Microalloyed Steels through History until 2018: Review of Chemical Composition, Processing and Hydrogen Service
Abstract
:1. Introduction
2. Chemical Composition of Microalloyed Steels
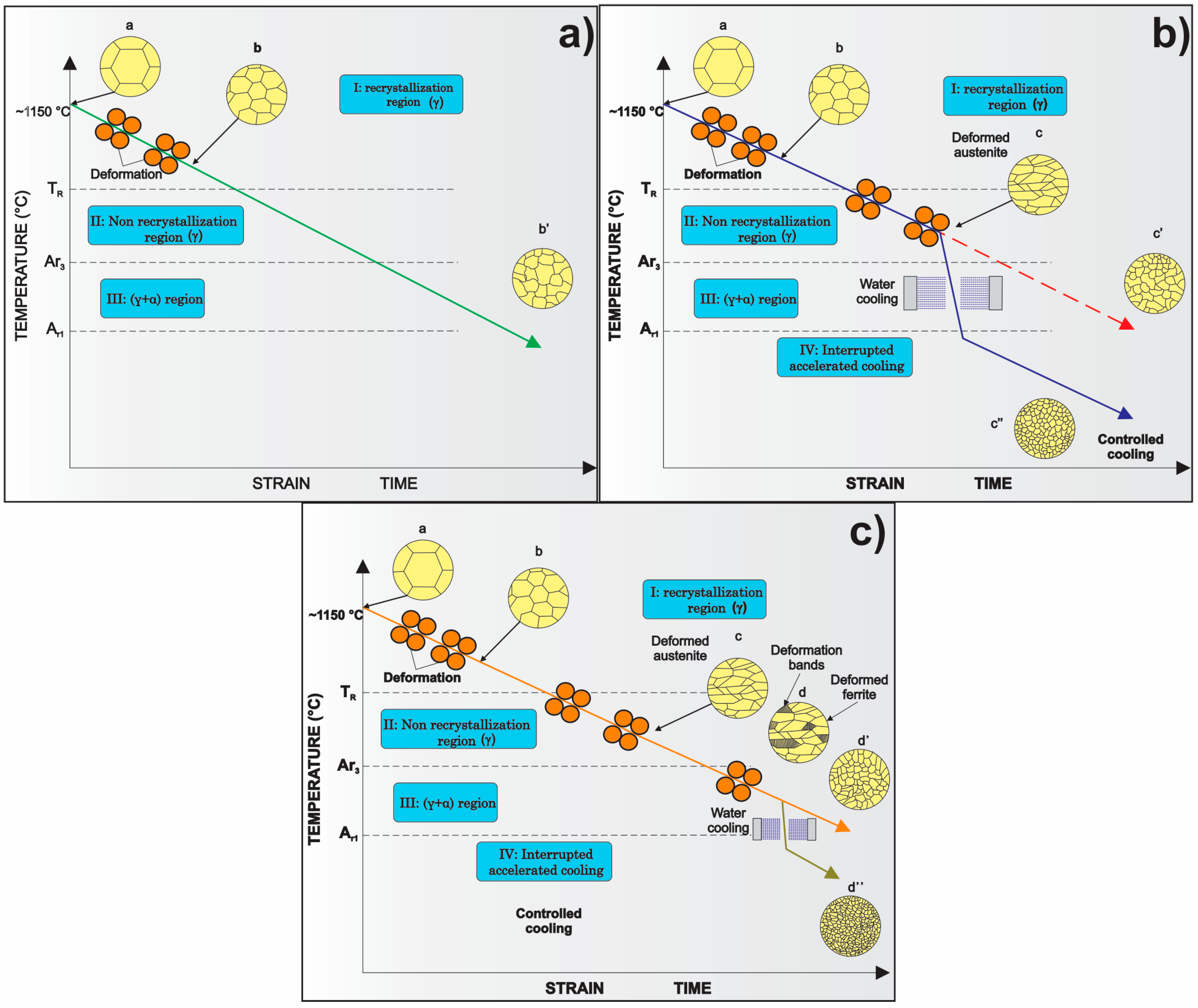
3. Processing of Microalloyed Steels
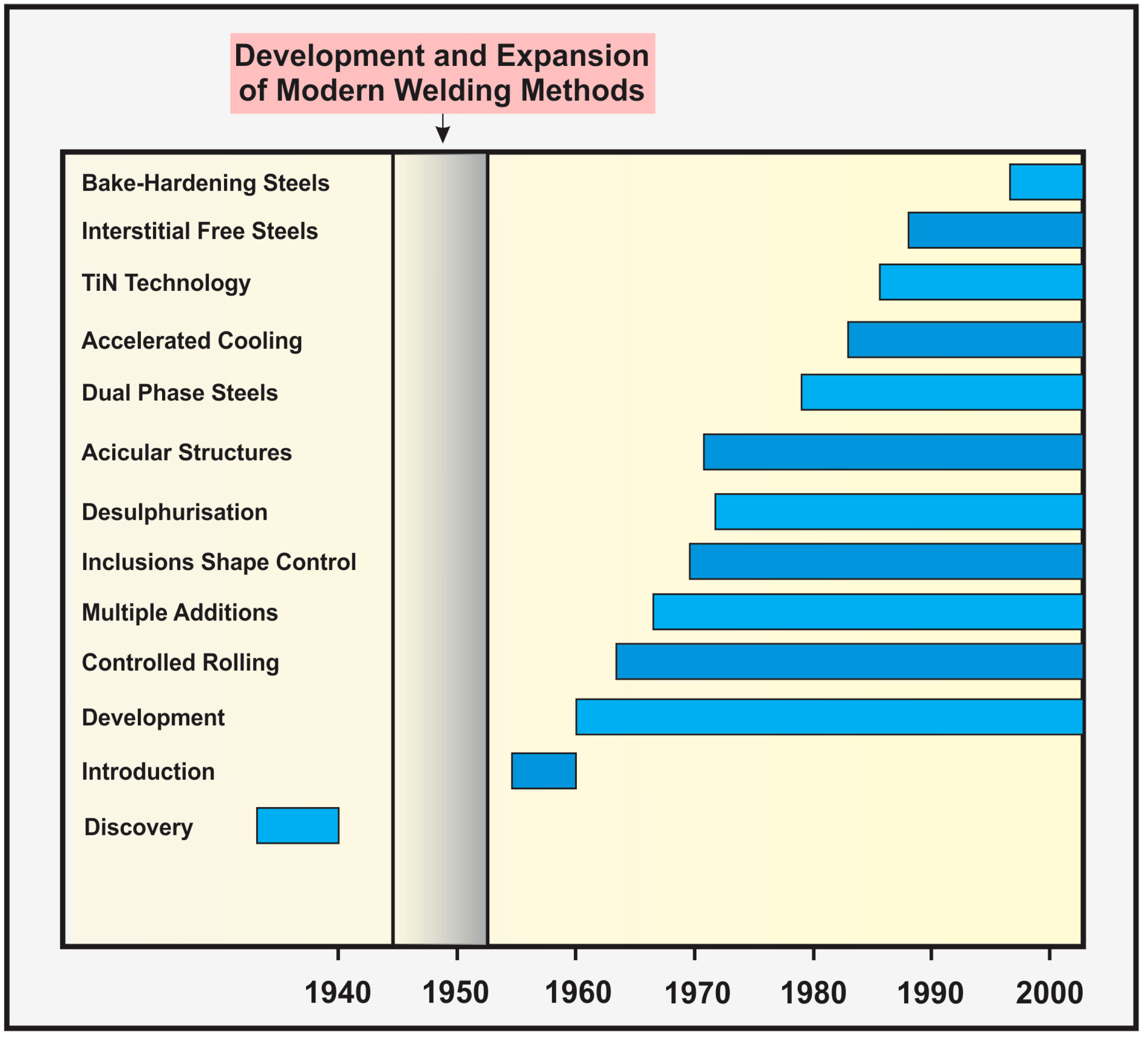
4. Development of Steels throughout History
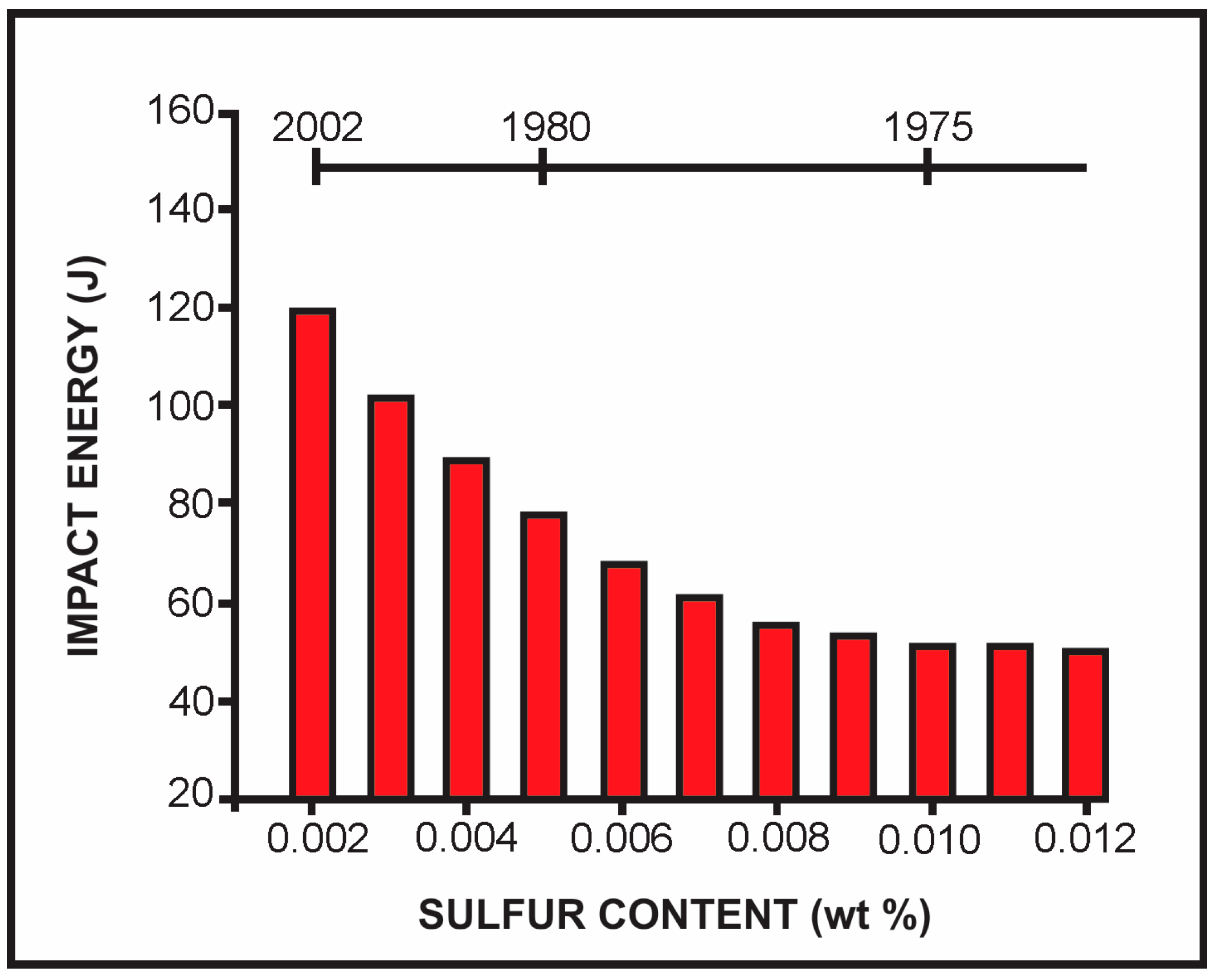
5. Sulfur Content in Microalloyed Steels
6. Mechanical Properties of Microalloyed Steels
7. Microalloyed Steels Welding
| 0.08–0.23% C | 0.010–0.050% S | 0.010–0.045% P | 0.15–0.65% Si | 0.45–1.6 Mn | 0–0.07% Nb |
| 1% Ni | 0.5% Cr | 0.4% Mo | 0.07% V | 0.3% Cu | 0.02% Ti | 0.03% Al | 0.002% B |
8. Hydrogen Embrittlement
- Quasi-brittle fracture in high strength materials that can occur with relatively low concentrations of hydrogen.
- Internal cracking and surface blistering in low strength materials (mainly C steels) due to very high internal hydrogen fugacity, allowing hydrogen pressure induced cracking, commonly referred to as HIC.
- The level of stress (or hardness) of the alloy;
- The microstructure;
- The amount of stress applied;
- The presence of localized tri-axial stress;
- The previous amount of cold work;
- The degree of stress segregation of low melting point elements such as: P, S, N, Ti or Sb at the grain boundaries.
8.1. Hydrogen Trapping
- Interstitial hydrogen, dissolved in solid solution in the steel matrix.
- Hydrogen associated with structural defects, such as dislocations or second phase particles.
- Hydrogen accumulated in voids or blisters in gaseous form.
8.2. Hydrogen Embrittlement Mechanisms
- (1)
- Hydrogen enhanced decohesion (HEDE). This mechanism proposes that hydrogen causes a reduction in the bond strength of metallic atoms, allowing weakness under tensile loads, in addition to promoting a propagation of fragile cracks [174].
- (2)
- Hydrogen enhanced local plasticity (HELP). This mechanism proposes that the presence of hydrogen increases the mobility of dislocations, causing a highly localized plastic deformation [175]. Because this deformation is concentrated in a small volume, the macroscopic ductility is low.
- (3)
- Absorption induced dislocation emission (AIDE). This mechanism is very similar to the HELP mechanism because it also involves localized plasticity. However, the main difference is that the AIDE mechanism proposes that localized plasticity occurs close to the surface in regions of stress concentration, such as cracks [176]. The hydrogen causes the movement of dislocations towards the crack tips, causing the growth of the same, as well as an intense deformation in the vicinity of the crack.
8.3. Hydrogen Entry
8.4. Hydrogen Gaseous Entry
- Physisorption: is the result of Van Der Waals forces between the metal surface and an adsorbent. It is completely reversible, and usually occurs instantly (direct adsorption of the hydrogen molecule on the surface).
- Chemisorption: is a chemical reaction that occurs between an atom of the metal surface and the adsorbent molecule. The chemical forces involved are short range and are limited to single layers. Chemisorption is usually slow and may be slowly reversible or irreversible. This process may be related to the formation of covalent bonds between an atom or adsorbent molecule and a surface atom (direct dissociation to atomic hydrogen).
- Absorption: it is a gas–solid interaction, which involves the incorporation of the products of the chemisorption within the crystalline network of the steel and its subsequent diffusion. Depending on the input mechanism, hydrogen absorption may be in atomic or ionic form (H+) [181].
8.5. Entry of Hydrogen into Aqueous Phase
8.6. Hydrogen Embrittlement Effect over Mechanical Properties of Tempered Treated Microalloyed Steels
8.7. Hydrogen Embrittlement Effect over the Mechanical Properties
9. Future Trends
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Stalheim, D.G.; Muralidharan, G. The Role of Continuous Cooling Transformation Diagrams in Material Design for High Strength Oil and Gas Transmission Pipeline Steels. In Proceedings of the IPC 2006, Calgary, AB, Canada, 25–29 September 2006. [Google Scholar]
- Takahashi, A.; Lino, M. Thermo-mechanical control process as a tool to grain-refine the low manganese containing steel for sour service linepipe. ISIJ Int. 1996, 36, 235–240. [Google Scholar] [CrossRef]
- Fu, J.; Wang, J.B.; Kang, Y.L. Research and development of HSLC steels produced by EAF-CSP technology. In Proceedings of the International Symposium on Thin Slab Casting and Rolling, Guangzhou, China, 3–5 December 2002. [Google Scholar]
- Xu, G.; Gan, X.; Ma, G.; Luo, F.; Zou, H. The development of Ti-alloyed high strength microalloy steel. Mater. Des. 2010, 31, 2891–2896. [Google Scholar] [CrossRef]
- Becket, F.M.; Russell, F. Steel. U.S. Patent 2,264,355, 2 December 1941. [Google Scholar]
- Gray, J.M.; Siciliano, F. High Strength Microalloyed Linepipe: Half a Century of Evolution. In Proceedings of the Pipeline Technology Meeting, Houston, TX, USA, 22–23 April 2009. [Google Scholar]
- Beiser, C.A. The Effect of Small Columbium Additions to semi-killed Medium-Carbon Steels. ASM Preprint No. 138. In Proceedings of the Regional Technical Meeting, Buffalo, NY, USA, 17–19 August 1959. [Google Scholar]
- Morrison, W.B. The Influence of Small Niobium Additions on the Properties of Carbon-Manganese Steels. ISIJ Int. 1963, 201, 317. [Google Scholar]
- Irani, J.J.; Burton, D.; Jones, J.D.; Rothwell, A.B. Beneficial Effects of Controlled Rolling in the Process of Structural Steels. In Strong Tough Structural Steels; Iron and Steel Institute Special Publication: Scarborough, UK, 1967. [Google Scholar]
- Iron and Steel Institute. Strong Tough Structural Steel. In Proceedings of the Joint Conference, Scarborough, UK, 4–6 April 1967. [Google Scholar]
- Cuddy, J.L. The Effect of Microalloy Concentration on the Recrystallisation of Austenite During Hot Deformation. In Thermomechanical Processing of Microalloyed Austenite; AIME: Pittsburgh, PA, USA, 1981; pp. 129–140. [Google Scholar]
- Tata Steel. Available online: http://www.tatasteeleurope.com (accessed on 16 January 2018).
- Starratt, F.W. Columbium-treated steels. J. Met. 1958, 10, 799. [Google Scholar]
- Oakes, G.; Barraclough, K.C. Steels; The Development of Gas Turbine Materials; Applied Science Publishers: London, UK, 1981; pp. 31–61. [Google Scholar]
- Llewellyn, D.T. Nitrogen in steels. Ironmak. Steelmak. 1993, 20, 35–41. [Google Scholar]
- Pressouyre, G.M.; Bernstein, I.M. A quantitative analysis of hydrogen trapping. Metall. Mater. Trans. A 1978, 9, 1571–1580. [Google Scholar] [CrossRef]
- DeArdo, A.J.; Hua, M.J.; Cho, K.G.; Garcia, C.I. On strength of microalloyed steels: An interpretive review. Mater. Sci. Technol. 2009, 25, 1074–1082. [Google Scholar] [CrossRef]
- Vervynckt, S.; Verbeken, K.; Lopez, B.; Jonas, J.J. Modern HSLA steels and role of non-recrystallisation temperature. Int. Mater. Rev. 2012, 57, 187–207. [Google Scholar] [CrossRef]
- Baker, T.N. Microalloyed Steels. Ironmak. Steelmak. 2016, 4, 264–307. [Google Scholar] [CrossRef]
- DeArdo, A.J. Microalloying ′95; ISS-AIME: Warrendale, PA, USA, 1995; Volume 15. [Google Scholar]
- DeArdo, A.J. Niobium in Modern Steels. Int. Mater. Rev. 2003, 48, 371–402. [Google Scholar] [CrossRef]
- Gilman, T. The Physical Metallurgy of Microalloyed Steel; Cambridge University Press: Cambridge, UK, 1957. [Google Scholar]
- Baker, T.N. Microalloyed Steel; Future Developments of Metals and Ceramics; Institute of Materials: London, UK, 1992; pp. 75–119. [Google Scholar]
- Gladman, T. Microalloyed Steels; Institute of Materials: London, UK, 1997. [Google Scholar]
- Xie, K.Y.; Zheng, T.; Cairney, J.M.; Kaul, H.; Williams, J.G.; Barbaro, F.J.; Killmore, C.R.; Ringer, S.P. Strengthening from Nb-rich clusters in a Nb-microalloyed steel. Scr. Mater. 2012, 66, 710–713. [Google Scholar] [CrossRef]
- Soto, R.; Saikaly, W.; Bano, X.; Issartel, C.; Rigaut, G.; Charai, A. Statistical and theoretical analysis of precipitates in dual-phase steels microalloyed with titanium and their effect on mechanical properties. Acta Mater. 1999, 47, 3475–3481. [Google Scholar] [CrossRef]
- Mangonon, P.L., Jr.; Heitmann, W.E. Proceeding Conference Microalloying; Union Carbide Corporation: Houston, TX, USA, 18–21 November 1977. [Google Scholar]
- Zhang, L.; Kannengiesser, T. Austenite grain growth and microstructure control in simulated heat affected zones of microalloyed HSLA steel. Mater. Sci. Eng. A 2014, 613, 326–335. [Google Scholar] [CrossRef]
- Gu, Y.; Tian, P.; Wang, X.; Han, X.; Liao, B.; Xiao, F. Non-isothermal prior austenite grain growth of a high-Nb X100 pipeline steel during a simulated welding heat cycle process. Mater. Des. 2016, 89, 589–596. [Google Scholar] [CrossRef]
- Karmakar, A.; Biswas, S.; Mukherjee, S.; Chakrabarti, D.; Kumar, V. Effect of composition and thermo-mechanical processing schedule on the microstructure, precipitation and strengthening of Nb-microalloyed steels. Mater. Sci. Eng. A 2017, 690, 158–169. [Google Scholar] [CrossRef]
- Chen, G.; Yang, W.; Guo, S.; Sun, Z. Effect of Nb on the transformation kinetics of low carbon (manganese) steel during deformation of undercooled austenite. J. Univ. Sci. Technol. Beijing Miner. Metall. Mater. 2006, 13, 411–415. [Google Scholar] [CrossRef]
- Baker, T.N. Titanium Technology in Microalloyed Steels, 2nd ed.; The Institute of Materials, Minerals and Mining: London, UK, 1997. [Google Scholar]
- Kojima, A.; Yoshii, K.; Hada, T. Development of high HAZ toughness steel plates for box columns with high heat input welding. Nippon Steel Tech. Rep. 2004, 90, 39–44. [Google Scholar]
- Chen, Y.; Zhang, D.T.; Liu, Y.C.; Li, H.J.; Xu, D.K. Effect of dissolution and precipitation of Nb on the formation of acicular ferrite/bainite ferrite in low-carbon HSLA steels. Mater. Charact. 2013, 84, 232–239. [Google Scholar] [CrossRef]
- Karjalainen, L.P.; Maccagno, T.M.; Jonas, J.J. Softening and Flow Stress Behaviour of Nb Microalloyed Steels during Hot Rolling Simulation. ISIJ Int. 1995, 35, 1523–1531. [Google Scholar] [CrossRef]
- Hansen, S.S.; Sande, J.B.V.; Cohen, M. Niobium Carbonitride Precipitation and Austenite Recrystallization in Hot-Rolled Microalloyed Steels. Metall. Trans. A 1987, 11, 387–402. [Google Scholar] [CrossRef]
- He, X.L.; Shang, C.; Yang, S. High Performance Low Carbon Bainitic Steel; Metallurgical Industry Press: Berjing, China, 2008. [Google Scholar]
- Hoogendoorn, T.M.; Spanraft, M.J. Quantifying the Effect of Microalloying Elements on Structures during Processing. In Proceedings of the International Symposium on High-Strength, Low-Alloy Steels, Washington, DC, USA, 1–3 October 1975. [Google Scholar]
- LeBon, A.B.; de Saint-Martin, L.N. Using Laboratory Simulations to Improve Rolling Schedules and Equipment. In Proceedings of the International Symposium on High-Strength, Low-Alloy Steels, Washington, DC, USA, 1–3 October 1975. [Google Scholar]
- Gauthier, G.; LeBon, A.B. Discussion: On the Recrystallization of Austenite. In Proceedings of the International Symposium on High-Strength, Low-Alloy Steels, Washington, DC, USA, 1–3 October 1975. [Google Scholar]
- Gauthier, G.; LeBon, A.B. Discussion: Techniques of Assessment of Precipitation Kinetics. In Proceedings of the International Symposium on High-Strength, Low-Alloy Steels, Washington, DC, USA, 1–3 October 1975. [Google Scholar]
- Tanaka, T.; Tabata, N.; Hatomura, T.; Shiga, C. Three Stages of the Controlled-Rolling Process. In Proceedings of the International Symposium on High-Strength, Low-Alloy Steels, Washington, DC, USA, 1–3 October 1975. [Google Scholar]
- Kozasu, I.; Ouchi, C.; Sampei, T.; Okita, T. Hot Rolling as a High-TemperatureThermo-Mechanical Process. In Proceedings of the International Symposium on High-Strength, Low-Alloy Steels, Washington, DC, USA, 1–3 October 1975. [Google Scholar]
- Gray, J.M.; Barbaro, F. Evolution of microalloyed steels since microalloying ’75 with specific emphasis on linepipe and plate. In HSLA Steels 2015, Microalloying 2015 & Offshore Engineering Steels 2015; The Minerals, Metals & Materials Society: Pittsburgh, PA, USA, 2016; pp. 53–70. [Google Scholar]
- Stalheim, D.G. The use of high temperature processing (HTP) steel for high strength oil and gas transmission pipeline application. Iron Steel 2005, 40, 699–704. [Google Scholar]
- Bracke, L.; de Wispelaere, N.; Ahmed, H.; Gungor, O.E. S700MC/Grade 100 in heavy gauges: Industrialisation at ArcelorMittal europe. In Proceedings of the International Symposium on Recent Developments in Plate Steels, Warrendale, PA, USA, 19–22 June 2011; pp. 131–138. [Google Scholar]
- Kim, S.J.; Lee, C.G.; Lee, T.H.; Lee, S. Effects of coiling temperature on microstructure and mechanical properties of high-strength hot-rolled steel plates containing Cu, Cr and Ni. ISIJ Int. 2000, 40, 692–698. [Google Scholar] [CrossRef]
- Misra, R.D.K.; Nathani, H.; Hartmann, J.E.; Siciliano, F. Microstructural evolution in a new 770 MPa hot rolled Nb–Ti microalloyed steel. Mater. Sci. Eng. A 2005, 394, 339–352. [Google Scholar] [CrossRef]
- Reip, C.P.; Shanmugam, S.; Misra, R.D.K. High strength microalloyed CMn (V–Nb–Ti) and CMn (V–Nb) pipeline steels processed through CSP thin-slab technology: Microstructure, precipitation and mechanical properties. Mater. Sci. Eng. A 2006, 424, 307–3017. [Google Scholar] [CrossRef]
- Shanmugam, S.; Misra, R.D.K.; Hartmann, J.E.; Jansto, S.G. Microstructure of high strength niobium-containing pipeline steel. Mater. Sci. Eng. A 2006, 441, 215–229. [Google Scholar] [CrossRef]
- Misra, R.D.K.; Jia, Z.; O’Malley, R.; Jansto, S.G. Precipitation behavior during thin slab thermomechanical processing and isothermal aging of copper-bearing niobium-microalloyed high strength structural steels: The effect on mechanical properties. Mater. Sci. Eng. A 2011, 528, 8772–8780. [Google Scholar] [CrossRef]
- Riva, R.; Mapelli, C.; Venturini, R. Effect of Coiling Temperature on Formability and Mechanical Properties of Mild Low Carbon and HSLA Steels Processed by Thin Slab Casting and Direct Rolling. ISIJ Int. 2007, 47, 1204–1213. [Google Scholar] [CrossRef]
- Misra, D.; Jansto, S.G. Niobium-based alloy design for structural applications. In HSLA Steels 2015, Microalloying 2015 & Offshore Engineering Steels 2015; The Minerals, Metals & Materials Society: Pittsburgh, PA, USA, 2016; pp. 261–266. [Google Scholar]
- Challa, V.S.A.; Zhou, W.H.; Misra, R.D.K.; O’Malley, R.; Jansto, S.G. The effect of coiling temperature on the microstructure and mechanical properties of a niobium–titanium microalloyed steel processed via thin slab casting. Mater. Sci. Eng. A 2014, 595, 143–153. [Google Scholar] [CrossRef]
- Maubane, R.; Banks, K.M.; Tuling, A.S. Hot strength during coiling of low C and Nb microalloyed steels. In HSLA Steels 2015, Microalloying 2015 & Offshore Engineering Steels 2015; The Minerals, Metals & Materials Society: Pittsburgh, PA, USA, 2016; pp. 323–328. [Google Scholar]
- Burgmann, F.A.; Xie, Y.; Cairney, J.M.; Ringer, S.P.; Killmore, C.R.; Barbaro, F.J.; Williams, J.G. The effect of niobium additions on ferrite formation in Castrip® steel. Mater. Forum 2008, 32, 9–12. [Google Scholar]
- Thelning, K.E. Steel and Its Heat; Butterworth & Co.: London, UK, 1984. [Google Scholar]
- Song, R.; Ponge, D.; Raabe, D. Influence of Mn content on microstructure and mechanical properties of ultrafine grained C-Mn Steels. ISIJ Int. 2005, 45, 1721–1726. [Google Scholar] [CrossRef]
- Shen, Y.F.; Zuo, L. High-strength low-alloy steel strengthened by multiply nanoscale microstructures. In HSLA Steels 2015, Microalloying 2015 & Offshore Engineering Steels 2015; The Minerals, Metals & Materials Society: Pittsburgh, PA, USA, 2016; pp. 187–193. [Google Scholar]
- Hall, E.O. The deformation and ageing of mild steel: 3 discussion of results. Proc. Phys. Soc. 1951, 64, 747–753. [Google Scholar] [CrossRef]
- Petch, N.J. The cleavage strength of polycrystals. J. Iron Steel Inst. 1953, 174, 25–28. [Google Scholar]
- Sanz, L.; Pereda, B.; López, B. Effect of thermomechanical treatment and coiling temperature on the strengthening mechanisms of low carbon steels microalloyed with Nb. Mater. Sci. Eng. A 2017, 685, 377–390. [Google Scholar] [CrossRef]
- Lucas, A.; Simon, P.; Bourdon, G.; Herman, J.C.; Riche, P.; Neutjens, J.; Harlet, P. Metallurgical Aspects of Ultra Fast Cooling in front of the Down-Coiler. Steel Res. Int. 2004, 75, 139–146. [Google Scholar] [CrossRef]
- Mohapatra, S.S.; Ravikumar, S.V.; Pal, S.K.; Chakraborty, S. Ultra Fast Cooling of a Hot Steel Plate by Using High Mass Flux Air Atomized Spray. Steel Res. Int. 2013, 84, 229–236. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Samanta, A.N.; Chakraborty, S. Spray evaporative cooling to achieve Ultra Fast cooling in runout table. Int. J. Therm. Sci. 2009, 48, 1741–1747. [Google Scholar] [CrossRef]
- Siciliano, F. High-Strength Linepipe Steels and Physical Simulation of Production Processes. In Advanced High Strength Steel; Springer: Singapore, 2018; pp. 71–78. [Google Scholar]
- Gilman, T. The Physical Metallurgy of Microalloyed Steel; Cambridge University Press: Cambridge, UK, 1977. [Google Scholar]
- Korchynsky, M. Microalloying 75; Union Carbide: New York, NY, USA, 1977. [Google Scholar]
- DeArdo, A.J.; Ratz, G.A.; Wray, J.P. Thermomechanical Processing of Microalloyed Austenite; Metallurgical Society of AIME: Warrendale, PA, USA, 1982. [Google Scholar]
- Korchynsky, M. (Ed.) Proceedings of the HSLA—Technology and Applications: Conference Proceedings of International Conference on Technology and Applications of HSLA Steels; American Society for Metals: Metals Park, OH, USA, 1983. [Google Scholar]
- Taylor, K.A.; Thompson, S.W.; Fletcher, F.B. Physical Metallurgy of Direct-Quenched Steels; Minerals, Metals and Materials Society: Chicago, IL, USA, 1993. [Google Scholar]
- Fazeli, F.; Amirkhiz, B.S.; Scott, C.; Arafin, M.; Collins, L. Kinetics and microstructural change of low-carbon bainite due to vanadium microalloying. Mater. Sci. Eng. A 2018, 720, 248–256. [Google Scholar] [CrossRef]
- Gray, J.M. An Independent View of Linepipe and Linepipe Steel for High Strength Pipelines: How to Get Pipe That’s Right for the Job at the Right Price; Microalloyed Steel Institute L.P.: Houston, TX, USA, 2002. [Google Scholar]
- Tsunekage, N.; Kobayashi, K.; Tsubakino, H. Influence of S and V on toughness of ferrite-pearlite or bainite steels. Curr. Adv. Mater. Processes 2000, 13, 534. [Google Scholar]
- Nomura, I. Metallurgical Technology in Microalloyed Steels for Automotive Parts. Mater. Jpn. 1995, 34, 705. [Google Scholar] [CrossRef]
- Tomita, Y.; Saito, N.; Tsuzuki, T.; Tokunaga, Y.; Okamoto, K. Improvement in HAZ Toughness of Steel by TiN-MnS Addition. ISIJ Int. 1994, 34, 829–835. [Google Scholar] [CrossRef]
- Suzuki, S.; Kuroki, K.; Kobayashi, H.; Takahashi, N. Sn Segregation at Grain Boundary and Interface between MnS and Matrix in Fe-3 mass%Si Alloys Doped with Tin. Mater. Trans. JIM 1992, 33, 1068–1076. [Google Scholar] [CrossRef]
- Tsunekage, N.; Tsubakino, H. Effects of Sulfur Content and Sulfide-forming Elements Addition on Impact Properties of Ferrite–Pearlitic Microalloyed Steels. ISIJ Int. 2001, 41, 498–505. [Google Scholar] [CrossRef]
- DeArdo, A.J. Microalloyed Steels: Past, Present and Future. In HSLA Steels 2015, Microalloying 2015 and Offshore Engineering Steels, 2015; The Minerals, Metals & Materials Society: Pittsburgh, PA, USA, 2015. [Google Scholar]
- Cottrell, A.H. Dislocations and Plastic Flow in Crystals; Clarendon Press: Oxford, UK, 1964. [Google Scholar]
- Korchynsky, M. Microalloyed Steels. In Proc. Conf. Microalloying ’75; Union Carbide Corp.: New York, NY, USA, 1977. [Google Scholar]
- Bai, D.; Collins, L.; Hamad, F.; Chen, X.; Klein, R. Microstructure and mechanical properties of high strength linepipes steels. In Proceedings of the Materials. Science & Technology Congress; AIST/ASM: Warrendale, PA, USA, 2007; pp. 355–366. [Google Scholar]
- Peters, P.A.; Gray, J.M. Genesis and Development of Specifications and Performance Requirements for Modern Linepipe—Strength, Toughness, Corrosion Resistance and Weldability; International Convention; Australian Pipeline Industry Association, Inc.: Hobart, Tasmania, Australia, 24–29 October 1992. [Google Scholar]
- Siwecki, T.; Eliasson, J.; Lagneborg, R.; Hutchinson, B. Vanadium microalloyed bainitic hot strip steels. ISIJ Int. 2010, 50, 760–767. [Google Scholar] [CrossRef]
- Kim, Y.W.; Song, S.W.; Seo, S.J.; Hong, S.G.; Lee, C.S. Development of Ti and Mo micro-alloyed hot-rolled high strength sheet steel by controlling thermomechanical controlled processing schedule. Mater. Sci. Eng. A 2013, 565, 430–438. [Google Scholar] [CrossRef]
- Juanhua, K.; Lin, Z.; Bin, G.; Pinghe, L.; Aihua, W.; Changsheng, X. Influence of Mo content on microstructures and mechanical properties of high strength pipeline steel. Mater. Des. 2004, 25, 723–728. [Google Scholar] [CrossRef]
- Xiao, F.R.; Liao, B.; Shan, Y.Y.; Qiao, G.Y.; Zhong, Y.; Zhang, C.; Yang, K. Challenge of mechanical properties of an acicular ferrite pipeline steel. Mater. Sci. Eng. A 2006, 431, 41–52. [Google Scholar] [CrossRef]
- Wang, W.; Shan, Y.; Yang, K. Study of high strength pipeline steels with different microstructures. Mater. Sci. Eng. A 2009, 502, 38–44. [Google Scholar] [CrossRef]
- Katsumata, M.; Machida, M.; Kaji, H. Recrystallization of Austenite in High-Temperature Hot-Rolling of Niobium Bearing Steels. In Proceedings of the International Conference on the Thermomechanical Austenite, Pittsburgh, PA, USA, 17–19 August 1981. [Google Scholar]
- Suikkanen, P.P.; Komi, J.I.; Karjalainen, L.P. Effect of austenite deformation and chemical composition on the microstructure and hardness of low-carbon and ultra low-carbon bainitic steels. Met. Sci. Heat Treat. 2005, 47, 507–511. [Google Scholar] [CrossRef]
- Gong, P.; Palmiere, E.J.; Rainforth, W.M. Thermomechanical processing route to achieve ultrafine grains in low carbon microalloyed steels. Acta Mater. 2016, 119, 43–54. [Google Scholar] [CrossRef]
- Dutta, B.; Valdes, E.; Sellars, C.M. Mechanism and kinetics of strain induced precipitation of Nb (C, N) in austenite. Acta Mater. 1992, 40, 653–662. [Google Scholar] [CrossRef]
- Gomez, M.; Valles, P.; Medina, S.F. Evolution of microstructure and precipitation state during thermomechanical processing of a X80 microalloyed steel. Mater. Sci. Eng. A 2011, 528, 4761–4773. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.C.; Yang, K.; Shan, Y. The effects of thermo-mechanical control process on microstructures and mechanical properties of a commercial pipeline steel. Mater. Sci. Eng. A 2002, 335, 14–20. [Google Scholar] [CrossRef]
- Zhao, M.C.; Yang, K.; Shan, Y.Y. Comparison on strength and toughness behaviors of microalloyed pipeline steels with acicular ferrite and ultrafine ferrite. Mater. Lett. 2003, 57, 1496–1500. [Google Scholar] [CrossRef]
- Xu, Y.B.; Yu, Y.M.; Xiao, B.L.; Liu, Z.Y.; Wang, G.D. Microstructural evolution in an ultralow-C and high-Nb bearing steel during continuous cooling. J. Mater. Sci. 2009, 44, 3928–3935. [Google Scholar] [CrossRef]
- Graf, M.K.; Hillenbrand, H.; Peters, P. Accelerated Cooling of Steel; TMS-AIME: Warrendale, PA, USA, 1986; pp. 165–179. [Google Scholar]
- Xiang-dong, H.; Lie-jun, L.; Zheng-wu, P.; Song-jun, C. Effects of TMCP schedule on precipitation, microstructure and properties of Ti-microalloyed high strength steel. J. Iron Steel Res. Int. 2016, 23, 593–601. [Google Scholar]
- Jorge, J.C.F.; Souza, L.F.G.; Rebello, J.M.A. The effect of chromium on the microstructure/toughness relationship of C- Mn weld metal deposits. Mater. Charact. 2001, 47, 195–205. [Google Scholar] [CrossRef]
- Beidokhti, B.; Koukabi, A.H.; Dolati, A. Influences of titanium and manganese on high strength low alloy SAW weld metal properties. Mater. Charact. 2009, 60, 225–233. [Google Scholar] [CrossRef]
- Dongsheng, L.; Binggui, C.; Yuanyan, C. Strengthening and Toughening of a Heavy Plate Steel for Shipbuilding with Yield Strength of Approximately 690 MPa. Metall. Mater. Trans. A 2013, 44, 440–445. [Google Scholar]
- Shin, S.Y.; Han, S.Y.; Hwang, B.C.; Lee, C.G.; Lee, S.H. Effects of Cu and B addition on microstructure and mechanical properties of high-strength bainitic steels. Mater. Sci. Eng. A 2009, 517, 212–218. [Google Scholar] [CrossRef]
- Han, S.Y.; Shin, S.Y.; Lee, S.H.; Kim, N.J.; Bae, J.H.; Kim, K. Effects of Cooling Conditions on Tensile and Charpy Impact Properties of API X80 Linepipe Steels. Metall. Mater. Trans. A 2010, 41, 329–340. [Google Scholar] [CrossRef]
- Kang, Y.L.; Han, Q.H.; Zhao, X.M.; Cai, M.H. Influence of nanoparticle reinforcements on the strengthening mechanism of an ultrafine-grained dual phase steel containing titanium. Mater. Des. 2013, 44, 331–339. [Google Scholar] [CrossRef]
- Wang, Y.W.; Feng, C.; Xu, F.Y.; Bai, B.Z.; Fang, H.Z. Influence of Nb on Microstructure and Property of Low-Carbon Mn-Series Air-Cooled Bainitic Steel. J. Iron Steel Res. Int. 2010, 17, 49–53. [Google Scholar] [CrossRef]
- Kong, J.H.; Xie, C.S. Effect of molybdenum on continuous cooling bainite transformation of low-carbon microalloyed Steel. Mater. Des. 2006, 27, 1169–1173. [Google Scholar] [CrossRef]
- Zackay, V.F.; Justusson, W.M. Special Report 76; Iron and Steel Institute: London, UK, 1962. [Google Scholar]
- Hara, T.; Asahi, H.; Uemori, R.; Tamehiro, H. Role of combined addition of niobium and boron and of molybdenum and boron on hardenability in low carbón steels. ISIJ Int. 2004, 44, 1431–1440. [Google Scholar] [CrossRef]
- Luo, H.; Karjalainen, L.P.; Porter, D.A.; Liimatainen, H.M.; Zhang, Y. The influence of Ti on the hot ductility of Nb-bearing steels in simulated continuous casting process. ISIJ Int. 2002, 42, 273–282. [Google Scholar] [CrossRef]
- Shukla, R.; Das, S.K.; Kumar, B.R.; Ghosh, S.K.; Kundu, S.; Chatterjee, S. An Ulta-low Carbon, Thermomechanically Controlled Processed Microalloyed Steel: Microstructure and Mechanical Properties. Metall. Mater. Trans. A 2012, 43, 4835–4845. [Google Scholar] [CrossRef]
- Li, C.N.; Ji, F.Q.; Yua, G.; Kang, J.; Misra, R.D.K.; Wang, G.D. The impact of thermos-mechanical controlled processing on structure-property relationship and strain hardening behavior in dual-phase steels. Mater. Sci. Eng. A 2016, 662, 100–110. [Google Scholar] [CrossRef]
- Oliver, S.; Jones, T.B.; Fourlaris, G. Dual phase versus TRIP strip steels: Microstructural changes as a consequence of quasi-static and dynamic tensile testing. Mater. Charact. 2007, 58, 390–400. [Google Scholar] [CrossRef]
- Li, X.L.; Lei, C.S.; Deng, X.T.; Wang, Z.D.; Yu, Y.G.; Wang, G.D.; Misra, R.D.K. Precipitation strengthening in titanium microalloyed high-strength steel plates with new generation-thermomechanical controlled processing (NG-TMCP). J. Alloys Compd. 2016, 689, 542–553. [Google Scholar] [CrossRef]
- Zhao, M.C.; Yang, K.; Xiao, F.R.; Shan, Y.Y. Continuous cooling transformation of undeformed and deformed low carbon pipeline steels. Mater. Sci. Eng. A 2003, 355, 126–136. [Google Scholar] [CrossRef]
- Bose-Filho, W.W.; Carvalho, A.L.M.; Strangw, M. Effects of alloying elements on the microstructure and inclusion formation in HSLA multipass welds. Mater. Charact. 2007, 58, 29–39. [Google Scholar] [CrossRef]
- Beidokhti, B.; Kokabi, A.H.; Dolati, A. A comprehensive study on the microstructure of high strength low alloy pipeline welds. J. Alloys Compd. 2014, 597, 142–147. [Google Scholar] [CrossRef]
- Bailey, N.; Jones, S.B. Solidification Cracking of Ferritic Steel during Submerged Arc-Welding; Welding Institute: Cambridge, UK, 1978; pp. 217–231. [Google Scholar]
- Mandelberg, S.L.; Rybacov, A.A.; Sidorenko, B.G. Resistance of pipe steel welded joints to solidification cracks. Avtom. Svarka 1972, 3, 1–5. [Google Scholar]
- Shiga, C. Effects of steelmaking, alloying and rolling variables on the HAZ structure and properties in microalloyed plate and line pipe. In Proceedings of the International Conference on The Metallurgy, Welding, and Qualification of Microalloyed (HSLA) Steel Weldments, Houston, TX, USA, 6–8 November 1990; pp. 327–350. [Google Scholar]
- Aihara, S.; Okamoto, K. Influence of Local Brittle Zone on HAZ Toughness of TMCP Steels. In Proceedings of the International Conference on The Metallurgy, Welding, and Qualification of Microalloyed (HSLA) Steel Weldments, Houston, TX, USA, 6–8 November 1990; pp. 402–426. [Google Scholar]
- García, R.; López, V.H.; Lazaro, Y.; Aguilera, J. Grain Refi nement in Electrogas Welding of Microalloyed Steels by Inducing a Centred Magnetic Field; Soldagem & Inspecao: Sao Paulo, Brasil, 2007; pp. 300–304. [Google Scholar]
- Loberg, B.; Nordgren, A.; Strid, J.; Easterling, K.E. The Role of Alloy Composition on the Stability of Nitrides in Ti-Microalloyed Steels during Weld Thermal Cycles. Metall. Trans. A 1984, 15, 33–41. [Google Scholar] [CrossRef]
- Zhang, W.; Elmer, J.W.; DebRoy, T. Modeling and real time mapping of phases during GTA welding of 1005 steel. Mater. Sci. Eng. A 2002, 333, 320–335. [Google Scholar] [CrossRef]
- Wan, X.L.; Wang, H.H.; Cheng, L.; Wu, K.M. The formation mechanisms of interlocked microstructures in low-carbon high-strength steel weld metals. Mater. Charact. 2012, 67, 41–51. [Google Scholar] [CrossRef]
- Terada, Y.; Tamehiro, H.; Morimoto, H.; Hara, T.; Tsuru, E.; Asahi, H. X100 Linepipe With Excellent HAZ Toughness and Deformability. In Proceedings of the 22nd International Conference on Offshore Mechanics and Arctic Engineering ASME, Cancun, Mexico, 8–13 June 2003; pp. 1–8. [Google Scholar]
- Matsuda, F.; Ikeuchi, K.; Liao, J.S.; Tanabe, H. Weld HAZ Toughness and Its Improvement of Low Alloy Steel SQV-2A for Pressure Vessels (Report 2): Microstructure and Charpy Impact Behavior of Intercritically Reheated Coarse Grained Heat Affected Zone (ICCGHAZ) (Materials, Metallurgy & Weldability). Trans. JWRI 1994, 23, 49–57. [Google Scholar]
- Shim, J.H.; Cho, Y.W.; Chung, S.H.; Shim, J.D.; Lee, D.N. Nucleation of intragranular ferrite at Ti2O3 particle in low carbon steel. Acta Mater. 1999, 47, 2751–2760. [Google Scholar] [CrossRef]
- Guo, A.M.; Li, S.R.; Guo, J.; Li, P.H.; Ding, Q.F.; Wu, K.M.; He, X.L. Effect of zirconium addition on the impact toughness of the heat affected zone in a high strength low alloy pipeline steel. Mater. Charact. 2008, 59, 134–139. [Google Scholar] [CrossRef]
- Lee, J.L.; Pan, Y.T. The Formation of Intragranular Acicular Ferrite in Simulated Heat-affected Zone. ISIJ Int. 1995, 35, 1027–1033. [Google Scholar] [CrossRef]
- Gianetto, J.A.; Braid, J.E.M.; Bowker, J.T.; Tyson, W.R. Heat-Affected Zone Toughness of a TMCP Steel Designed for Low-Temperature Applications. Trans. ASME 1997, 119, 134–144. [Google Scholar] [CrossRef]
- Matsuda, F.; Ikeuchi, K.; Fukada, Y.; Horii, Y.; Okada, H.; Shiwaku, T.; Shiga, C.; Suzuki, S. Review of Mechanical and Metallurgical Investigations of M-A Constituent in Welded Joint in Japan. Trans. JWRI 1995, 24, 1–24. [Google Scholar]
- Lan, L.; Qiu, C.; Zhao, D.; Gao, X.; Du, L. Microstructural characteristics and toughness of the simulated coarse grained heat affected zone of high strength low carbon bainitic steel. Mater. Sci. Eng. A 2011, 529, 192–200. [Google Scholar] [CrossRef]
- Hunt, A.C.; Kluken, A.O.; Edwards, G.R. Heat input and dilution effects in microalloyed steel weld metals. Weld. J. 1994, 73, 9–15. [Google Scholar]
- Karabulut, H.; Türkmen, M.; Erden, M.A.; Gündüz, S. Effect of Different Current Values on Microstructure and Mechanical Properties of Microalloyed Steels Joined by the Submerged Arc Welding Method. Metals 2016, 6, 281. [Google Scholar] [CrossRef]
- Zhang, W.; Elmer, J.W.; DebRoy, T. Kinetics of ferrite to austenite transformation during welding of 1005 steel. Scr. Mater. 2002, 46, 753–757. [Google Scholar] [CrossRef]
- Ekicia, M.; Ozsarac, U. Investigation of Mechanical Properties of Microalloyed Steels Joined by GMAW and Electrical Arc Welding. Acta Phys. Pol. A 2013, 123, 289–290. [Google Scholar] [CrossRef]
- Dunđer, M.; Vuherer, T.; Samardžić, I. Weldability of microalloyed high strength steels TStE 420 and S960QL. Metalurgija 2014, 53, 335–338. [Google Scholar]
- Sharma, V.; Shahi, A.S. Quenched and tempered steel welded with micro-alloyed based ferritic fillers. J. Mater. Process. Technol. 2018, 253, 2–16. [Google Scholar] [CrossRef]
- Liu, H.J.; Shen, J.J.; Zhou, L.; Zhao, Y.Q.; Li, C. Microstructural characterisation and mechanical properties of friction stir welded joints of aluminium alloy to copper. Sci. Technol. Weld. Join. 2011, 16, 92–99. [Google Scholar] [CrossRef]
- Hart, P.H.M.; Harrison, P.L. Compositional Parameters for HAZ Cracking and Hardening in C-Mn Steels. In Proceedings of the 67th Annual AWS Meeting, Atlanta, GA, USA, 14–16 April 1986; pp. 13–18. [Google Scholar]
- Wang, S.H.; Luu, W.C.; Ho, K.F.; Wu, J.K. Hydrogen permeation in a submerged arc weldment of TMCP steel. Mater. Chem. Phys. 2002, 77, 447–454. [Google Scholar] [CrossRef]
- Sun, Q.; Di, H.; Li, J.; Wu, B.; Misra, R. A comparative study of the microstructure and properties of 800 MPa microalloyed C-Mn steel weld joints by laser and gas metal arc welding. Mater Sci Eng A 2016, 669, 150–158. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, S.; Zhou, J.; Zhang, M.; Chen, C.; Misra, R. Effect of heat input on microstructure and properties of hybrid fiber laser-arc weld joints of the 800 MPa hot-rolled Nb-Ti-Mo microalloyed steels. Opt. Lasers Eng. 2017, 91, 86–96. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, X.; Zhu, G.; Chen, C.; Hou, J.; Zhang, S.; Jing, H. Effect of laser welding process parameters on microstructure and mechanical properties on butt joint of new hot-rolled nano-scale precipitate strenthen steel. Acta Metall. Sin. 2014, 27, 521–529. [Google Scholar] [CrossRef]
- Bhadesia, H. Bainite in Steels; The Institute of Materials: London, UK, 1992. [Google Scholar]
- Pamnani, R.; Karthik, V.; Jayamukar, T.; Vasudevan, M.; Sakthivel, T. Evaluation of Mechanical Properties across Micro Alloyed HSLA Steel Weld Joints using Atomated Ball Indentation. Mater. Sci. Eng. A 2015, 651, 214–223. [Google Scholar] [CrossRef]
- Chatzidouros, E.V.; Papazoglou, V.J.; Tsiourva, T.E.; Pantelis, D.I. Hydrogen effect on fracture toughness of pipeline steel welds, with insitu hydrogen charging. Int. J. Hydrog. Energy 2011, 36, 12626–12643. [Google Scholar] [CrossRef]
- Demofonti, G.; Mannucci, G.; Di Biagio, M.; Hillenbrand, H.G.; Harris, D. Fracture propagation resistance evaluation of X100 TMCP steel pipes for high pressure gas transportation pipelines by full scale burst tests. Pipeline Technol. Proc. 2004, 1, 467–482. [Google Scholar]
- Yakubtsov, I.A.; Poruks, P.; Boyd, J.D. Microstructure and mechanical properties of bainitic low carbon high strength plate steels. Mater. Sci. Eng. A. 2008, 480, 109–116. [Google Scholar] [CrossRef]
- Woodtli, J.; Kieselbach, R. Damage due to hydrogen embrittlement and stress corrosion cracking. Eng. Fail. Anal. 2000, 7, 427–450. [Google Scholar] [CrossRef]
- Hui, W.J.; Weng, Y.Q.; Dong, H. Steels for High Strength Fastener; Metallurgical Industry Press: Beijing, China, 2009. [Google Scholar]
- Cottis, R.A. Hydrogen Embrittlement; School of Materials: Manchester, UK, 2010. [Google Scholar]
- Domizzi, G.; Anteri, G.; Ovejero-García, J. Influence of sulphur content and inclusion distribution on the hydrogen induced blister cracking in pressure vessel and pipeline steels. Corros. Sci. 2001, 43, 325–339. [Google Scholar] [CrossRef]
- Hardie, D.; Charles, E.A.; Lopez, A.H. Hydrogen embrittlement of high strength pipeline steels. Corros. Sci. 2006, 48, 4378–4385. [Google Scholar] [CrossRef]
- Shin, S.Y.; Hwang, B.; Lee, S.; Kim, N.J.; Ahn, S.S. Correlation of microstructure and charpy impact properties in API X70 and X80 line-pipe steels. Mater. Sci. Eng. A 2007, 458, 281–289. [Google Scholar] [CrossRef]
- Shterenlikht, A.; Hashemi, S.H.; Howard, I.C.; Yates, J.R.; Andrews, R.M. A specimen for studying the resistance to ductile crack propagation in pipes. Eng. Fract. Mech. 2004, 71, 1997–2013. [Google Scholar] [CrossRef]
- Asoaka, T.; Lapasset, G.; Aucouturier, M.; Lacombe, P. Observation of hydrogen trapping in Fe-0.15 wt % Ti alloy by high resolution autoradiography. Corrosion 1978, 34, 39–47. [Google Scholar] [CrossRef]
- Qian, L.; Andrej, A. Reversible hydrogen trapping in a 3.5 NiCrMoV medium strength steel. Corros. Sci. 2015, 96, 112–120. [Google Scholar]
- Mohsen, D.; Martin, M.L.; Nagao, A.; Sofronis, P.; Robertson, I.M. Modeling hydrogen transport by dislocations. J. Mech. Phys. Solids 2015, 78, 511–525. [Google Scholar]
- Marchetti, L.; Herms, E.; Lagoutaris, P.J. Hydrogen embrittlement susceptibility of tempered 9% Cr, 1% Mo steel. Int. J. Hydrog. Energy 2011, 34, 15880–15887. [Google Scholar] [CrossRef]
- Druce, P.D.; Daniel, H.; Ahmed, A.S.; Ayesha, J.H.; Andrzej, C.; Pereloma, V.E. Investigation of the effect of electrolytic hydrogen charging of x70 steel: I. The effect of microstructure on hydrogen-induced cold cracking and blistering. Int. J. Hydrog. Energy 2016, 41, 12411–12423. [Google Scholar]
- Liu, Q.; Venezuela, J.; Zhang, M.; Zhou, Q.; Atrens, A. Hydrogen trapping in some advanced high strength Steels. Corros. Sci. 2016, 111, 770–785. [Google Scholar] [CrossRef]
- Hirth, J. Effects of hydrogen on the properties of iron and steel. Metall. Trans. A 1980, 11, 861–890. [Google Scholar] [CrossRef]
- Thomas, R.L.S.; Li, D.; Gangloff, R.P.; Scully, J.R. Trap-governed hydrogen diffusivity and uptake capacity in ultrahigh-strength AERMET 100 steel. Metall. Mater. Trans. A 2002, 33, 1991–2004. [Google Scholar] [CrossRef]
- Venezuela, J.; Liu, Q.; Zhang, M.; Zhou, Q.; Atrens, A. The influence of hydrogen on the mechanical and fracture properties of some martensitic advanced high strength steels studied using the linearly increasing stress test. Corros. Sci. 2015, 99, 98–117. [Google Scholar] [CrossRef]
- Wei, F.G.; Tsuzaki, K. Quantitative analysis on hydrogen trapping of TiC particles in steel. Metall. Mater. Trans. A 2006, 37, 331–353. [Google Scholar] [CrossRef]
- Depover, T.; Escobar, D.P.; Wallaert, E.; Zermout, Z.; Verbeken, K. Effect of hydrogen charging on the mechanical properties of advanced high strength steels. Int. J. Hydrog Energy 2014, 39, 4647–4656. [Google Scholar] [CrossRef]
- Wallaert, E.; Depover, T.; Arafin, M.; Verbeken, K. Thermal desorption spectroscopy evaluation of the hydrogen-trapping capacity of NbC and NbN precipitatesMetall. Mater. Trans. A 2014, 45, 2412–2420. [Google Scholar] [CrossRef]
- Takahashi, J.; Kawakami, K.; Kobayashi, Y.; Tarui, T. The first direct observation of hydrogen trapping sites in TiC precipitation-hardening steel through atom probe tomography. Scr. Mater. 2010, 63, 261–264. [Google Scholar] [CrossRef]
- Valentini, R.; Solina, A.; Matera, S.; de Gregorio, P. Influence of titanium and carbon contents on the hydrogen trapping of microalloyed steels. Metall. Mater. 1996, 27, 3773–3780. [Google Scholar] [CrossRef]
- McNabb, A.; Foster, P.K. A new analysis of the diffusion of hydrogen in iron and ferritic steels. Trans. Metall. Soc. AIME 1963, 227, 618–627. [Google Scholar]
- Gangloff, R.P. Hydrogen Assisted Cracking of High Strength Alloys; In Comprehensive Structural Integrity; Elsevier: New York, NY, USA, 2003; pp. 31–101. [Google Scholar]
- Lynch, S. Hydrogen embrittlement phenomena and mechanisms. Corros. Rev. 2012, 30, 105–123. [Google Scholar] [CrossRef]
- Oriani, R.A. A mechanistic theory of hydrogen embrittlement of steels. Berich. Bunsengesellsch. Phys. Chem. 1972, 76, 848–857. [Google Scholar]
- Beachem, C.D. A new model for hydrogen assisted cracking (Hydrogen embrittlement). Metall. Trans. A 1972, 3, 437–451. [Google Scholar] [CrossRef]
- Lynch, S.P. Environmentally assisted cracking: Overview of evidence for an adsorption-induced localized-slip process. Acta Metall. 1988, 20, 2639–2661. [Google Scholar] [CrossRef]
- Tehemiro, H.; Takeda, T.; Matsuda, S.; Yamamoto, K.; Komura, H. Effect of accelerated cooling after controlled rolling on the hydrogen-induced cracking resistance of pipeline steels. Trans. Iron Steel Inst. Jpn. 1985, 25, 982–988. [Google Scholar]
- Ejim, F. Hydrogen Diffusion in Pipeline Steels. Ph.D. Thesis, Dipartimento di Chimica, Materiali e Ingegneria Chimica “Giulio Natta”, Milan, Italy, 2011. [Google Scholar]
- Pasco, R.W.; Ficalora, P.J. Hydrogen Degradation of Ferrous Alloys; Noyes Publications: Park Ridge, NJ, USA, 1984; pp. 199–214. [Google Scholar]
- Afrooz, B. Hydrogen Embrittlement; Saarland University: Saarbrücken, Germany, 2011. [Google Scholar]
- Myers, S.M.; Baskes, M.L.; Brinbaum, H.K.; Corbett, J.W.; Deleo, G.G.; Estreicher, S.K.; Haller, E.E.; Jena, P.; Johnson, N.M.; Kirchheim, R.; et al. Hydrogen interactions with defects in crystalline solids. Rev. Mod. Phys. 1992, 64, 559. [Google Scholar] [CrossRef]
- Xu, K. Hydrogen Embrittlement of Carbon Steels and Their Welds; Gaseous Hydrogen Embrittlement of Materials in Energy Technology; Woodhead: Philadelphia, IL, USA, 2012; pp. 526–561. [Google Scholar]
- Walter, R.J.; Chandler, W.T. Effects of High Pressure Hydrogen on Metals at Ambient Temperature; No. N-70-18637; NASA-CR-102425; Rocketdyne: Canoga Park, CA, USA, 1968. [Google Scholar]
- Thompson, A.W.; Bernstein, I.M. Selection of structural materials for hydrogen pipelines and storage vessels. Int. J. Hydrog. Energy, 1977, 2, 163–173. [Google Scholar] [CrossRef]
- Imbihl, R.; Behm, R.J.; Christmann, K.; Ertl, G.; Matsushima, T. Phase transitions of a two-dimensional chemisorbed system: H on Fe(110). Surface Sci. 1982, 117, 257–266. [Google Scholar] [CrossRef]
- Shanmugan, S.; Ramisetti, N.K.; Mirsa, R.D.K. Microstructure and High Strenght-Toughness Combination of a New 700 MPa Nb Microalloyed Pipeline Steel. Mater. Sci. Eng. 2008, 478, 26–37. [Google Scholar] [CrossRef]
- Liu, D.; Xu, H.; Yang, K.; Fang, H. Effect of bainite/Martensite Mixed Microstructure on the strenghth and toughness of low carbon alloy steels. Acta Metall. Sin. 2004, 40, 882–886. [Google Scholar]
- Nayak, S.S.; Misra, R.D.K.; Hartmann, J.; Siciliano, F.; Gray, J.M. Microestructure and properties of low manganese and niobium containing HIC pipeline Steel. Mater. Sci. Eng. A 2008, 494, 456–463. [Google Scholar] [CrossRef]
- Uranga, P.; Fernandez, A.I.; Lopez, B.; Rodriguez-Ibabe, J.M. Transition between static and metadynamic recrystallization kinetics in coarse Nb microalloyed austenite. Mater. Sci. Eng. A 2003, 345, 319–327. [Google Scholar] [CrossRef]
- Zhou, M.; Du, L.X.; Liu, X.H. Relationship among Microstructure and properties and heat treatment of Ultra-High Strenght X120 pipeline steel. J. Iron Steel Res. Int. 2011, 18, 59–64. [Google Scholar] [CrossRef]
- Ping, Z. Microstructure and Mechanical Properties in Isothermal Tempering of High Co-Ni Secondary Hardening Ultrahigh Strength Steel. Sch. Mater. Sci. Eng. Beijing 2007, 14, 292–295. [Google Scholar]
- Asahi, H.; Ueneo, M.; Yonezawa, T. Prediction of Sulfide Stress Cracking in High Strenght Tubulars. Corrosion 1994, 50, 537. [Google Scholar] [CrossRef]
- Lunarska, E.; Zielinski, A. Hydrogen Degradation of Ferrous Alloys; Noyes Publications: Park Ridge, NJ, USA, 1985. [Google Scholar]
- Park, G.T.; Koh, S.U.; Jung, H.G.; Kim, K.Y. Effect of microstructure on the hydrogen trapping Efficiency induced Cracking of linepipe Steel. Corros. Sci. 2008, 50, 1865–1871. [Google Scholar] [CrossRef]
- Liu, D.; Bai, B.; Fang, H.; Zhang, W.; Gu, J.; Chang, K. Effect of tempering and Carbide Free Bainite on the Mechanical Characteristics of a High Strenght Low Alloy Steel. Mater. Sci. Eng. 2004, 371, 40–44. [Google Scholar] [CrossRef]
- Dong, C.F.; Xiao, K.; Liu, Z.Y.; Yang, W.J.; Li, X.G. Hydrogen Induced Cracking of X80 pipeline Steel. Int. J. Miner. Metall. Mater. 2010, 17, 579–586. [Google Scholar] [CrossRef]
- Das, A.K. The Present and the Future of Line Pipe Steels for Petroleum Industry. Mater. Manuf. Processes 2010, 25, 1–3. [Google Scholar] [CrossRef]
- Meimeth, S.; Grimpe, F.; Meuser, H.; Siegel, H.; Stallybrass, C.; Heckmann, C.J. Development, state of the art and future trends in design and production of heavy plates in X80 steel-grades, steel rolling 2006. In Proceedings of the 9th International & 4th European Conferences, Paris, France, 19–21 June 2006. [Google Scholar]
- Madías, J. Nuevas Plantas Latinoamericanas; Industria del Acero: San Nicolás, Argentina, 2017; pp. 28–43. [Google Scholar]
- Jansto, S. Current development in niobium high carbon applications. In Proceedings of the MS&T 2011, Materials Science & Technology 2011 Conference and Exhibition (MS&T Partner Societies), Columbus, OH, USA, 16–20 October 2011. [Google Scholar]
- Jansto, S. Applied metallurgy of the microniobium R alloy approach in long and plate products. In Proceedings of the METAL, Brno, Czech Republic, 23–25 May 2012. [Google Scholar]
- Jansto, S. Metallurgical mechanism and niobium efects on improved mechanical properties in high carbon steels. In Proceedings of the Microalloying 2015 & O shore Engineering Steels 2015, Hangzhou, China, 11–13 November 2015; pp. 981–986. [Google Scholar]

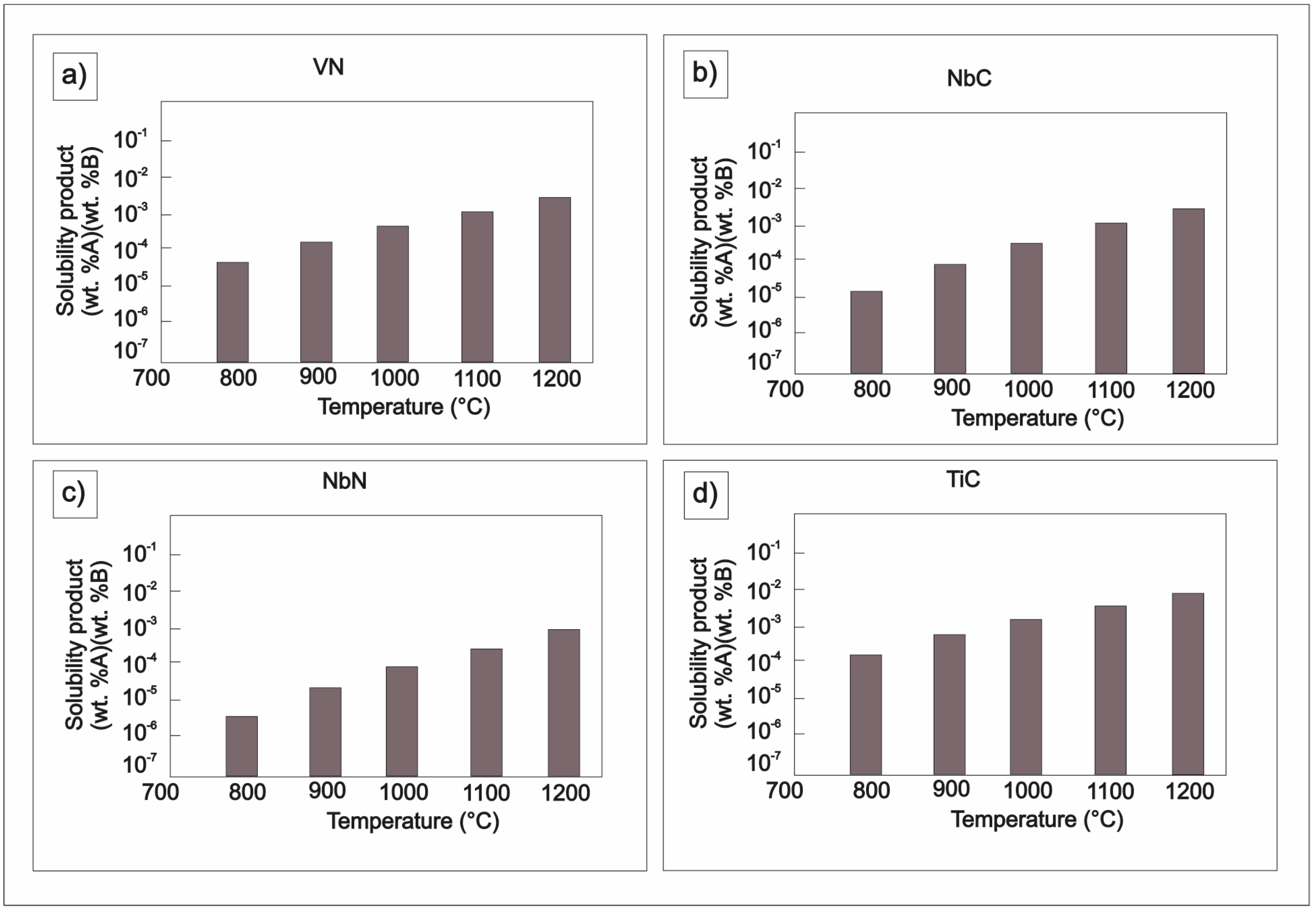











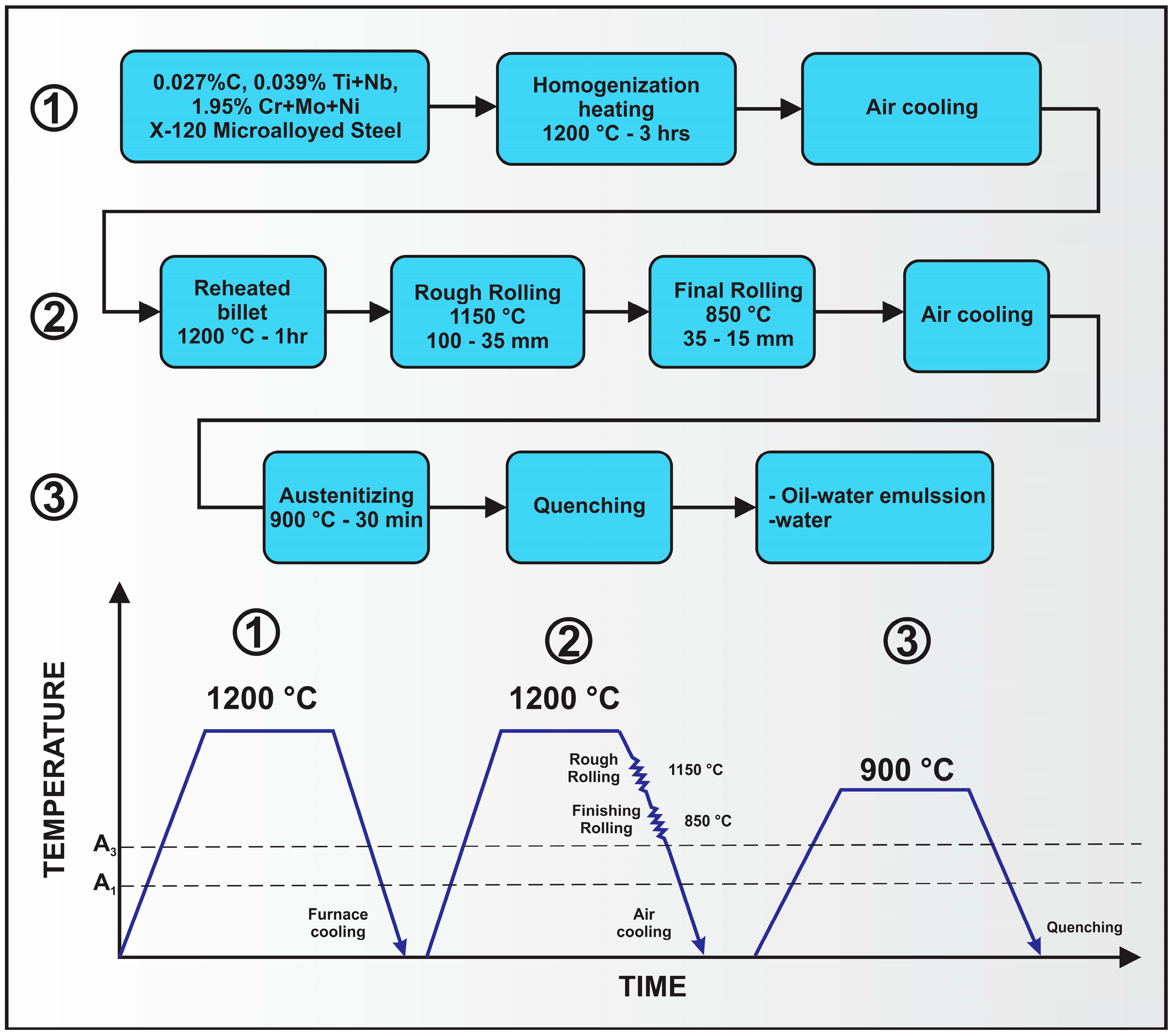





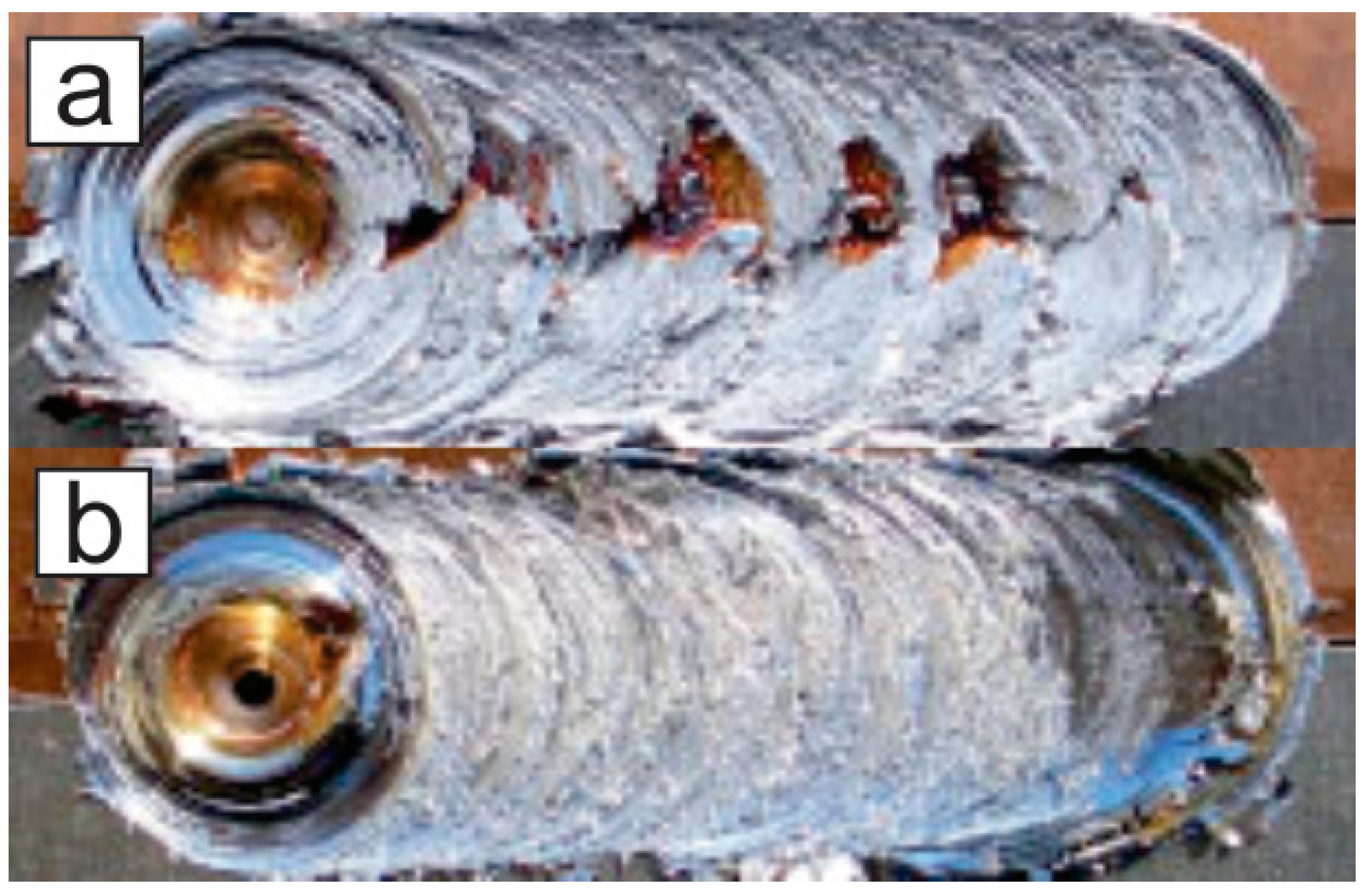







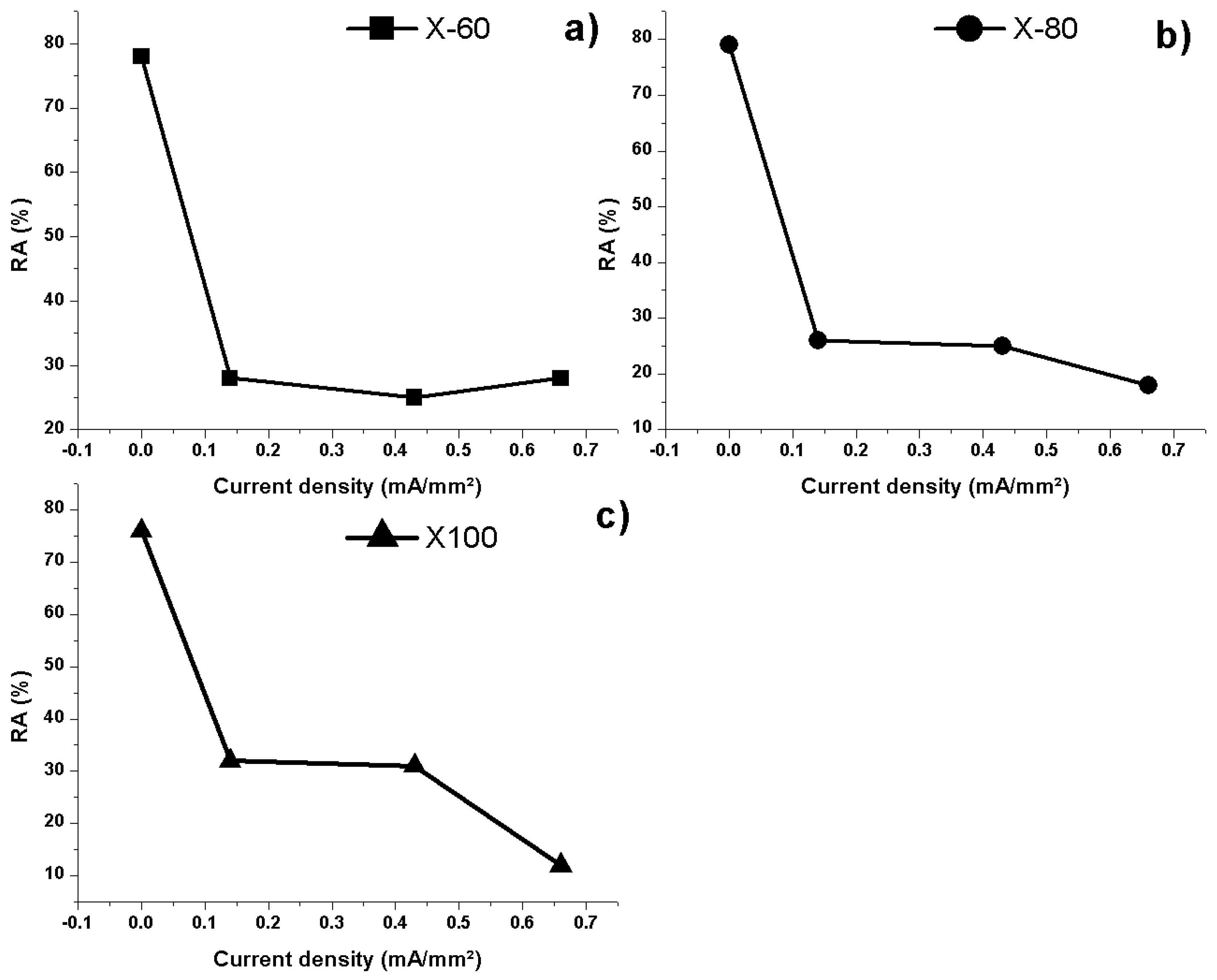
| Element | wt % | Effect |
|---|---|---|
| C | <0.25 | Strengthener |
| Mn | 0.5–2.0 | Retards the austenite decomposition during accelerated cooling Decreases ductile to brittle transitions temperature Strong sulfide former |
| Si | 0.1–0.5 | Deoxidizer in molten steel Solid solution strengthener |
| Al | <0.02 | Deoxidizer Limits grain growths as aluminum nitride |
| Nb | 0.02–0.06 | Very strong ferrite strengthener as niobium carbides/nitrides Delays austenite-ferrite transformation |
| Ti | 0–0.06 | Austenite grain control by titanium nitrides Strong ferrite strengthener |
| V | 0–0.10 | Strong ferrite strengthener by vanadium carbonitrides |
| Zr | 0.002–0.05 | Austenite grain size control Strong sulfide former |
| N | <0.012 | Strong former of nitrides and carbonitrides with microalloyed elements |
| Mo | 0–0.3 | Promotes bainite formations Ferrite strengthener |
| Ni | 0–0.5 | Increase fracture toughness |
| Cu | 0–0.55 | Improves corrosion resistance Ferrite strengthener |
| Cr | 0–1.25 | In the presence of copper, increase atmospheric corrosion resistance |
| B | 0.0005 | Promotes bainite formation |
| C | Mn | S | Si | Cu | Mo | Nb | V | Ti | Al | Cr | Ni | B | Grade |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.041–0.17 | 0.30–1.68 | 0.0002–0.03 | 0.02–1.39 | 0.02–0.31 | 0.005–0.14 | 0.018–0.06 | 0.042–0.21 | 0.002–0.01 | - | 0.02–0.157 | 0.005–0.8 | - | X-65 |
| 0.037–0.125 | 1.44–1.76 | 0.001–0.015 | 0.14–0.44 | 0.006–0.27 | 0.01–0.3 | 0.051–0.092 | 0.001–0.095 | 0.009–0.03 | - | 0.007–0.266 | 0.02–0.23 | - | X-70 |
| 0.028–0.142 | 1.52–1.90 | 0.001–0.009 | 0.17–0.31 | 0.015–0.20 | 0.05–0.3 | 0.038–0.090 | 0.002–0.1 | 0.007–0.024 | - | 0.015–0.12 | 0.02–0.75 | - | X-80 |
| 0.025–0.1 | 1.56–2.0 | 0.001–0.0024 | 0.1–0.25 | 0.25–0.46 | 0.19–0.43 | 0.043–0.089 | 0.003–0.07 | 0.011–0.02 | 0.006–0.030 | 0.016–0.42 | 0.13–0.54 | - | X-100 |
| 0.027–0.05 | 0.54–2.14 | 0.001–0.004 | 0.08–0.31 | 0.010–0.015 | 0.001–0.40 | 0.048–0.1 | <0.025 | <0.015 | 0.045–0.04 | 0.22–0.42 | 0.017–1.35 | 0.0013–0.0017 | X-120 |
| Stage | Description |
|---|---|
| 1 | Formed during the liquid phase or after solidification process. Very stable precipitates, generally too coarse to influence on austenite recrystallization. Smallest can retard austenite coarsening during reheating. |
| 2 | Precipitation induced by strain during controlled rolling, retarding the austenite recrystallization and causing grain refinement. |
| 3 | Formed during or after austenite-ferrite transformation, nucleation in austenite-ferrite interfase or ferrite. Fine precipitation is observed. |
| Precipitate Type | Effect |
|---|---|
| Nb | Control austenite transformation during hot rolling processing |
| TIN | Pin and refine the grain size during high temperature austenitizing |
| VN, NbCN, TiC | Refines steels microstructure and grain size |
| Nb, NbCN | Increase recrystallization temperature during hot rolling |
| VC | Induces precipitation strengthening after normalizing |
| VN, VC, NbCN, TiC | Induce precipitation strengthening after hot rolling |
| Temperature | 579 °C | 621 °C |
| Yield strength | 701–728 MPa | 749–821 MPa |
| Tensile strength | 996–997 MPa | 821–876 MPa |
| Elongation | 21–23% | 19–25% |
| Decade | Relevant Topics |
|---|---|
| 1940s | Patent No. 2,264,355, “Steel” by F.M. Becket and F. Russell. |
| 1950s | Deformation and ageing of mild steel. Cleavage strength. Metallurgy of Microalloyed steel. Columbium(Nb)-treated steels. Effect of small Nb additions to steels. |
| 1960s | Small Nb addition to C-Mg steels. Dislocations and plastic flow. Effects of controlled rolling. Strong Tough Structural Steel. |
| 1970s | Theory of hydrogen embrittlement. New model for hydrogen embrittlement. Assessment of precipitation kinetics. Stages of the controlled-rolling. Control of inclusions. Controlling inclusions by injecting Ca. Materials for hydrogen pipelines. Analysis of hydrogen trapping. |
| 1980s | Recrystallization of austenite during hot deformation. Hydrogen degradation. Effect of accelerated cooling. Niobium carbonitride precipitation. Environmentally assisted cracking. |
| 1990s | Specifications requirements for modern linepipe. Strain induced precipitation of Nb in austenite. Hydrogen interactions with defect. Nitrogen in steels. TiN-MnS Addition for Improvement toughness. Sulfide Stress Cracking. Softening and flow stress behavior. Influence of titanium and carbon contents. Titanium technology. |
| 2000s | Effects of coiling temperature on microstructure. Effects of sulfide-forming elements. Effect of chromium on the microstructure. Hydrogen induced blister cracking. Effects of thermo-mechanical control process. Influence of Ti on the hot ductility. Trap-governed hydrogen diffusivity. Comparison of acicular ferrite and ultrafine ferrite. Influence of Mo content. Ultra-Fast Cooling. Role of Nb, B and Mo hardenability. Ductile crack propagation in pipes. Effect of bainite/martensite mixed microstructure. Effect of tempering and carbide free bainite. Microstructural evolution. Influence of Mn content. Steels processed through CSP thin-slab technology. Effect of Mo on continuous cooling bainite transformation. Correlation of microstructure and Charpy impact properties. Dual phase versus TRIP strip steels. The effect of niobium in Castrip® steel. Spray evaporative cooling. |
| 2010s | Ti-alloyed high strength microalloyed steel. Hot strip steels. The first direct observation of hydrogen trapping sites. Evolution during thermomechanical processing. Ultra-High Strength X120 pipeline steel. Niobium high carbon applications. Modern HSLA steels. Ultra-low Carbon steels. Ultra-Fast Cooling. Mechanical anisotropy in steels. Effect of dissolution and precipitation of Nb. Influence of nanoparticle reinforcements. Reversible hydrogen trapping. Strengthening by multiply nanoscale microstructures. Effects of TMCP schedule on precipitation. |
| Welding Zone | Microhardness (HV) |
|---|---|
| Fusion zone | 356 |
| Heat affected zone | 325 |
| Base metal | 298 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villalobos, J.C.; Del-Pozo, A.; Campillo, B.; Mayen, J.; Serna, S. Microalloyed Steels through History until 2018: Review of Chemical Composition, Processing and Hydrogen Service. Metals 2018, 8, 351. https://doi.org/10.3390/met8050351
Villalobos JC, Del-Pozo A, Campillo B, Mayen J, Serna S. Microalloyed Steels through History until 2018: Review of Chemical Composition, Processing and Hydrogen Service. Metals. 2018; 8(5):351. https://doi.org/10.3390/met8050351
Chicago/Turabian StyleVillalobos, Julio C., Adrian Del-Pozo, Bernardo Campillo, Jan Mayen, and Sergio Serna. 2018. "Microalloyed Steels through History until 2018: Review of Chemical Composition, Processing and Hydrogen Service" Metals 8, no. 5: 351. https://doi.org/10.3390/met8050351





