Agglomeration Behavior of Non-Metallic Particles on the Surface of Ca-Treated High-Carbon Liquid Steel: An In Situ Investigation
Abstract
:1. Introduction
2. Experimental Procedure
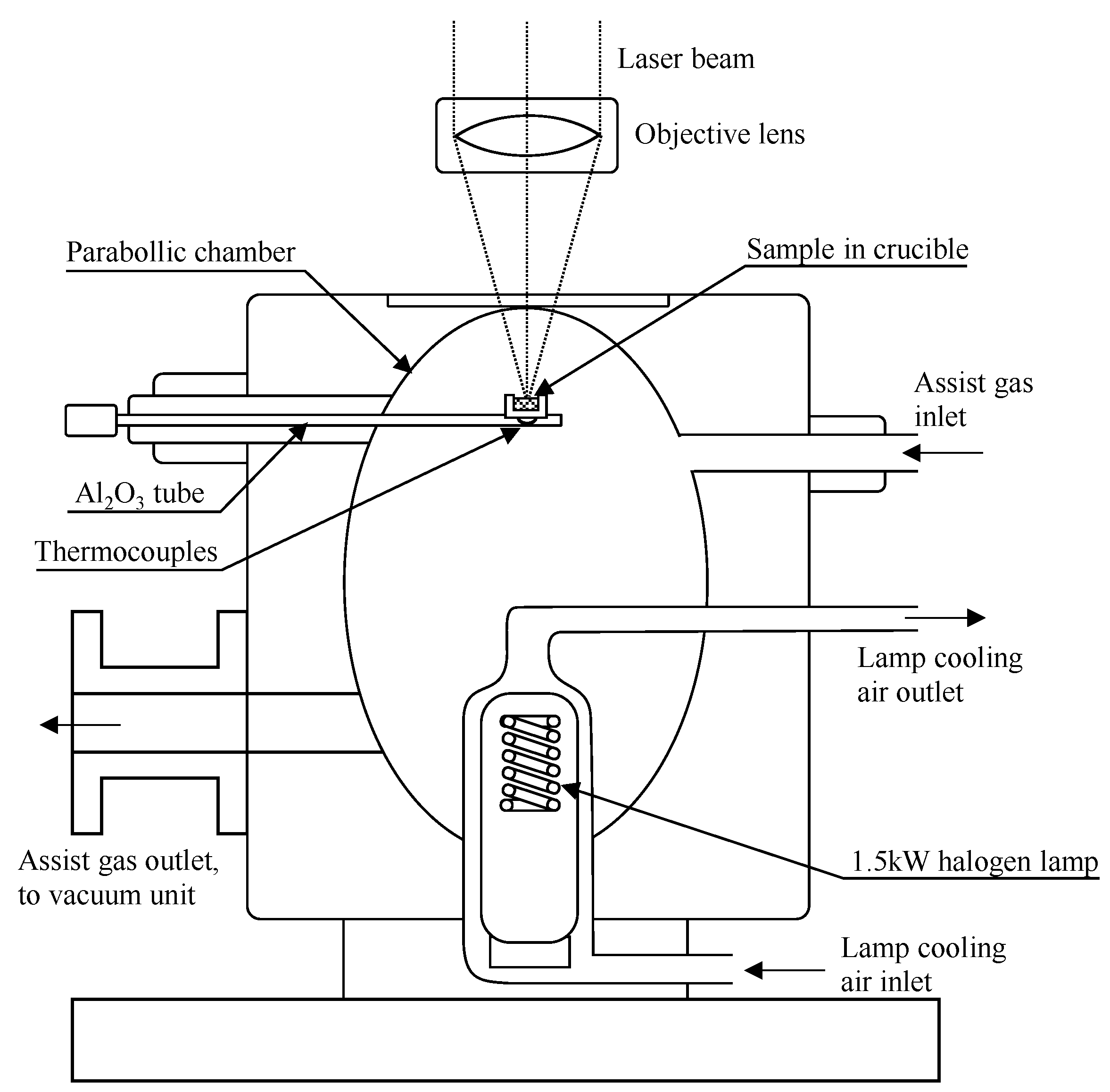
2.1. Equipment for In Situ Observation
2.2. Materials and Heating Pattern
3. Results and Discussion
3.1. Chemical Compositions
3.2. Morphologies in Confocal Scanning Laser Microscope (CSLM)
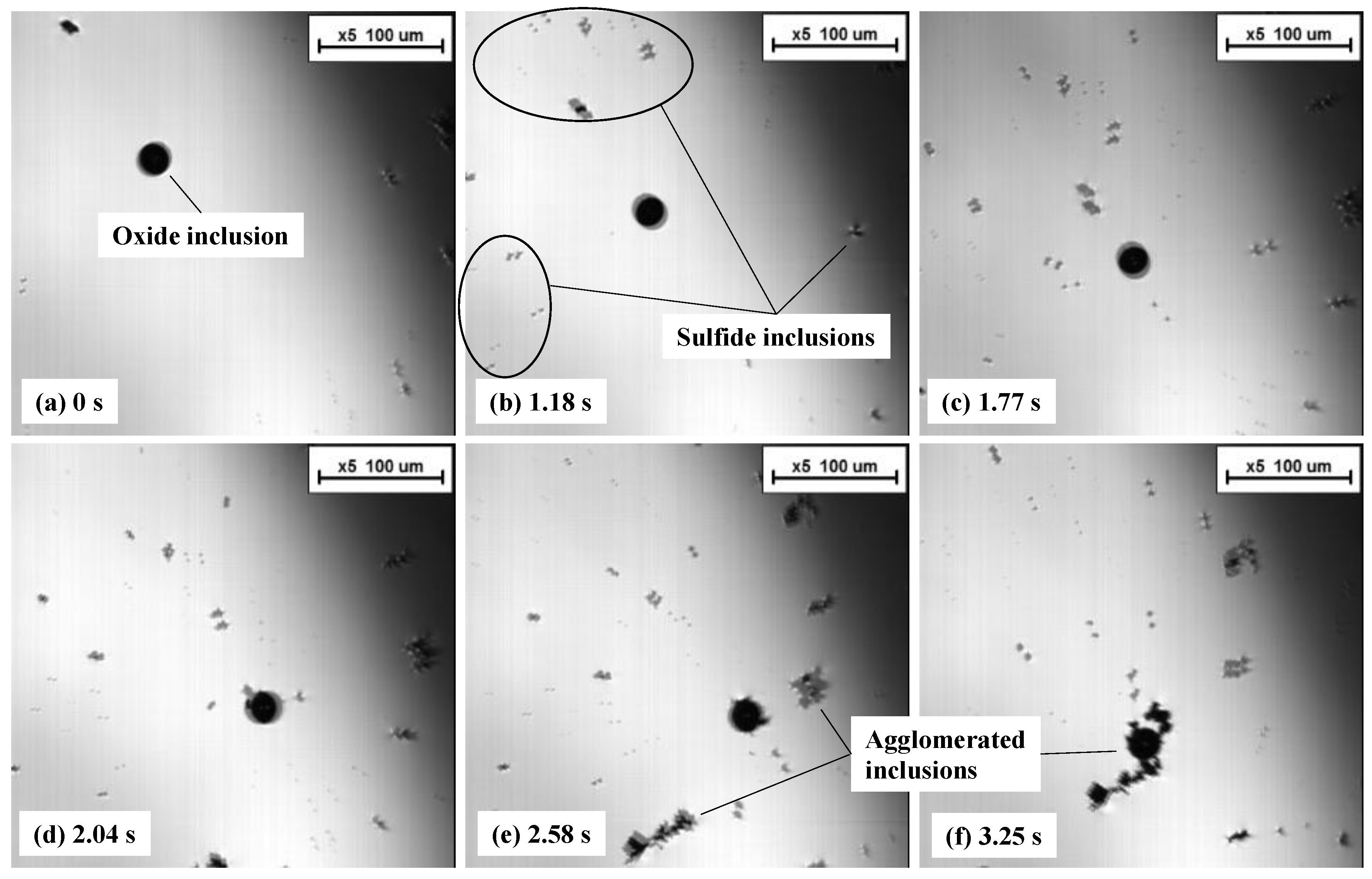
3.3. Agglomeratrion Behaviors
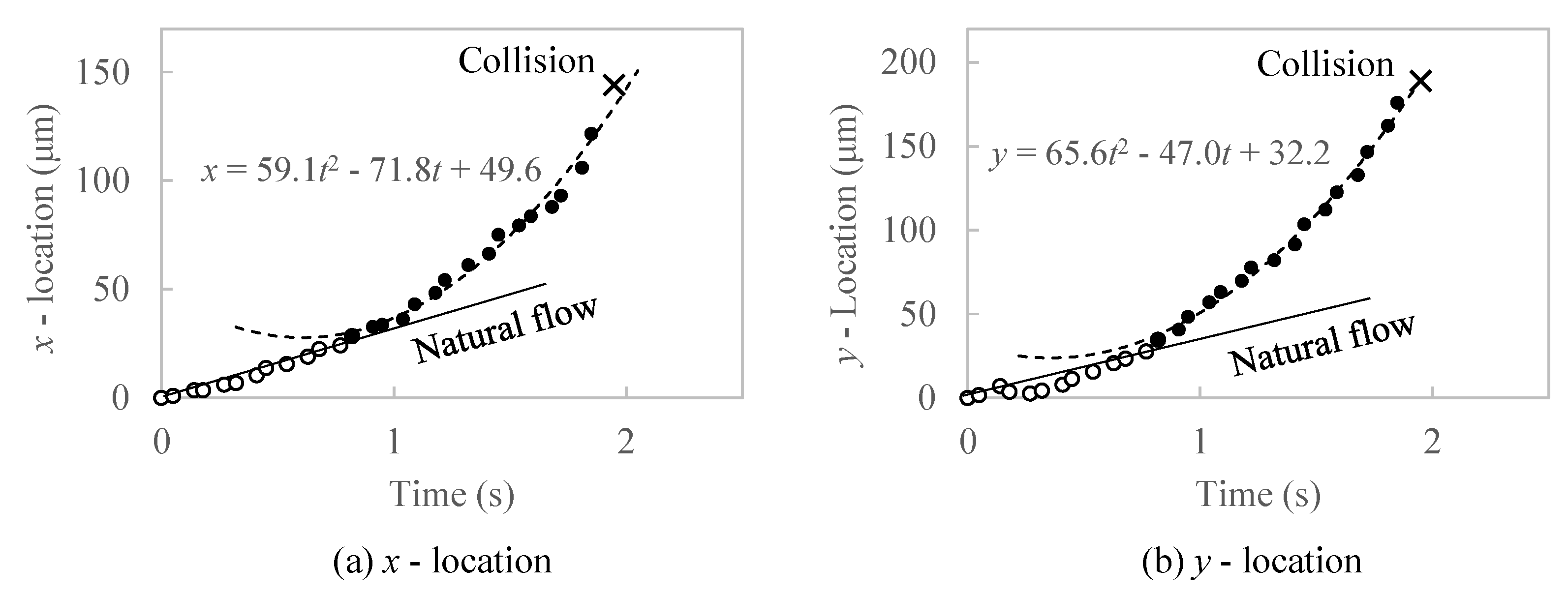
3.4. Measurement of Attraction Force and Acting Length from CSLM Video File
3.5. Type of Attraction Force
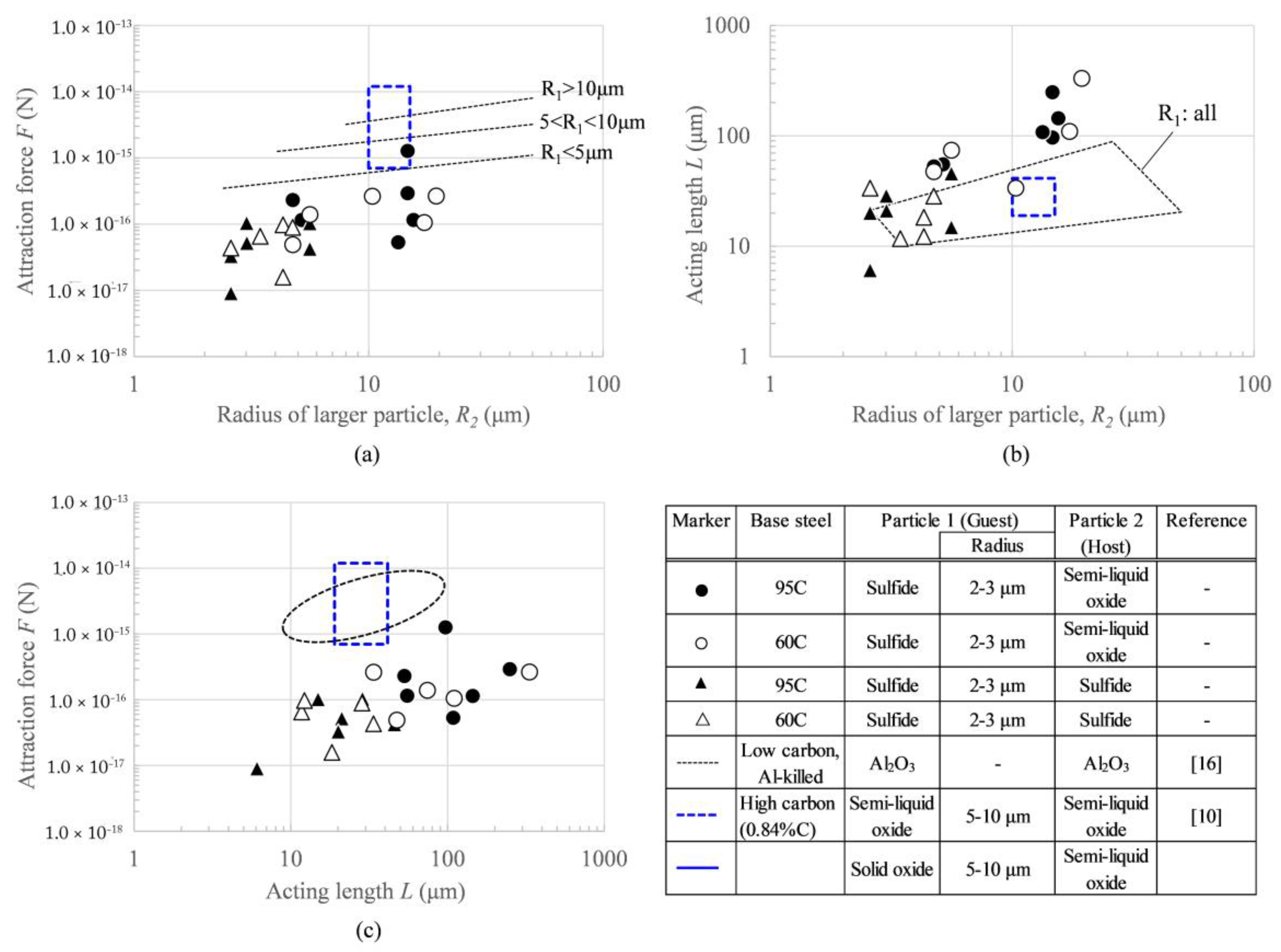
3.6. Comparison with Other Materials
3.7. Agglomeration Behavior Caused by Capillary Force
4. Conclusions
- The agglomeration behaviors of sulfide/sulfide particle pairs and sulfide/semi-liquid oxide pairs on the surface of liquid high-carbon steels were observed in situ by using CSLM. Agglomerations between oxide/oxide pairs were not detected because they were surrounded by sulfide particles shortly after floating up to the surface.
- Attraction forces between the surface particles were calculated from acceleration, estimated by tracking the trajectories of each particle in CSLM video files. The acting length is defined as the distance between particles when they start to accelerate.
- Estimated attraction forces and identified acting length are quite close to the values of the capillary phenomenon estimated in recent studies.
- The attraction force of sulfide/sulfide and sulfide/semi-liquid oxide pairs on the surface of high-carbon steel was revealed to be slightly weaker than that of the alumina particle pair on the surface of low-carbon steel. Possible reasons could be the particle size and wettability, which is affected by the temperature and carbon percentage in liquid steel and the surface roughness of solid particle.
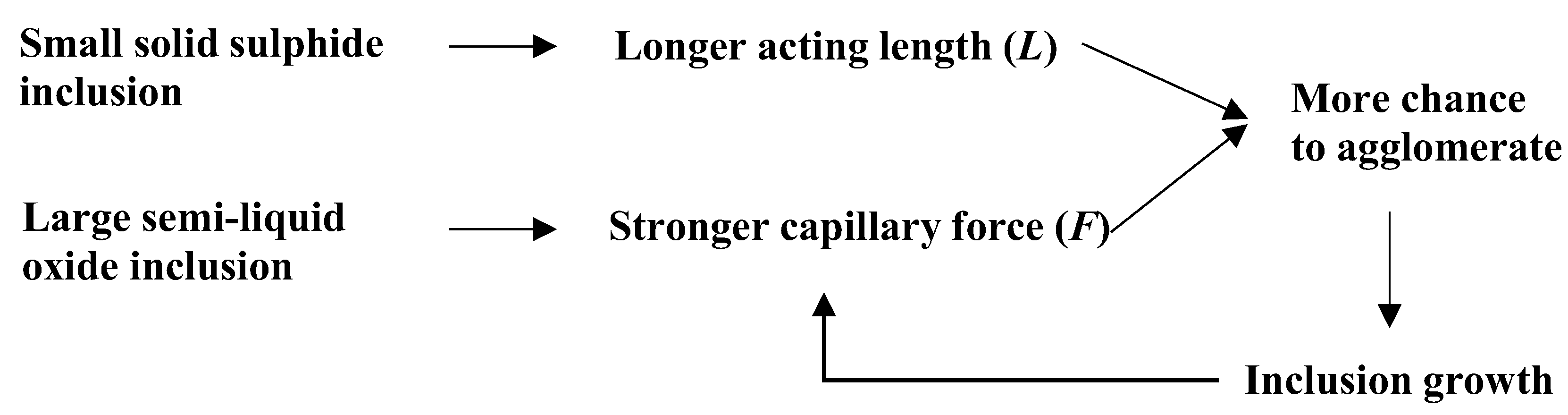
- Semi-liquid oxide particles can grow through surface agglomeration, leading to further, stronger capillary forces. It is indicated that agglomeration among non-metallic surface particles is more likely to occur under conditions where small sulfide particles and large semi-liquid oxide particles that are capable of growing co-exist with each other.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sasai, K. Direct Measurement of Agglomeration Force Exerted between Alumina Particles in Molten Steel. ISIJ Int. 2014, 54, 2780–2789. [Google Scholar] [CrossRef]
- Mizoguchi, T.; Ueshima, Y.; Sugiyama, M.; Mizukami, K. Influence of Unstable Non-equilibrium Liquid Iron Oxide on Clustering of Alumina Particles in Steel. Tetsu-to-Hagané 2013, 99, 601–609. [Google Scholar] [CrossRef]
- Sasai, K. Interaction between Alumina Inclusions in Molten Steel Due to Cavity Bridge Force. ISIJ Int. 2016, 56, 1013–1022. [Google Scholar] [CrossRef]
- Zheng, L.; Malfliet, A.; Wollants, P.; Blanpain, B.; Guo, M. Effect of Alumina Morphology on the Clustering of Alumina Inclusions in Molten Iron. ISIJ Int. 2016, 56, 926–935. [Google Scholar] [CrossRef]
- Fuchigami, K.; Wakoh, M.; Imamura, N.; Endoh, K.; Kiyose, A.; Sawada, I. Analysis of Inclusion Behavior in a Ladle Refining Process by a Newly Developed Coagulation Model. Tetsu-to-Hagané 1999, 85, 368–374. [Google Scholar] [CrossRef]
- Arai, H.; Nakamura, Y.; Shimasaki, S.; Taniguchi, S. Theoretical Model on Heterocoagulation of Inclusion in Molten Steel and its Experimental Verification: Part I. The Theoretical Model. Tetsu-to-Hagané 2015, 101, 129–138. [Google Scholar] [CrossRef]
- Nakaoka, T.; Taniguchi, S.; Matsumoto, K.; Johansen, S.T. Particle-Size-Grouping Method of Inclusion Agglomeration and its Application to Water Model Experiments. ISIJ Int. 2001, 41, 1103–1111. [Google Scholar] [CrossRef]
- Higashitani, K.; Ogawa, R.; Hosokawa, G.; Matsuno, Y. Kinetic Theory of Shear Coagulation for Particles in a Viscous Fluid. J. Chem. Eng. Jpn. 1982, 15, 299–304. [Google Scholar] [CrossRef]
- Yin, H.; Shibata, H.; Emi, T.; Suzuki, M. “In-Situ” Observation of Collision, Agglomeration and Cluster Formation of Alumina Inclusion Particles on Steel Melts. ISIJ Int. 1997, 37, 936–945. [Google Scholar] [CrossRef]
- Yin, H.; Shibata, H.; Emi, T.; Suzuki, M. Characteristics of Agglomeration of Various Inclusion Particles on Molten Steel Surface. ISIJ Int. 1997, 37, 946–955. [Google Scholar] [CrossRef]
- Bikerman, J.J. Physical Surfaces; Academic Press: New York, NY, USA, 1970; Chapter 1. [Google Scholar]
- Kang, Y.; Sahebkar, B.; Scheller, P.R.; Morita, K.; Sichen, D. Observation on Physical Growth of Nonmetallic Inclusion in Liquid Steel during Ladle Treatment. Metall. Mater. Trans. B 2011, 42, 522–534. [Google Scholar] [CrossRef]
- Aneziris, C.G.; Schroeder, C.; Emmel, M.; Schmidt, G.; Heller, H.P.; Berek, H. In Situ Observation of Collision between Exogenous and Endogenous Inclusions on Steel Melts for Active Steel Filtration. Metall. Trans. B 2013, 44, 954–968. [Google Scholar] [CrossRef]
- Aneziris, C.G.; Schroeder, C.; Fischer, U.; Berek, H.; Emmel, M.; Kortus, J.; Amirkhanyan, L.G.; Weißbach, T. Interactions between Exogenous Spinel Inclusions with Endogenous Inclusions in a Steel Melt. Adv. Eng. Mater. 2013, 15, 1169–1176. [Google Scholar] [CrossRef]
- Schröder, C.; Fischer, U.; Schmidt, A.; Schmidt, G.; Volkova, O.; Aneziris, C.G. Interactions between Exogenous Magnesia Inclusions with Endogenous Inclusions in a High Alloyed Steel Melt. Adv. Eng. Mater. 2017, 19. [Google Scholar] [CrossRef]
- Shibata, H.; Yin, H.; Emi, T. The Capillary Effect Promoting Collision and Agglomeration of Inclusion Particles at the Inert Gas-Steel Interface. Philos. Trans. R. Soc. Lond. A 1998, 356, 957–966. [Google Scholar] [CrossRef]
- Kawai, Y.; Mori, K.; Kishimoto, M.; Ishikura, K.; Shimada, T. Surface Tension of Liquid Fe-C-Si Alloys. Tetsu-to-Hagané 1974, 60, 29–37. [Google Scholar] [CrossRef]
- Nakajima, K. Estimation of Interfacial Tensions between Phases in the Molten Iron-Slag-Inclusion (Alumina) System. Tetsu-to-Hagané 1994, 80, 383–388. [Google Scholar] [CrossRef]




| Steel | C (wt %) | Si (wt %) | Mn (wt %) | P (wt %) | S (wt %) | Ni (wt %) | Cr (wt %) | Mo (wt %) | Al (wt %) | T.Ca (ppm) | T.O (ppm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 60C | 0.58 | 0.26 | 0.74 | 0.014 | 0.022 | 0.07 | 0.20 | 0.02 | 0.004 | 8.8 | 30.9 |
| 95C | 0.95 | 0.23 | 0.88 | 0.012 | 0.028 | 0.06 | 0.23 | 0.12 | 0.002 | 7.9 | 16.0 |
| Steel | Crucible | Holding Time (min) | Holding Temperature (°C) | Average Composition | Liquid Fraction (wt %) | ||
|---|---|---|---|---|---|---|---|
| CaO (wt %) | SiO2 (wt %) | Al2O3 (wt %) | |||||
| 60C | MgO | 5 | 1540 | 38.9 | 27.6 | 33.6 | 76.1 |
| 95C | MgO | 5 | 1515 | 56.8 | 30.8 | 12.4 | 5.7 |
| Steel | Crucible | Holding Time (min) | Holding Temperature (°C) | Average Composition | |
|---|---|---|---|---|---|
| CaS (wt %) | MnS (wt %) | ||||
| 60C | MgO | 5 | 1540 | 49.6 | 50.4 |
| 95C | MgO | 5 | 1515 | 88.9 | 11.1 |
| Particle Type | 60C Steel | 95C Steel |
|---|---|---|
| Oxide particle |  |  |
| Sulfide particle |  |  |
| Agglomerated particle |  |  |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, Y.; Pahlevani, F.; Sahajwalla, V. Agglomeration Behavior of Non-Metallic Particles on the Surface of Ca-Treated High-Carbon Liquid Steel: An In Situ Investigation. Metals 2018, 8, 176. https://doi.org/10.3390/met8030176
Tanaka Y, Pahlevani F, Sahajwalla V. Agglomeration Behavior of Non-Metallic Particles on the Surface of Ca-Treated High-Carbon Liquid Steel: An In Situ Investigation. Metals. 2018; 8(3):176. https://doi.org/10.3390/met8030176
Chicago/Turabian StyleTanaka, Yasuhiro, Farshid Pahlevani, and Veena Sahajwalla. 2018. "Agglomeration Behavior of Non-Metallic Particles on the Surface of Ca-Treated High-Carbon Liquid Steel: An In Situ Investigation" Metals 8, no. 3: 176. https://doi.org/10.3390/met8030176






