaline Solution. J. Colloid Interface Sci. 1994, 166, 133–142. [Google Scholar] [CrossRef]
- Salmani Nuri, O.; Allahkarami, E.; Irannajad, M.; Abdollahzadeh, A. Estimation of selectivity index and separation efficiency of copper flotation process using ANN model. Geosyst. Eng. 2017, 20, 41–50. [Google Scholar] [CrossRef]
- Xing, W.D.; Lee, M.S. Leaching of gold and silver from anode slime with a mixture of hydrochloric acid and oxidizing agents. Geosyst. Eng. 2017, 20, 216–223. [Google Scholar] [CrossRef]
- Hirato, T.; Awakura, Y.; Majima, H. The leaching of sintered CuS disks with ferric chlorides. Metall. Trans. B 1989, 20, 485–491. [Google Scholar] [CrossRef]
- Palache, C.; Berman, H.; Frondel, C. Dana’s System of Mineralogy, 7th ed.; Wiley: New York, NY, USA, 1944; Volume 1, p. 248. [Google Scholar]
- Ammou-Chokroum, M.; Sen, P.K.; Fouques, F. Electrooxidation of chalcopyrite in acid chloride medium: Kinetics, stoichiometry and reaction mechanism. In Proceedings of the 13th International Mineral Processing Congress, Warsaw, Poland, 4–9 June 1979; p. 527. [Google Scholar]
- Parker, A.J.; Paul, R.L.; Power, G.P. Electrochemical aspects of leaching copper from chalcopyrite in ferric and cupric salt solutions. Aust. J. Chem. 1981, 34, 13–34. [Google Scholar] [CrossRef]
- Downes, K.W.; Bruce, R.W. The recovery of elemental sulfur from pyrite and pyrrhotite. Trans. Can. Inst. Min. Met. 1955, 58, 77–82. [Google Scholar]
- Sherman, M.I.; Strickland, J.D.H. Dissolution of Lead Sulfide Ores in Acid Chlorine Solutions. J. Met. 1957, 9, 795–800. [Google Scholar] [CrossRef]
- Forward, F.A.; Veltman, H. Direct leaching zinc-sulfide concentrates by Sherritt Gordon. J. Met. 1959, 11, 836–840. [Google Scholar] [CrossRef]
- Peters, E.; Loewen, F. Pressure leaching of copper minerals in perchloric acid solutions. Metall. Trans. B 1979, 4, 5–14. [Google Scholar] [CrossRef]
- Ghali, E.; Dandapani, B.; Lewenstam, A. Electrodissolution of synthetic covellite in hydrochloric acid. J. Appl. Electrochem. 1982, 12, 369–376. [Google Scholar] [CrossRef]
- Vereš, J.; Lovás, M.; Jakabský, Š.; Šepelák, V.; Hredzák, S. Characterization of blast furnace sludge and removal of zinc by microwave. Hydrometallurgy 2012, 129, 67–73. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.; Kingman, S.; Bradshaw, S. The reality of non-thermal effects in microwave assisted leaching systems. Hydrometallurgy 2006, 84, 1–13. [Google Scholar] [CrossRef]
- Palaniandy, S. Impact of mechanochemical effect on chalcopyrite leaching. Int. J. Miner. Process 2015, 136, 56–65. [Google Scholar] [CrossRef]
- Baláž, P.; Achimovičová, M. Mechano-chemical leaching in hydrometallurgy of complex sulphides. Hydrometallurgy 2006, 84, 60–68. [Google Scholar] [CrossRef]
- Hu, H.P.; Chen, Q.Y.; Yin, Z.L.; He, Y.H.; Huang, B.Y. Mechanism of mechanical activation for sulfide ores. Trans. Nonferr. Met. Soc. China 2007, 17, 205–213. [Google Scholar] [CrossRef]
- Abdel Gawwad, H.A.; Abd El-Aleem, S.; Faried, A.S. Effect of internal sulfate attack on the properties of sulfate-resisting cement and alkali-activated slag. Geosyst. Eng. 2017, 20, 195–206. [Google Scholar] [CrossRef]
- Minjigmaa, A.; Oyun-Erdene, G.; Zolzaya, T.; Davaabal, B.; Amgalan, J.; Temuujin, J. Phosphorus fertilizer prepared from natural Burenkhaan phosphorite (Mongolia) by a mechanical activation. Geosyst. Eng. 2016, 19, 119–124. [Google Scholar] [CrossRef]
- Warris, C.J.; McCormick, P.G. Mechanochemical processing of refractory pyrite. Miner. Eng. 1997, 10, 1119–1125. [Google Scholar] [CrossRef]
- Kim, J.K.; Han, S.H.; Lee, K.M. Estimation of compressive strength by a new apparent activation energy function. Cem. Concr. Res. 2001, 31, 217–225. [Google Scholar] [CrossRef]
- Banza, A.N.; Gock, E. Mechanochemical processing of chrysocolla with sodium sulphide. Miner. Eng. 2003, 16, 1349–1354. [Google Scholar] [CrossRef]
- Guo, X.; Xiang, D.; Duan, G.; Mou, P. A review of mechanochemistry applications in waste management. Waste Manag. 2010, 30, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Bujňáková, Z.; Baláž, P.; Zorkovská, A. Enargite concentrate processing by the combination of mechanochemical, hydrometallurgical and precipitation methods. Int. J. Miner. Process. 2014, 127, 28–36. [Google Scholar] [CrossRef]
- Bredenhann, R.; Van Vuuren, C.P.J. Technical note the leaching behavior of a nickel concentrate in an oxidative sulphuric acid solution. Miner. Eng. 1999, 12, 687–692. [Google Scholar] [CrossRef]
- Vračar, R.; Vučković, N.; Kamberović, Ž. Leaching of copper(I) sulphide by sulphuric acid solution with addition of sodium nitrate. Hydrometallurgy 2003, 70, 143–151. [Google Scholar] [CrossRef]
- Sokić, M.; Vračar, R.; Ilić, I.; Marković, B. Leaching of polymetallic sulphide Cu-Zn-Pb concentrate with sulphuric acid in sodium nitrate presence. CIM Mag. 2008, 101, 1–9. [Google Scholar]
- Cheng, C.Y.; Lawson, F. The kinetics of leaching covellite in acidic oxygenated sulphate-chloride solutions. Hydrometallurgy 1991, 27, 269–284. [Google Scholar] [CrossRef]
- Thomas, G.; Ingraham, T.R.; MacDonald, R.J.C. Kinetics of dissolution of synthetic digenite and chalcocite in aqueous acidic ferric sulphate solutions. Can. Metall. Q. 1967, 6, 281–292. [Google Scholar] [CrossRef]
- Mulak, W.; Niemiec, J. Kinetics of Cu2S dissolution in acidic solutions of ferric sulphate. ROCZNIKI CHEMII 1969, 43, 1387. [Google Scholar]
- King, J.A.; Burkin, A.R.; Ferreira, R.C.H. Leaching of chalcocite by acidic ferric chloride solutions. In Leaching and Reduction in Hydrometallurgy; Burkin, A.R., Ed.; Camelot Press: London, UK, 1975; pp. 36–45. [Google Scholar]
- Warren, I.H. A study of the acid pressure leaching of chalcopyrite, chalcocite and covellite. Aust. J. Appl. Sci. 1958, 9, 36–51. [Google Scholar]
- Gharabaghi, M.; Noaparast, M.; Irannajad, M. Selective leaching kinetics of low-grade calcareous phosphate ore in acetic acid. Hydrometallurgy 2009, 95, 341–345. [Google Scholar] [CrossRef]
- Sokić, M.D.; Marković, B.; Živković, D. Kinetics of chalcopyrite leaching by sodium nitrate in sulphuric acid. Hydrometallurgy 2009, 95, 273–279. [Google Scholar] [CrossRef]
- Souza, A.D.; Pina, P.S.; Lima, E.V.O.; Da Silva, C.A.; Leão, V.A. Kinetics of sulphuric acid leaching of a zinc silicate calcine. Hydrometallurgy 2007, 87, 337–345. [Google Scholar] [CrossRef]
- Sancho, J.P.; Ayala, J.; García, M.P.; Fernández, B. Leaching behaviour of a Bayer electrofilter fines in sulphuric acid. Hydrometallurgy 2009, 96, 35–41. [Google Scholar] [CrossRef]
- Lee, I.H.; Wang, Y.J.; Chern, J.M. Extraction kinetics of heavy metal-containing sludge. J. Hazard. Mater. 2005, 123, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Uçar, G. Kinetics of sphalerite dissolution by sodium chlorate in hydrochloric acid. Hydrometallurgy 2009, 95, 39–43. [Google Scholar] [CrossRef]
- Abdel-Aal, E.A.; Rashad, M.M. Kinetic study on the leaching of spent nickel oxide catalyst with sulfuric acid. Hydrometallurgy 2004, 74, 189–194. [Google Scholar] [CrossRef]
- Safarzadeh, M.S.; Moradkhani, D.; Ojaghi-Ilkhchi, M. Kinetics of sulfuric acid leaching of cadmium from Cd–Ni zinc plant residues. J. Hazard. Mater. 2009, 163, 880–890. [Google Scholar] [CrossRef] [PubMed]
- Baba, A.A.; Ayinla, K.I.; Adekola, F.A.; Bale, R.B.; Ghosh, M.K.; Alabi, A.F.; Sheik, A.; Folorunso, I.O. Hydrometallurgical application for treating a Nigerian chalcopyrite ore in chloride medium: Part I. Dissolution kinetics assessment. Int. J. Miner. Metall. Mater. 2013, 20, 1021–1028. [Google Scholar] [CrossRef]
- Bell, S.L.; Welch, G.D.; Bennett, P.G. Development of ammoniacal lixiviants for the in-situ leaching of chalcopyrite. Hydrometallurgy 1995, 39, 11–23. [Google Scholar] [CrossRef]
- Baba, A.A.; Balogun, A.F.; Olaoluwa, D.T.; Bale, R.B.; Adekola, F.A.; Alabi, A.G.F. Leaching kinetics of a Nigerian complex covellite ore by the ammonia-ammonium sulfate solution. Korean J. Chem. Eng. 2017, 34, 1133–1140. [Google Scholar] [CrossRef]
- Dutrizac, J.E.; MacDonald, R.J.C. Kinetics of dissolution of covellite in acidified ferric sulphate solutions. Can. Metall. Q. 1974, 13, 423–433. [Google Scholar] [CrossRef]

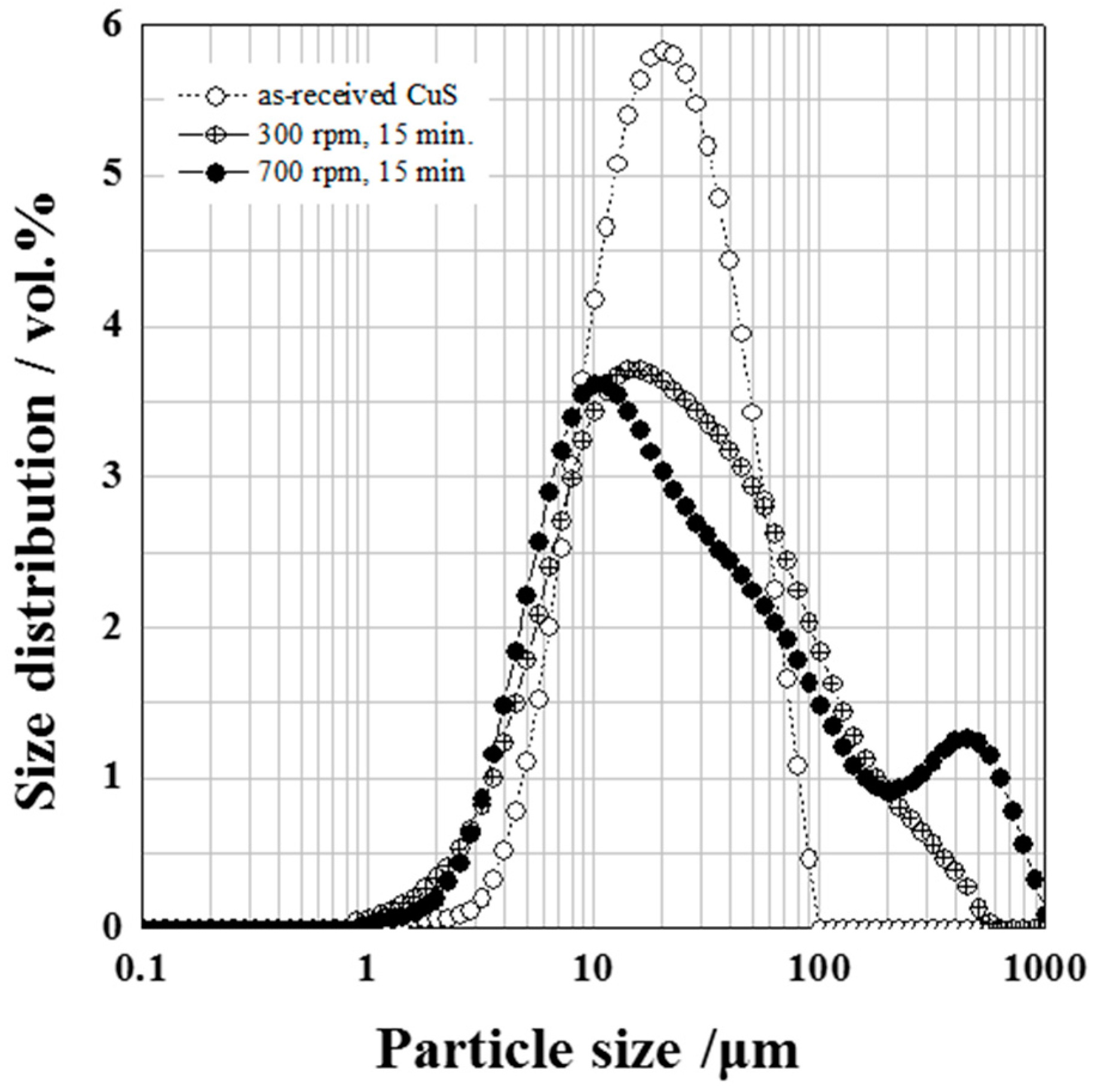
| Starting Materials | d10 (µm) | d50 (µm) | d90 (µm) | SSA (m2/g) |
|---|---|---|---|---|
| CuS as received | 9.10 | 23.80 | 59.66 | 1.11 |
| CuS ground at 300 rpm for 15 min | 5.87 | 23.80 | 124.68 | 2.82 |
| CuS ground at 700 rpm for 15 min | 5.73 | 23.36 | 316.43 | 2.08 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Kim, S.; Kim, B.; Lee, J.-c. Effect of Mechanical Activation on the Kinetics of Copper Leaching from Copper Sulfide (CuS). Metals 2018, 8, 150. https://doi.org/10.3390/met8030150
Lee J, Kim S, Kim B, Lee J-c. Effect of Mechanical Activation on the Kinetics of Copper Leaching from Copper Sulfide (CuS). Metals. 2018; 8(3):150. https://doi.org/10.3390/met8030150
Chicago/Turabian StyleLee, Jaeryeong, Suyun Kim, Byoungjin Kim, and Jae-chun Lee. 2018. "Effect of Mechanical Activation on the Kinetics of Copper Leaching from Copper Sulfide (CuS)" Metals 8, no. 3: 150. https://doi.org/10.3390/met8030150






