The Influence of Cu Addition on Dispersoid Formation and Mechanical Properties of Al-Mn-Mg 3004 Alloy
Abstract
:1. Introduction
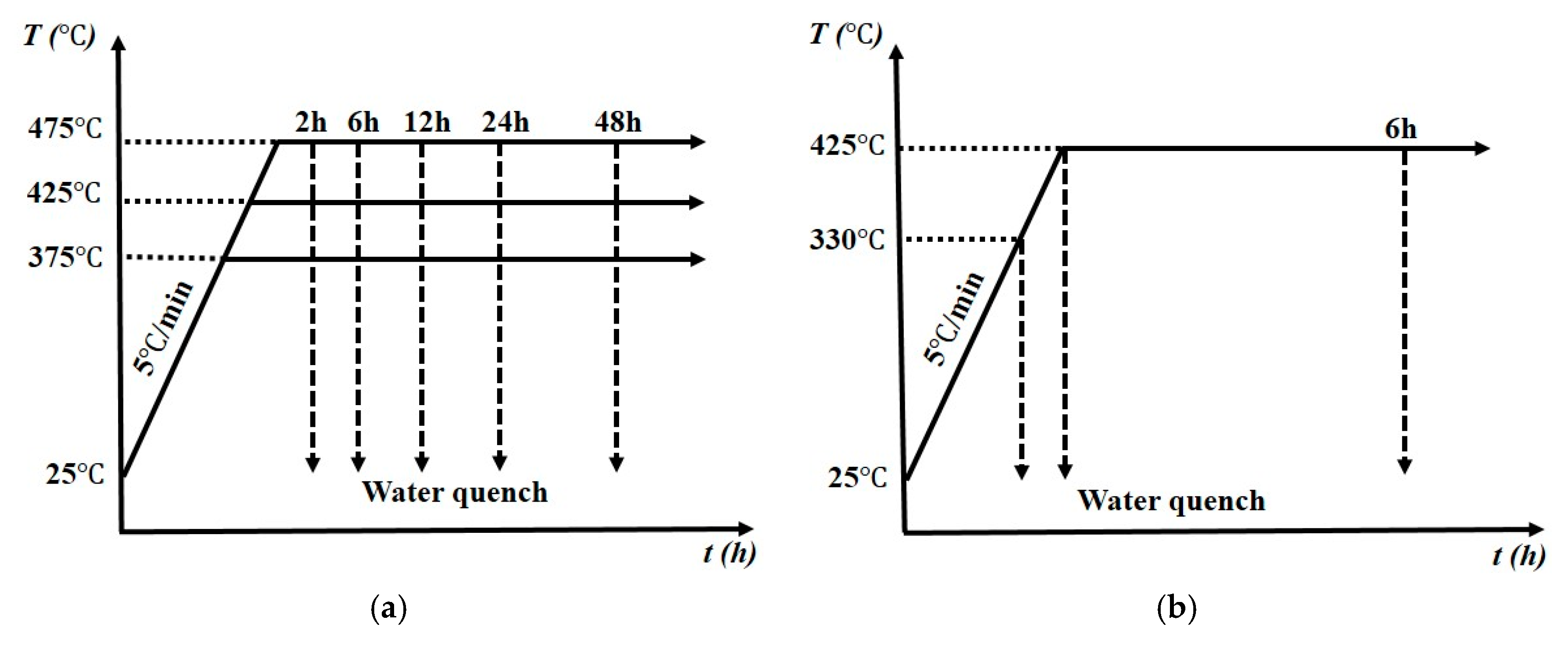
2. Materials and Methods
3. Results and Discussion
3.1. Influence of Cu on Microstructure
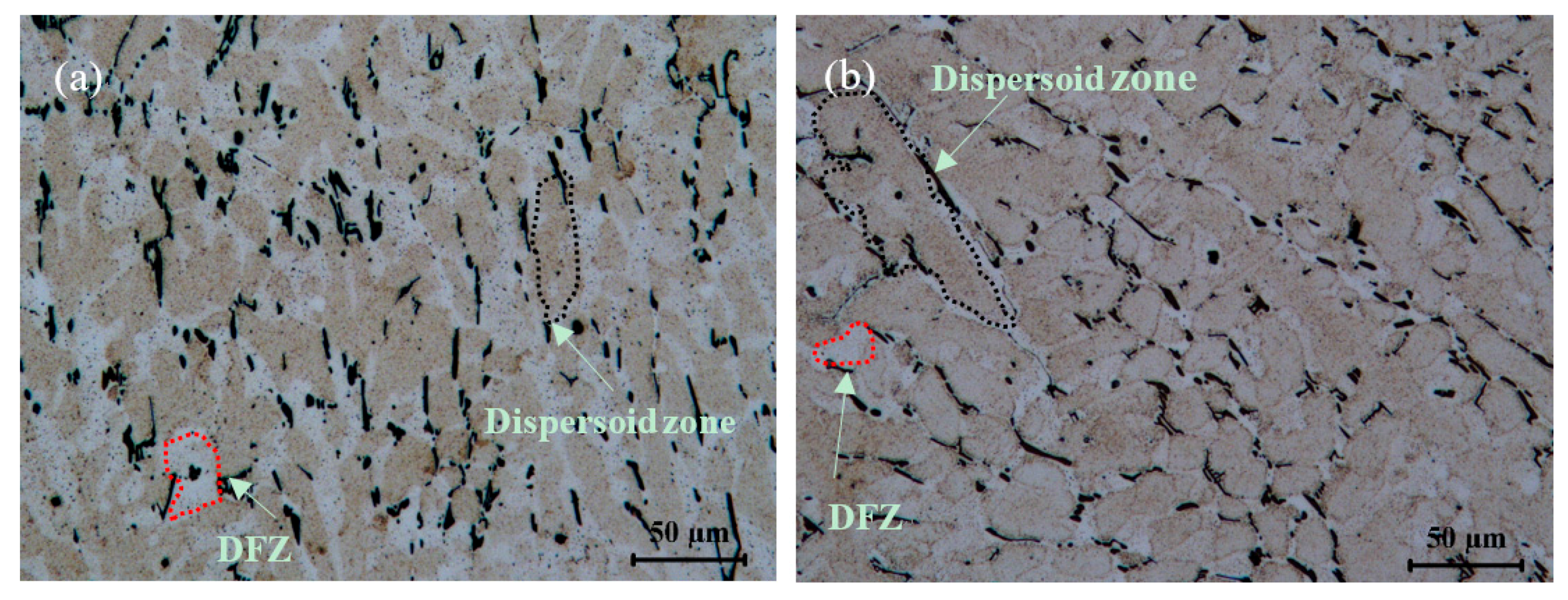
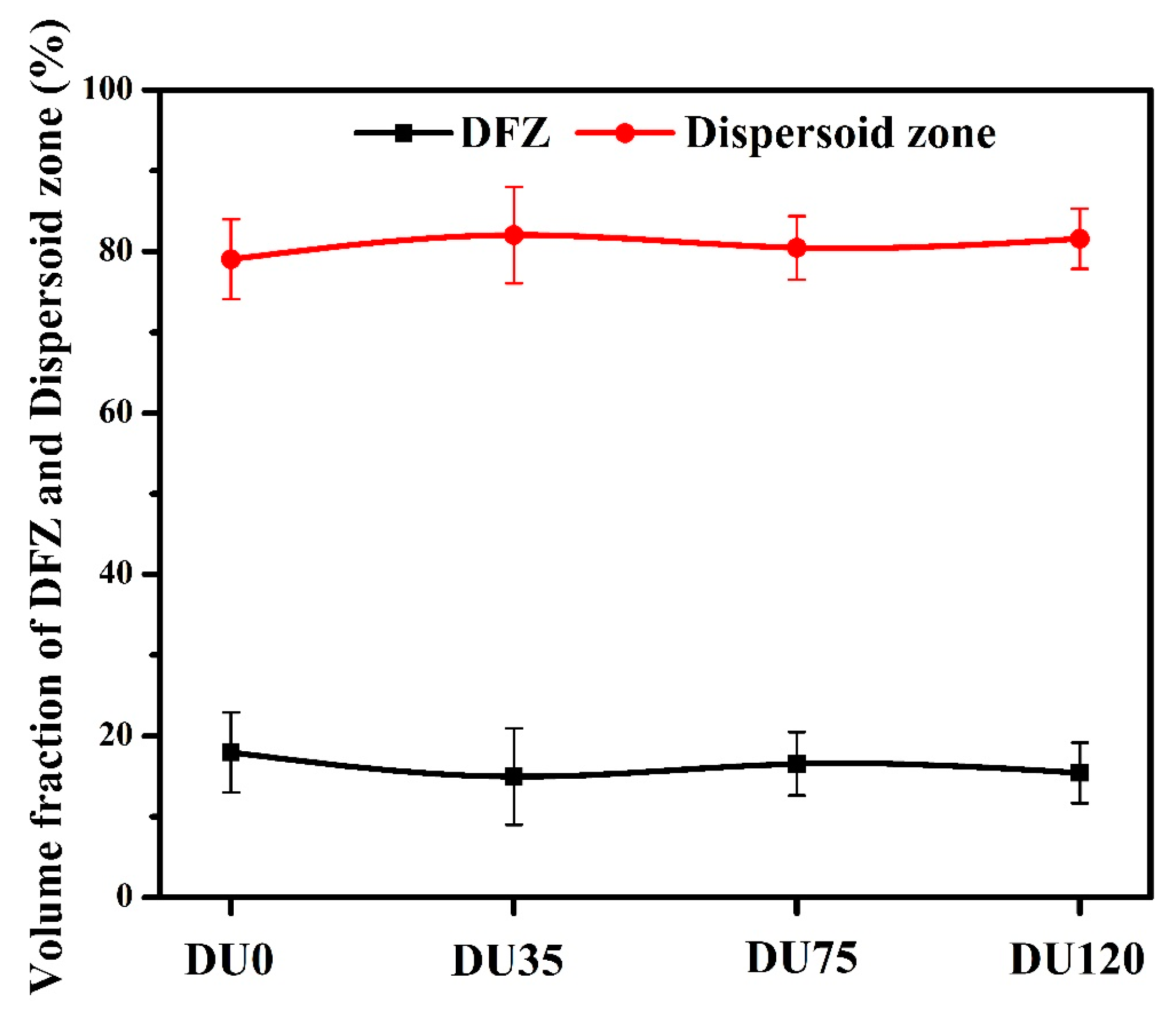
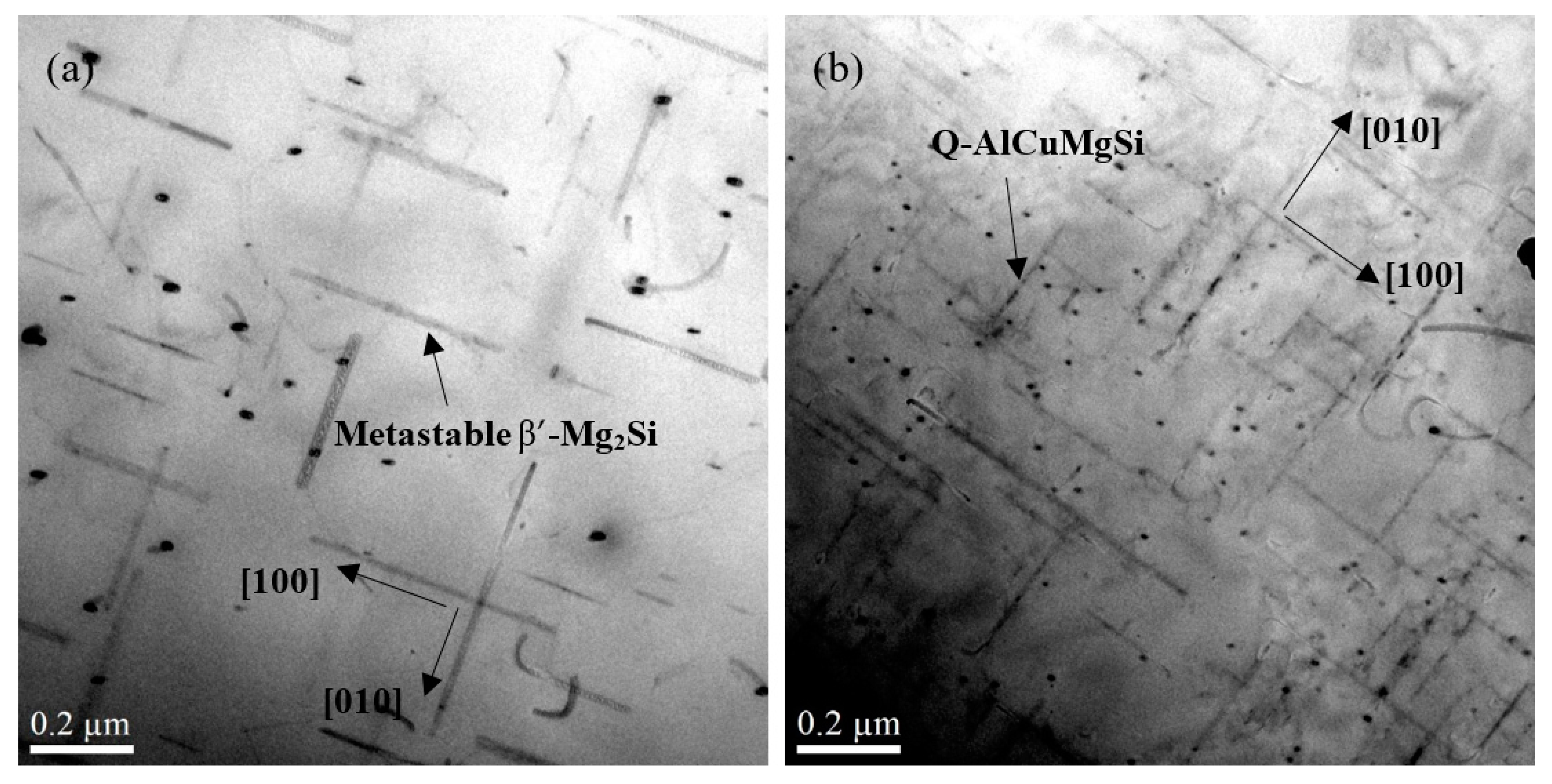
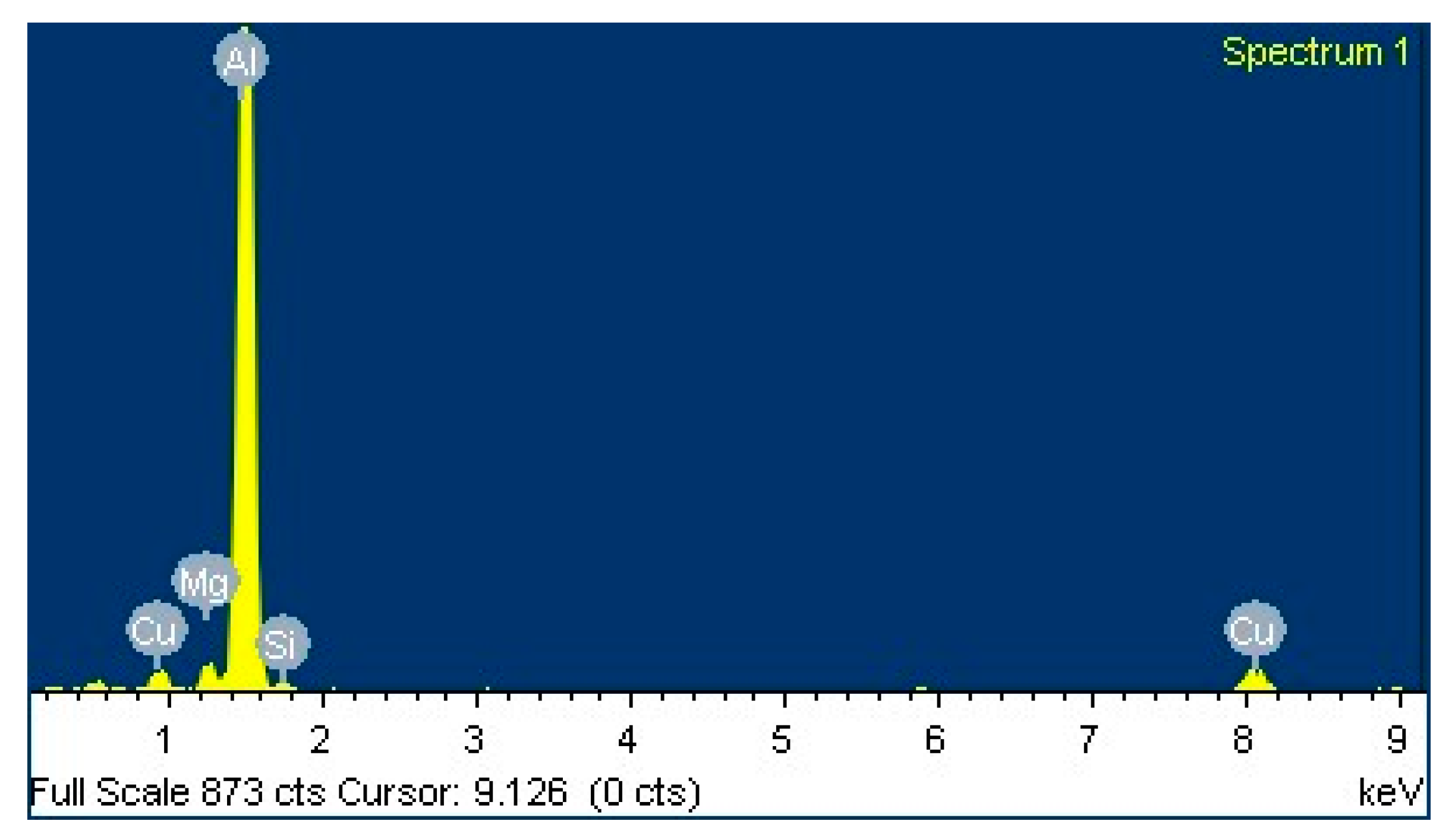
3.1.1. Influence of Cu on Intermetallic Phases and Dispersoid Distribution
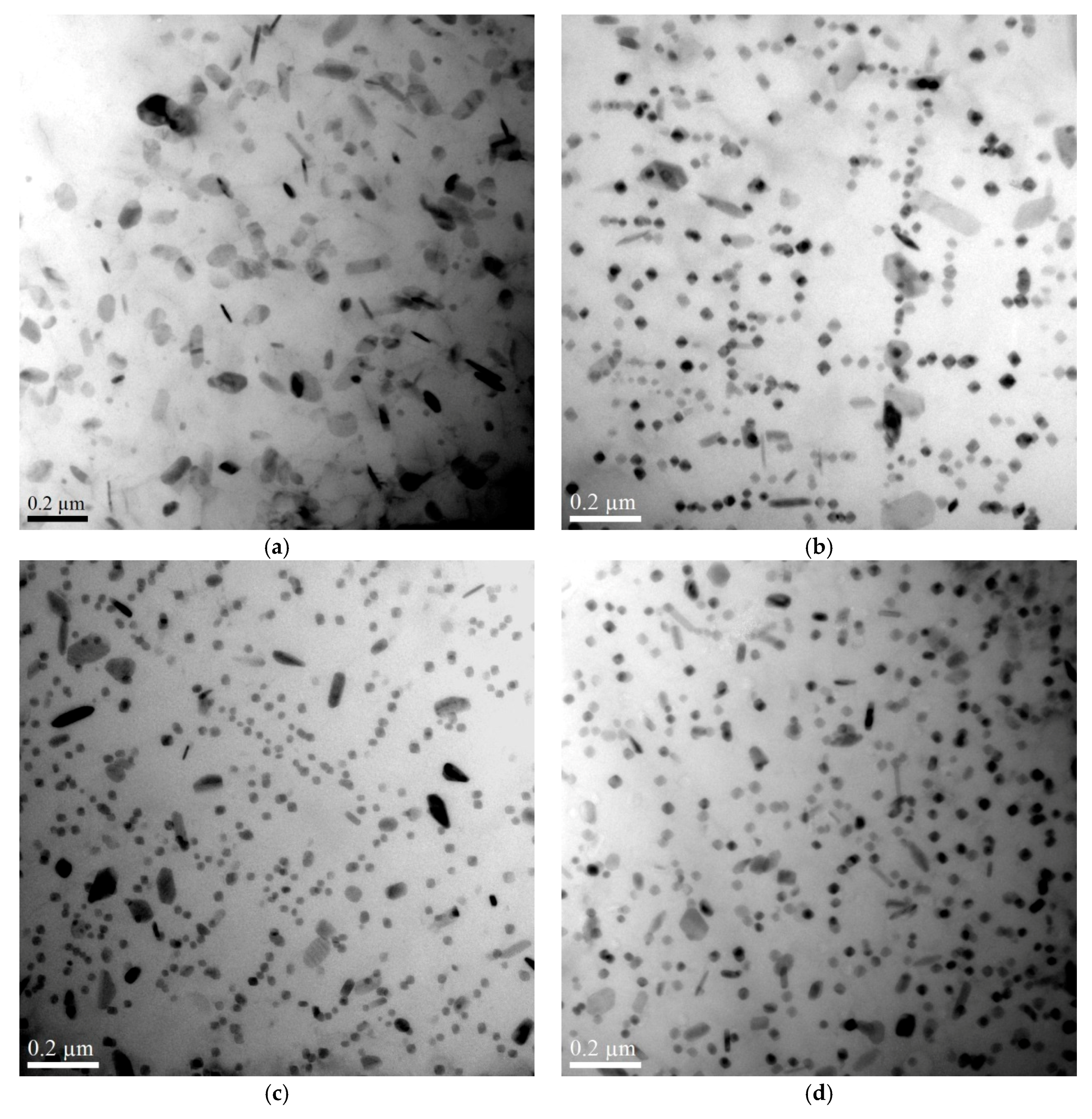
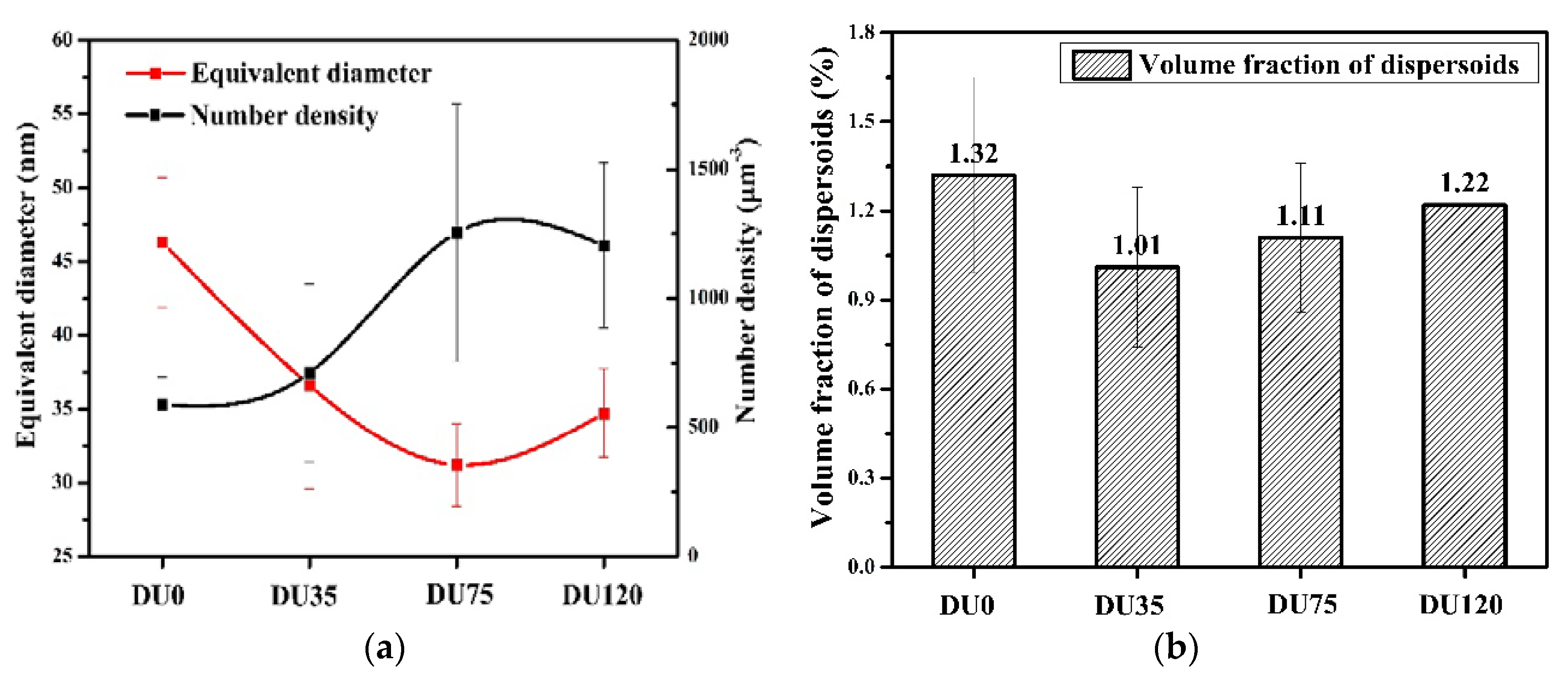
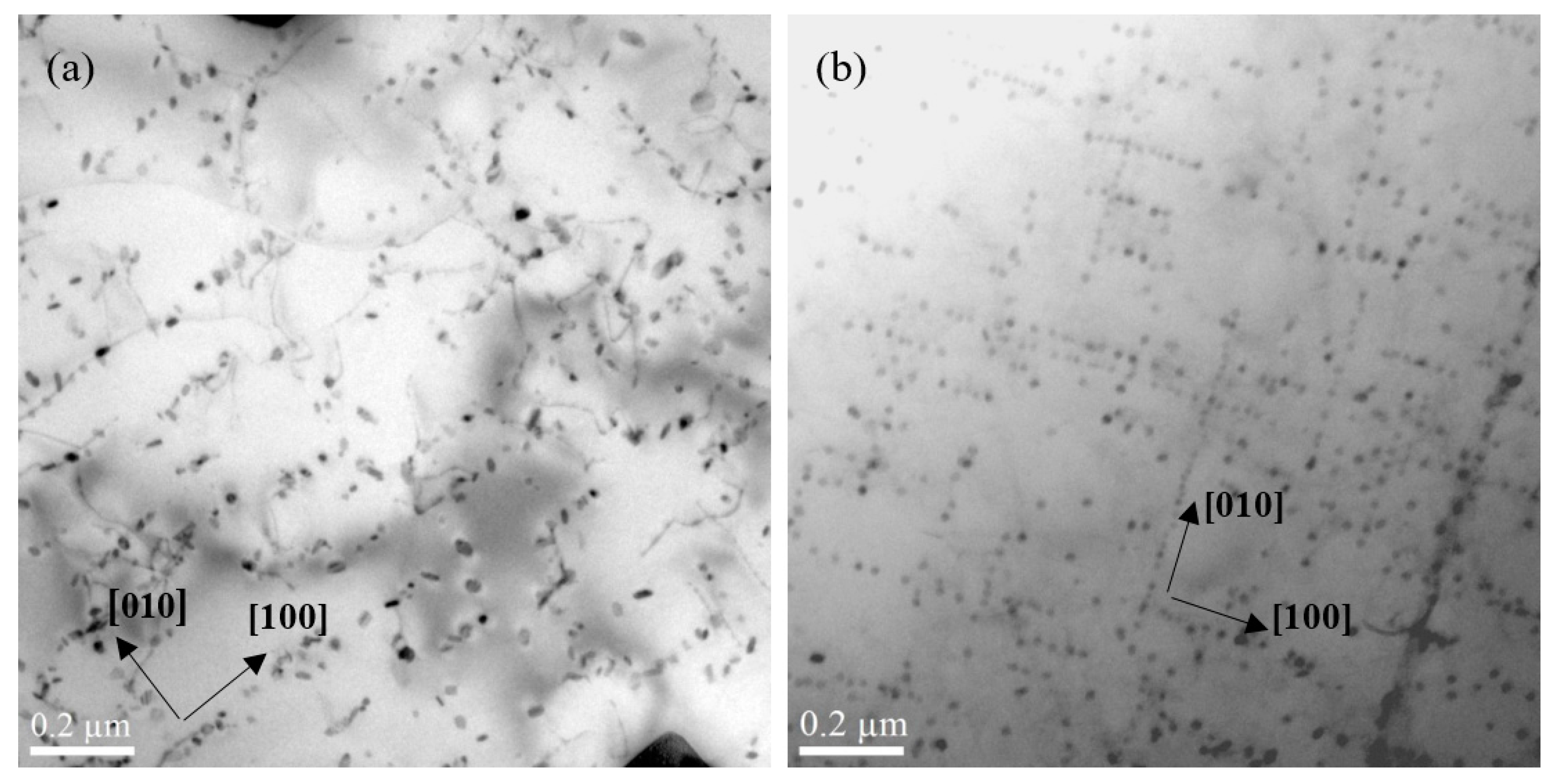
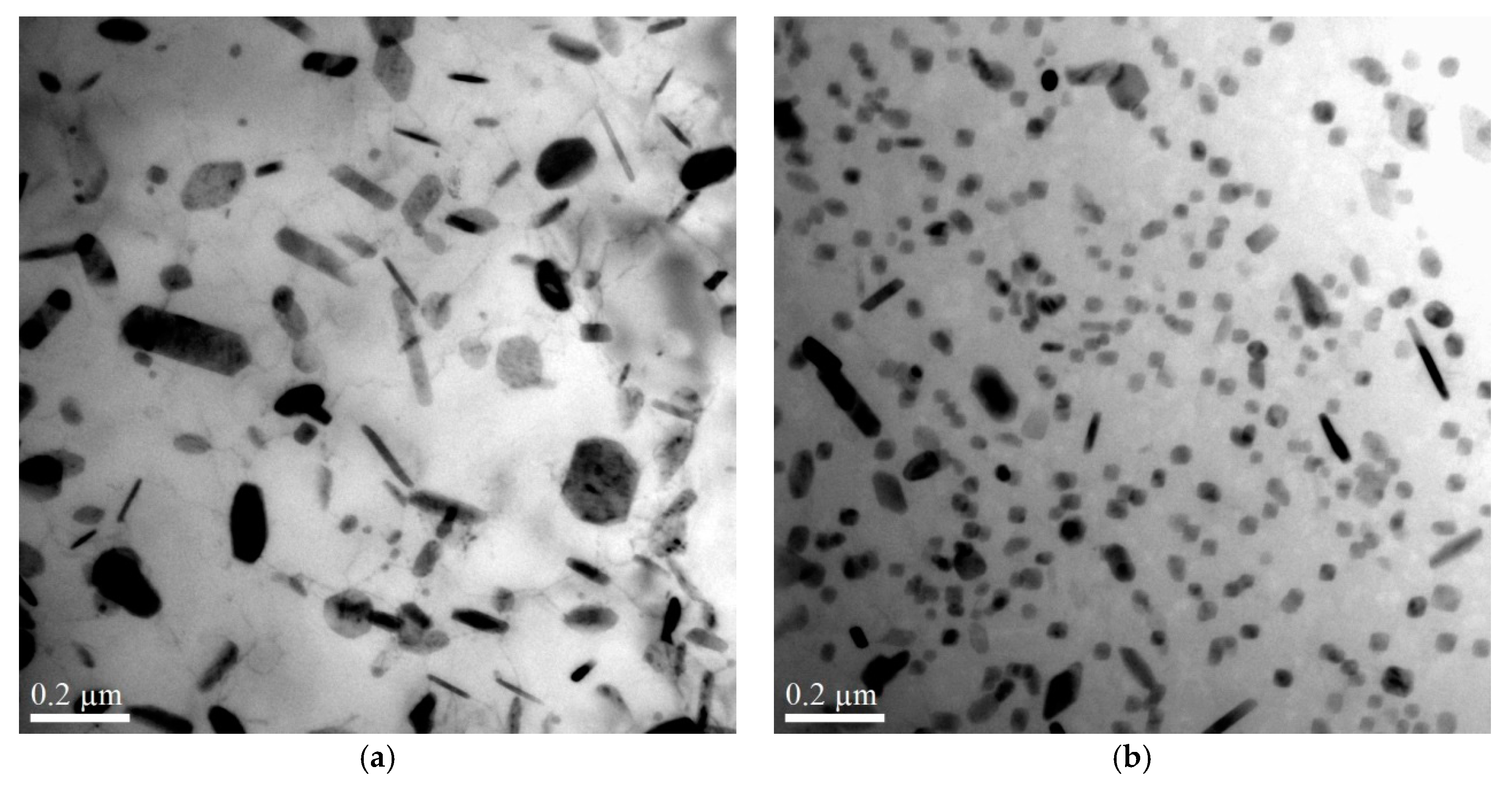
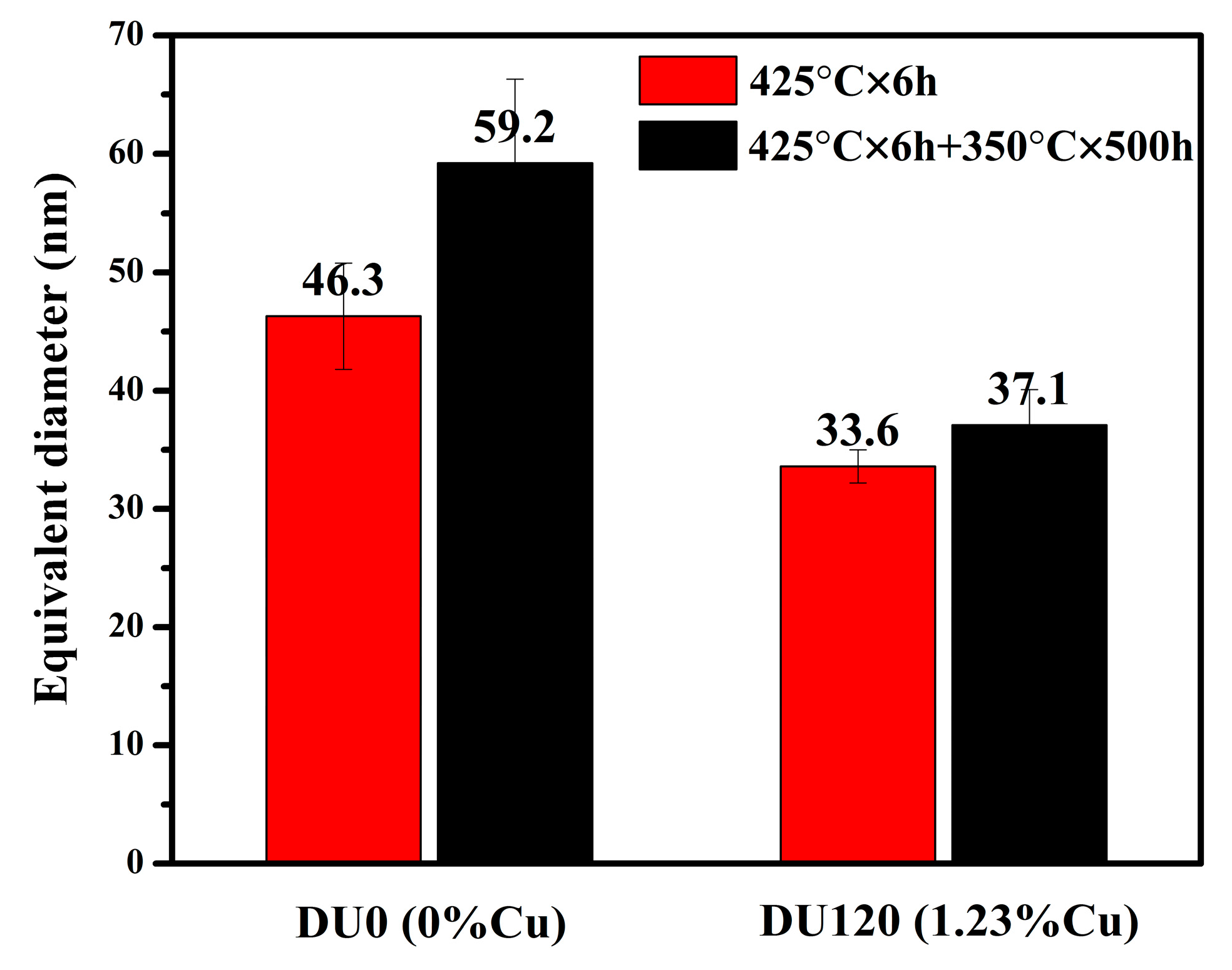
3.1.2. The Influence of Cu on Dispersoid Features and Thermal Stability
3.2. Influence of Cu on Mechanical Properties
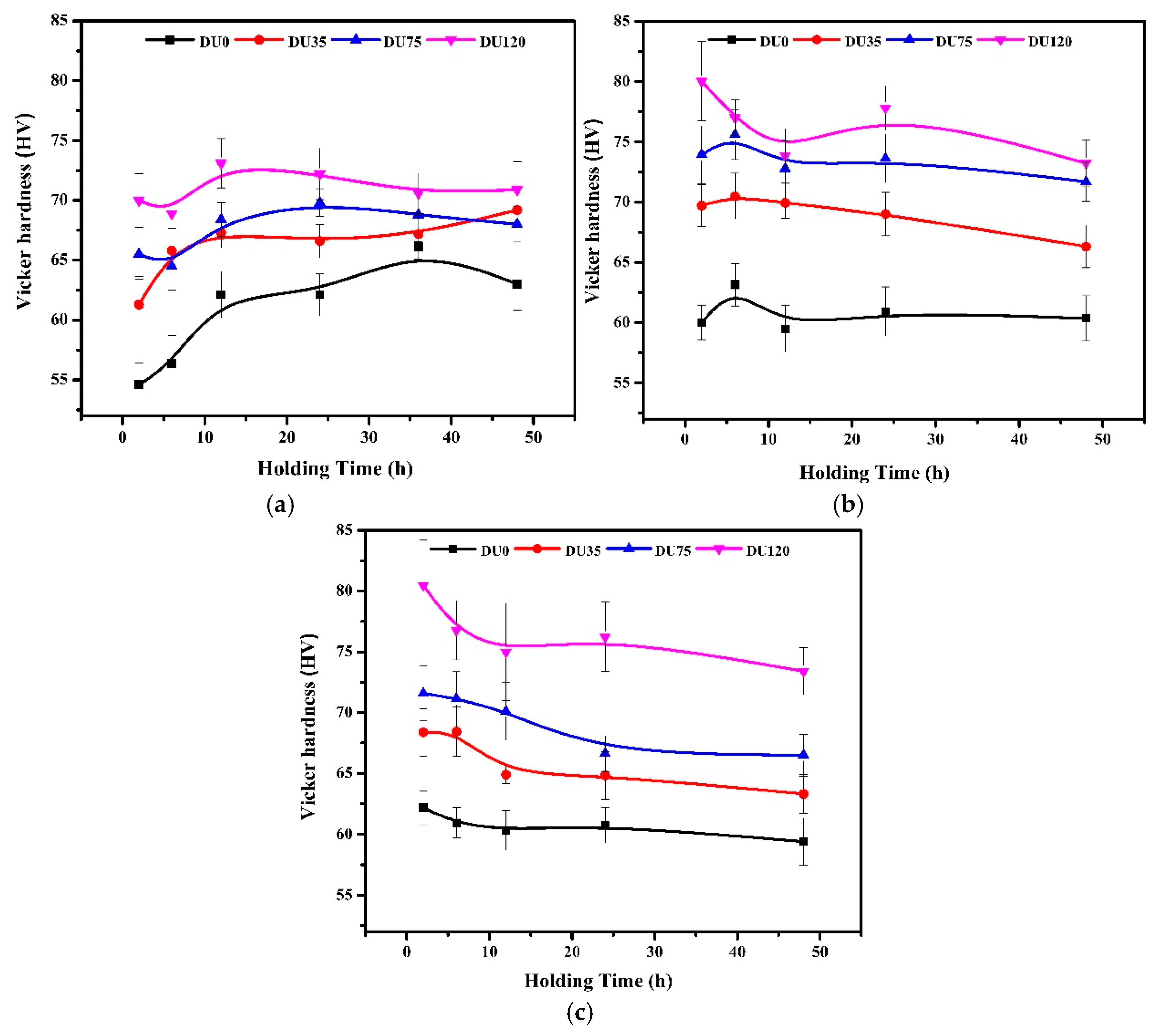
3.2.1. Influence of Cu on Microhardness at Ambient Temperature
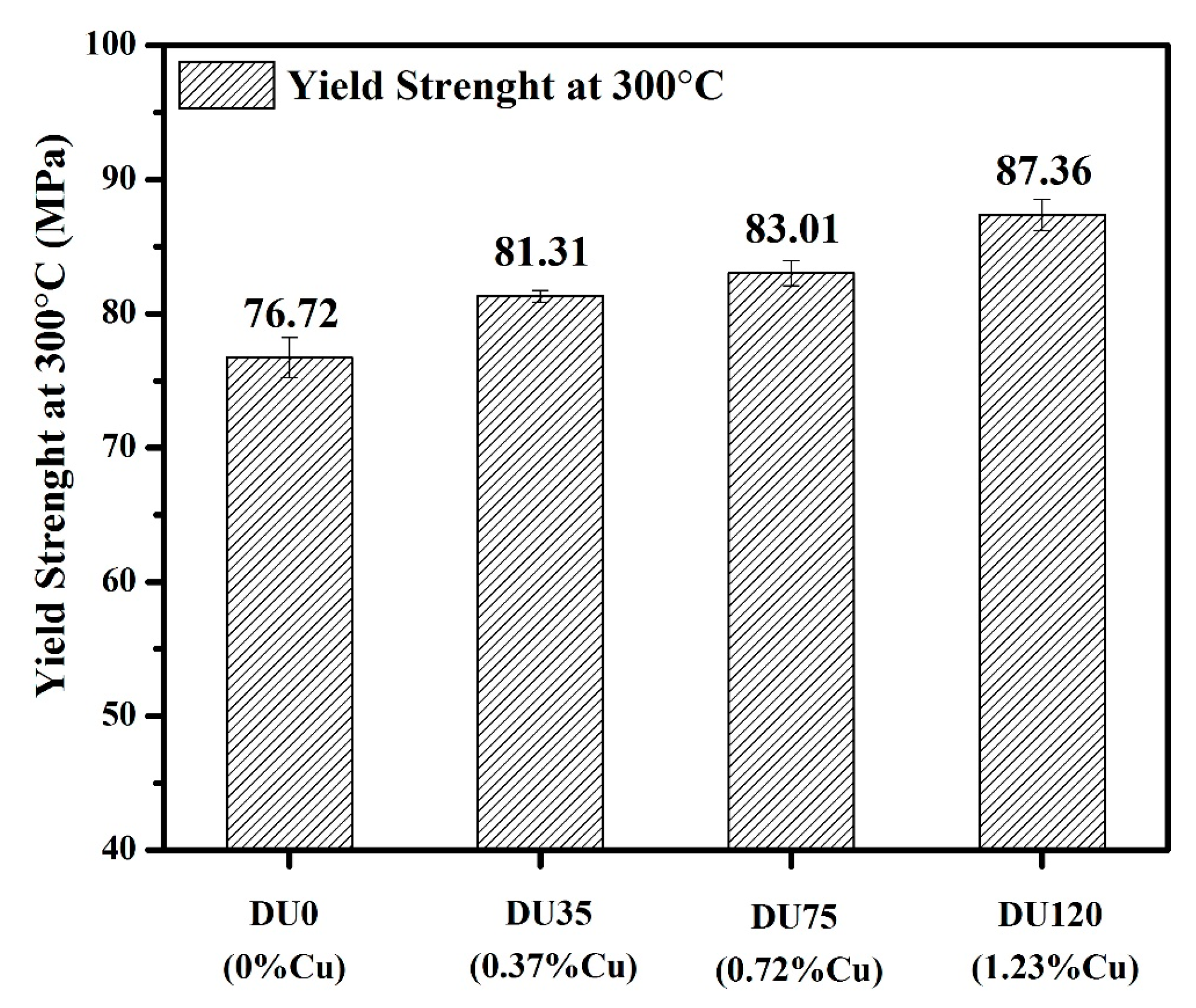
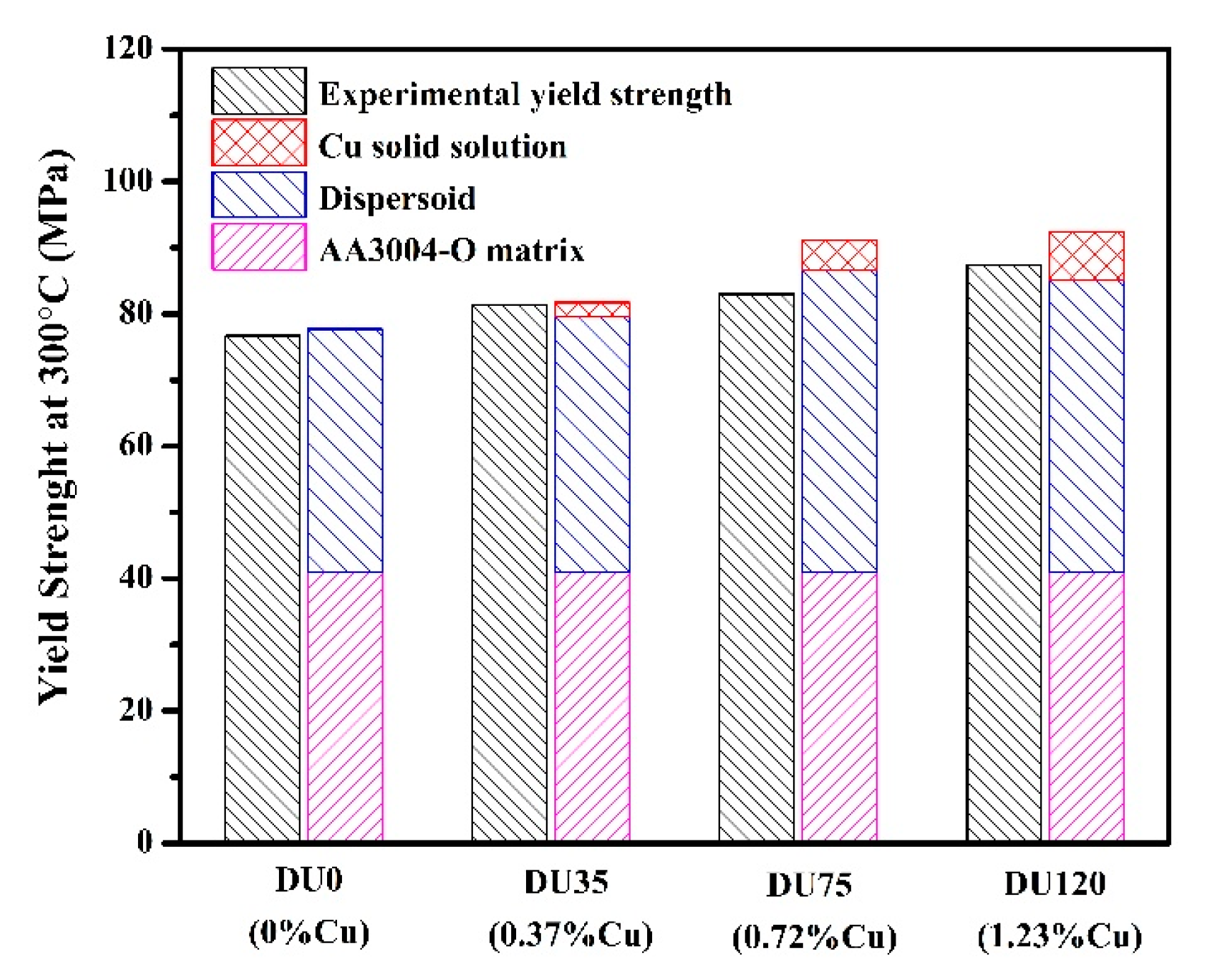
3.2.2. Influence of Cu on Yield Strength at Elevated Temperature
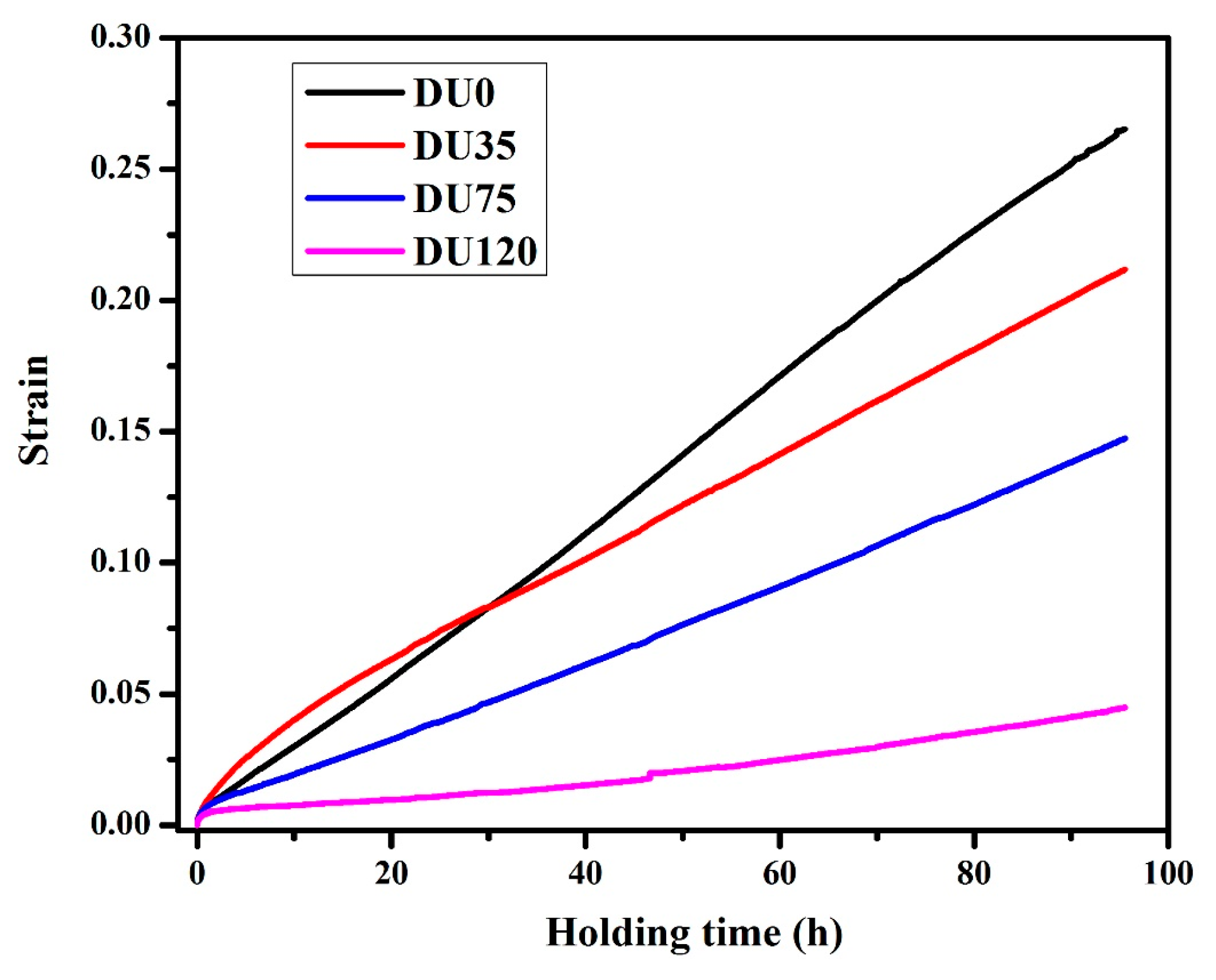
3.2.3. Influence of Cu on Creep Resistance
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, Y.J.; Muggerud, A.M.F.; Olsen, A.; Furu, T. Precipitation of partially coherent α-Al(Mn,Fe)Si dispersoids and their strengthening effect in AA 3003 alloy. Acta Mater. 2012, 60, 1004–1014. [Google Scholar] [CrossRef]
- Dehmas, M.; Aeby-Gautier, E.; Archambault, P.; Serrière, M. Interaction between Eutectic Intermetallic Particles and Dispersoids in the 3003 Aluminum Alloy during Homogenization Treatments. Metall. Mater. Trans. A 2013, 44, 1059–1073. [Google Scholar] [CrossRef]
- Li, Y.J.; Arnberg, L. Quantitative study on the precipitation behavior of dispersoids in DC-cast AA3003 alloy during heating and homogenization. Acta Mater. 2003, 51, 3415–3428. [Google Scholar] [CrossRef]
- Huang, H.-W.; Ou, B.-L. Evolution of precipitation during different homogenization treatments in a 3003 aluminum alloy. Mater. Des. 2009, 30, 2685–2692. [Google Scholar] [CrossRef]
- Muggerud, A.M.F.; Walmsley, J.C.; Holmestad, R.; Li, Y. Combining HAADF STEM tomography and electron diffraction for studies of α-Al(Fe,Mn)Si dispersoids in 3xxx aluminium alloys. Philos. Mag. 2015, 95, 744–758. [Google Scholar] [CrossRef]
- Liu, K.; Chen, X.G. Development of Al–Mn–Mg 3004 alloy for applications at elevated temperature via dispersoid strengthening. Mater. Des. 2015, 84, 340–350. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Chen, X.G. Effect of magnesium on dispersoid strengthening of Al–Mn–Mg–Si (3xxx) alloys. Trans. Nonferr. Met. Soc. China 2016, 26, 2793–2799. [Google Scholar] [CrossRef]
- Muggerud, A.M.F.; Mørtsell, E.A.; Li, Y.; Holmestad, R. Dispersoid strengthening in AA3xxx alloys with varying Mn and Si content during annealing at low temperatures. Mater. Sci. Eng. A 2013, 567, 21–28. [Google Scholar] [CrossRef]
- Liu, K.; Chen, X.-G. Evolution of Intermetallics, Dispersoids, and Elevated Temperature Properties at Various Fe Contents in Al-Mn-Mg 3004 Alloys. Metall. Mater. Trans. B 2016, 47, 3291–3300. [Google Scholar] [CrossRef]
- Lodgaard, L.; Ryum, N. Precipitation of dispersoids containing Mn and/or Cr in Al–Mg–Si alloys. Mater. Sci. Eng. A 2000, 283, 144–152. [Google Scholar] [CrossRef]
- Hirasawa, H. Precipitation process of Al-Mn and Al-Cr supersaturated solid solution in presence of age hardening phases. Scr. Metall. 1975, 9, 955–958. [Google Scholar] [CrossRef]
- Liu, K.; Ma, H.; Chen, X.G. Enhanced elevated-temperature properties via Mo addition in Al-Mn-Mg 3004 alloy. J. Alloys Compd. 2017, 694, 354–365. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Chen, X.G. Microstructure, elevated-temperature mechanical properties and creep resistance of dispersoid-strengthened Al-Mn-Mg 3xxx alloys with varying Mg and Si contents. Mater. Sci. Eng. A 2017, 708, 383–394. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Chen, X.G. Effect of metastable Mg2Si and dislocations on α-Al(MnFe)Si dispersoid formation in Al-Mn-Mg 3xxx alloys. Metall. Mater. Trans. A 2018. submitted. [Google Scholar]
- Marlaud, T.; Deschamps, A.; Bley, F.; Lefebvre, W.; Baroux, B. Influence of alloy composition and heat treatment on precipitate composition in Al–Zn–Mg–Cu alloys. Acta Mater. 2010, 58, 248–260. [Google Scholar] [CrossRef]
- Marioara, C.; Andersen, S.; Stene, T.; Hasting, H.; Walmsley, J.; Van Helvoort, A.; Holmestad, R. The effect of Cu on precipitation in Al–Mg–Si alloys. Philos. Mag. 2007, 87, 3385–3413. [Google Scholar] [CrossRef]
- Wang, X.; Esmaeili, S.; Lloyd, D.J. The sequence of precipitation in the Al-Mg-Si-Cu alloy AA6111. Metall. Mater. Trans. A 2006, 37, 2691–2699. [Google Scholar] [CrossRef]
- Marioara, C.D.; Andersen, S.J.; Røyset, J.; Reiso, O.; Gulbrandsen-Dahl, S.; Nicolaisen, T.-E.; Opheim, I.-E.; Helgaker, J.F.; Holmestad, R. Improving Thermal Stability in Cu-Containing Al–Mg–Si Alloys by Precipitate Optimization. Metall. Mater. Trans. A 2014, 45, 2938–2949. [Google Scholar] [CrossRef]
- Man, J.; Jing, L.; Jie, S.G. The effects of Cu addition on the microstructure and thermal stability of an Al–Mg–Si alloy. J. Alloys Compd. 2007, 437, 146–150. [Google Scholar] [CrossRef]
- Edwards, G.A.; Stiller, K.; Dunlop, G.L.; Couper, M.J. The precipitation sequence in Al–Mg–Si alloys. Acta Mater. 1998, 46, 3893–3904. [Google Scholar] [CrossRef]
- Vissers, R.; van Huis, M.A.; Jansen, J.; Zandbergen, H.W.; Marioara, C.D.; Andersen, S.J. The crystal structure of the β′ phase in Al–Mg–Si alloys. Acta Mater. 2007, 55, 3815–3823. [Google Scholar] [CrossRef]
- Qin, J.; Zhang, Z.; Chen, X.-G. Mechanical Properties and Strengthening Mechanisms of Al-15 Pct B4C Composites with Sc and Zr at Elevated Temperatures. Metall. Mater. Trans. A 2016, 47, 4694–4708. [Google Scholar] [CrossRef]
- Huskins, E.L.; Cao, B.; Ramesh, K.T. Strengthening mechanisms in an Al–Mg alloy. Mater. Sci. Eng. A 2010, 527, 1292–1298. [Google Scholar] [CrossRef]
- Ryen, Ø.; Holmedal, B.; Nijs, O.; Nes, E.; Sjölander, E.; Ekström, H.-E. Strengthening mechanisms in solid solution aluminum alloys. Metall. Mater. Trans. A 2006, 37, 1999–2006. [Google Scholar] [CrossRef]
- Kaufman, J.G. Properties of Aluminum Alloys: Tensile, Creep, and Fatigue Data at High and Low Temperatures; ASM International: Materials Park, OH, USA, 1999; pp. 9, 41, 102. ISBN 978-0-87170-632-4. [Google Scholar]


| Alloy Code | Cu | Si | Fe | Mn | Mg | Al |
|---|---|---|---|---|---|---|
| DU0 (base) | 0 | 0.24 | 0.49 | 1.23 | 0.97 | Bal. |
| DU35 | 0.37 | 0.27 | 0.53 | 1.25 | 1.03 | Bal. |
| DU75 | 0.72 | 0.24 | 0.53 | 1.24 | 0.99 | Bal. |
| DU120 | 1.23 | 0.26 | 0.48 | 1.27 | 1.04 | Bal. |
| Treatment Temperature (°C) | Properties | DU0 | DU35 | DU75 | DU120 |
|---|---|---|---|---|---|
| 375 | Peak hardness | 65 HV/36 h | 68 HV/48 h | 70 HV/24 h | 73 HV/12 h |
| EC (MS/m) | 23.0 | 22.8 | 22.0 | 21.0 | |
| 425 | Peak hardness | 62 HV/6 h | 70 HV/6 h | 75 HV/6 h | 80 HV/2 h |
| EC (MS/m) | 22.5 | 22.2 | 21.6 | 20.5 | |
| 475 | Peak hardness | 62 HV/2 h | 68 HV/2 h | 72 HV/2 h | 80 HV/2 h |
| EC (MS/m) | 21.8 | 21.5 | 21.2 | 20.2 |
| Strength Contribution | DU0 | DU35 | DU75 | DU120 |
|---|---|---|---|---|
| AA3004-O matrix (MPa) | 41 | 41 | 41 | 41 |
| Dispersoids (MPa) | 36.7 | 38.6 | 45.7 | 44.2 |
| Cu solid solution (MPa) | 0 | 2.1 | 4.5 | 7.2 |
| The total calculated results (MPa) | 77.7 | 81.7 | 91.2 | 92.4 |
| The experimental results (MPa) | 76.7 | 81.3 | 83.0 | 87.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zhang, Z.; Chen, X.-G. The Influence of Cu Addition on Dispersoid Formation and Mechanical Properties of Al-Mn-Mg 3004 Alloy. Metals 2018, 8, 155. https://doi.org/10.3390/met8030155
Li Z, Zhang Z, Chen X-G. The Influence of Cu Addition on Dispersoid Formation and Mechanical Properties of Al-Mn-Mg 3004 Alloy. Metals. 2018; 8(3):155. https://doi.org/10.3390/met8030155
Chicago/Turabian StyleLi, Zhen, Zhan Zhang, and X.-Grant Chen. 2018. "The Influence of Cu Addition on Dispersoid Formation and Mechanical Properties of Al-Mn-Mg 3004 Alloy" Metals 8, no. 3: 155. https://doi.org/10.3390/met8030155




