Synthesis of Complex-Alloyed Nickel Aluminides from Oxide Compounds by Aluminothermic Method
Abstract
:1. Introduction
2. Materials and Methods
- -
- X-ray spectral microanalysis was performed on the alloy complexes to determine the content of elements in different structural components using field emission scanning electron microscope (FE-SEM) Hitachi SU-70 (Tokyo, Japan) with attachments for energy-dispersive (Thermo Scientific Ultra Dry) and wave (Thermo Scientific Magna Ray) X-ray spectral microanalyzer;
- -
- X-ray diffraction analysis, which was performed on a diffractometer “DRON-7” in copper radiation using a diffraction database.
3. Results and Discussion
3.1. Alloy No. 1. Ni-Al-Mo
- -
- In β′-solid solution based on NiAl (points 3–5 on Figure 1a), the following elements are dissolved, atom %: 61.35 Ni; 38.04 Al; β′ = Ni61.35Al38.04 = Ni1,6Al. In this phase, 0.24 atom % Mo is dissolved;
- -
- In the quasi-eutectic (points 1, 2, 6 on Figure 1a) the content of Ni and Al decreases, and the concentration of Mo increased substantially in comparison with the β′ phase, atom %: 58.64 Ni; 3.65 Al; 37.69 Mo. It can be assumed that the intermetallide phase MoNi (β′ + MoNi) crystallizes in quasi-eutectic.
3.2. Alloy No. 2. Ni-Al-W
- -
- In β′ phase of the variable composition (points 4–6 on Figure 1b), 65.22 atom % Ni and 34.44 atom % Al are dissolved, but W is not dissolved in this phase; β′ = Ni65.22Al34.44 = Ni1,9Al;
- -
- In quasi-eutectic (points 1–3 on Figure 1b), 12.81 atom % Ni and 87.18 atom % W are dissolved, but Al does not dissolve in this phase; W is in the form of a rounded, independent phase in the eutectic, since Ni does not dissolve in W.
3.3. Alloy No. 3. Ni-Al-Cr
- -
- In β′ phase (points 1–3, 7–10 on Figure 1c), the dissolved elements are, atom %: 51.08 Ni; 43.32 Al; 5.58 Cr; β′ = Ni51.08Al43.32 = Ni1.18Al with 5.58 atom % Cr;
- -
- In quasi-eutectic (points 4–6 on Figure 1c), the dissolved elements are, atom %: 17.7 Ni; 5.35 Al; 76.93 Cr. The assumed β′ phase of the variable composition has the corresponding stoichiometric ratio, atom %: β′ = Ni51.08Al43.32 = Ni1.18Al; 5.58 atom % Cr is dissolved in nickel aluminide;
- -
- In eutectic (points 4, 5, 6), the dissolved elements are, atom %: 17.7 Ni; 5.35 Al; 76.93 Cr. According to the phase diagrams of the alloys Ni–Cr and Al–Cr, nickel and aluminum can be dissolved in chromium, thus forming a β-solid solution based on chromium.
3.4. Alloy No. 4. Ni-Al-Ti
- -
- In β′ phase (Figure 1d, points 1–3), the following elements are dissolved, atom %: 52.33 Ni; 41.35 Al; 6.31 Ti; β′ = Ni52.33Al41.35 = Ni1.27Al with 6.31 atom % Ti; at the points 4–5, the mixture of nickel aluminide and titanium aluminide is formed, atom %: 29.06 Ni; 35.75 Al; 35.23 Ti;
- -
- In quasi-eutectic (Figure 1d, points 6–9), Ni and Al content decreased, and Ti concentration increased substantially in comparison with the β′ phase, atom %: 33.84 Ni; 36.07 Al; 30.08 Ti. Therefore, intermetallides, nickel aluminides and titanium nickelides may be the second phases in the eutectic.
3.5. Alloys No. 5. Ni-Al-Cr-Mo-W and No. 6. Ni-Al-Cr-Mo-W-Ti
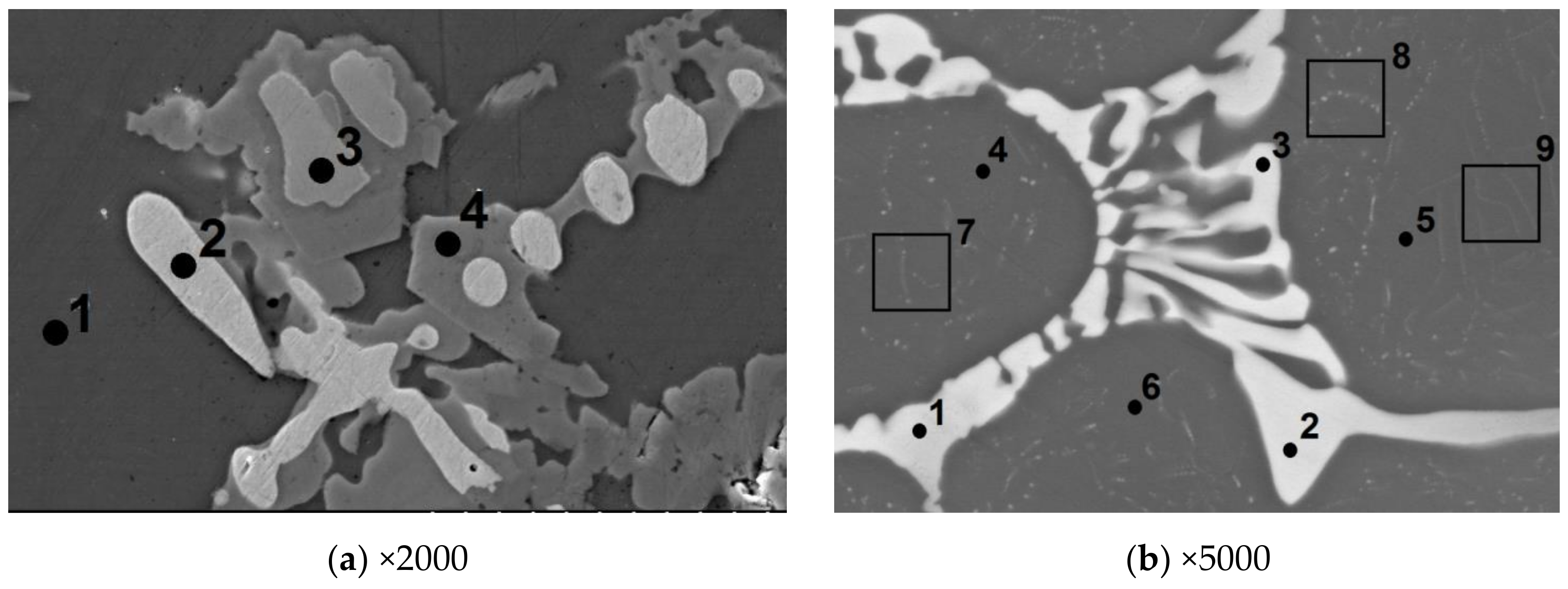
3.6. Alloy No. 7. Ni-Al-Cr-V and Alloy No. 8. Ni-Al-Cr-Mo-V
- -
- In β′ phase (points 4–9 in Figure 2b), the following elements are dissolved, atom %: 50.35 Ni; 41.57 Al; 4.67 Cr; 0.53 Mo; 2.87 V; Ni50.35Al41.57 = Ni1.21Al with alloying elements Cr, Mo and V;
- -
- In quasi-eutectic (points 1–3 on Figure 2b), the following elements are dissolved, atom %: 10.2 Ni; 5.88 Al; 31.94 Cr; 32.68 Mo; 19.28 V. According to the phase diagram of V–Cr, these elements form continuous solid solutions.
- system Ni-Al-Mo: 63.33 Ni; 39.69 Al; 5.97 Mo;
- system Ni-Al-W: 62.95 Ni; 33.96 Al; 3.19 W;
- system Ni-Al-Cr: 37.29 Ni; 32.81 Al; 29.90 Cr;
- system Ni-Al-Ti: 49.61 Ni; 40.34 Al; 10.05 Ti.
4. Conclusions
- (1)
- Nickel aluminides doped with chromium, molybdenum, tungsten, titanium and vanadium have been successfully obtained in the joint aluminothermic reduction of nickel oxide and transition metal oxides. The thermodynamic feasibility of joint reduction of oxides of alloying metals is consistent with the results of the experiments made on complex-alloyed nickel aluminides. Conditions for the alloys’ synthesis have been studied and the obtained alloys have been identified by the methods of elemental and X-ray phase analysis. The microstructure investigations show that all the alloys obtained are formed on the basis of two structural components: nickel aluminide (NiAl) with variable content of alloying elements (β′ phase) and quasi-eutectic consisting of β′ phase and intermetallic phases.
- (2)
- The obtained intermetallic alloys (Ni–Al, Ni-Al-Cr, Ni-Al-Mo, Ni-Al-W, Ni-Al-Cr-Mo-W) have been used as anode materials for the creation of heat-resistant coatings by the ESD method on steel C1030 grade, which actually display increased heat resistance by 7.5 times, while the Ni-Al-Cr-Mo-W alloy coating practically does not oxidize under the selected test conditions.
- (3)
- The use of intermetallic NiAl as a modifying additive in tin bronze allows increase of the microhardness of the α-solid solution by 1.9 times and the microhardness of the eutectoid (α + β phase) by 2.7 times, when adding of 0.15 wt. % master alloy.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bochenek, K.; Basista, M. Advances in processing of NiAl intermetallic alloys and composites for high temperature aerospace applications. Prog. Aerosp. Sci. 2015, 79, 136–146. [Google Scholar] [CrossRef]
- Guo, J.; Wang, Z.; Sheng, L.; Zhou, L.; Yuan, C.; Chen, Z.; Song, L. Wear properties of NiAl based materials. Prog. Nat. Sci. 2012, 22, 414–425. [Google Scholar] [CrossRef]
- Bhaskar, M.S. Quantitative phase field modelling of precipitate coarsening in Ni-Al-Mo alloys. Comp. Mat. Sci. 2018, 146, 102–111. [Google Scholar] [CrossRef]
- Petzow, G.; Effenberg, G. (Eds.) Ternary Alloys: A Comprehensive Compendium of Results, Constitutional Data and Phase Diagrams; VCH: Weinheim, Germany; Basel, Switzerland; Cambridge, UK; New York, NY, USA, 1992; Volume 7. [Google Scholar]
- Hyde, K.B.; Norman, A.F.; Prangnell, P.B. The Effect of Ti on Grain Refinement in Al-Sc Alloys. Mater. Sci. Forum 2002, 396–402, 39–44. [Google Scholar] [CrossRef]
- Song, M.; He, Y.; Fang, S. Effects of Zr Content on the Yield Strength of an Al-Sc Alloy. J. Mater. Eng. Perform. 2010, 20, 377–381. [Google Scholar] [CrossRef]
- Dalen, M.E.; Dunand, D.C.; Seidman, D.N. Effects of Ti additions on the nanostructure and creep properties of precipitation-strengthened Al-Sc alloys. Acta Mater. 2005, 53, 4225–4235. [Google Scholar] [CrossRef]
- Royset, J.; Ryum, N. Scandium in Aluminum Alloys. Int. Mater. Rev. 2005, 50, 19–44. [Google Scholar] [CrossRef]
- Ghosh, G.; Asta, M. First-principles calculation of structural energetics of Al-TM (TM = Ti, Zr, Hf) intermetallics. Acta Mater. 2005, 53, 3225–3252. [Google Scholar] [CrossRef]
- Marquis, E.A.; Seidman, D.N. Nanoscale structural evolution of Al3Sc precipitates in Al(Sc) alloys. Acta Mater. 2001, 49, 1909–1919. [Google Scholar] [CrossRef]
- Fuller, C.B.; Murray, J.L.; Seidman, D.N. Temporal evolution of the nanostructure of Al(Sc,Zr) alloys: Part I—Chemical compositions of Al3(Sc1-xZrx) precipitates. Acta Mater. 2005, 53, 5401–5413. [Google Scholar] [CrossRef]
- Knipling, K.E.; Karnesky, R.A.; Lee, C.P.; Dunand, D.C.; Seidman, D.N. Precipitation evolution in Al–0.1Sc, Al–0.1Zr and Al–0.1Sc–0.1Zr (at.%) alloys during isochronal aging. Acta Mater. 2010, 58, 5184–5195. [Google Scholar] [CrossRef]
- Zakharov, V.V. About alloying of aluminum alloys with transition metals. Met. Sci. Heat Treat. 2017, 59, 67–71. [Google Scholar] [CrossRef]
- Malek, P.; Janecek, M.; Smola, B.; Bartuska, P.; Plestil, J. Structure and properties of rapidly solidified Al-Zr-Ti alloys. J. Mater. Sci. 2000, 35, 2625–2633. [Google Scholar] [CrossRef]
- Popova, E.A.; Shubin, A.B.; Kotenkov, P.V.; Pastukhov, E.A.; Bodrova, L.E.; Fedorova, O.M. Al-Ti-Zr master alloys: Structure formation. Russ. Metall. (Met.) 2012, 5, 357–361. [Google Scholar] [CrossRef]
- Popova, E.A.; Kotenkov, P.V.; Pastukhov, E.A.; Shubin, A.B. Master alloys Al-Sc-Zr, Al-Sc-Ti, and Al-Ti-Zr: Their manufacture, composition, and structure. Russ. Metall. (Met.) 2013, 8, 590–594. [Google Scholar] [CrossRef]
- Popova, E.A.; Kotenkov, P.V.; Pastukhov, E.A. Synergetic effect in modifying with master alloys having an aluminide cubic structure. Russ. Metall. (Met.) 2016, 2, 189–193. [Google Scholar] [CrossRef]
- Wang, X.; Chen, G.; Li, B.; Wu, L.; Jiang, D. Effects of Sc, Zr and Ti on the microstructure and properties of Al alloys with high Mg content. Rare Metals 2010, 29, 66–71. [Google Scholar] [CrossRef]
- Xu, C.; Du, R.; Wang, X.; Hanada, S.; Yamagata, H.; Wang, W.; Ma, C. Effect of cooling rate on morphology of primary particles in Al-Sc-Zr master alloy. Trans. Nonferr. Met. Soc. China 2014, 24, 2420–2426. [Google Scholar] [CrossRef]
- Lapshin, O.V.; Ovcharencko, V.E.; Boyangin, E.N. Thermokinetic and Thermo-physical Parameters of High-Temperature Synthesis of Intermetallide Ni3Al by Thermal Shock of a Powder Mixture of Pure Elements. Combust. Expl. Shock Waves 2002, 38, 430–434. [Google Scholar] [CrossRef]
- Xie, Y.J.; Wang, M.C. Microstructural morphology of electrospark deposition layer of a high gamma prime superalloy. Surf. Coat. Technol. 2006, 201, 691–698. [Google Scholar] [CrossRef]
- Reynold, J.L.; Holdren, L.R.; Brown, L.E. Electro-Spark Deposition. Adv. Mater. Process. 2003, 161, 35. [Google Scholar]
- Oniashvili, G.S.H.; Aslamazashvili, Z.G.; Zakharov, G.V.; Tavadze, G.F.; Chikhradze, M.N.; Dzigrashvili, T.A.; Berner, A. SHS of fine-grained ceramics containing carbides, nitrides, and borides. Int. J. Self Propag. High Temp. Synth. 2013, 22, 185–188. [Google Scholar] [CrossRef]
- Shekari, M.; Adeli, M.; Khobzi, A.; Kobashi, M.; Kanetake, N. Induction-activated self-propagating, high-temperature synthesis of nickel aluminide. Adv. Powder Technol. 2017, 28, 2974–2979. [Google Scholar] [CrossRef]
- Gostishchev, V.V.; Astapov, I.A.; Medneva, A.V.; Ri, H.; Khimukhin, S.N. Fabrication of Alloyed Aluminum Nickelides by Metallothermy of Metals Oxides. Russ. J. Non-Ferr. Met. 2016, 57, 41–46. [Google Scholar] [CrossRef]
- Gostishchev, V.V.; Astapov, I.A.; Seredyuk, A.V.; Khimukhin, S.N.; Ri, H. High-Temperature Synthesis of Composites Based on Nickel Aluminides. Inorg. Mater. 2016, 4, 419–422. [Google Scholar] [CrossRef]
- Vrel, D.; Langlois, P.; Heian, E.M.; Karnatak, N.; Dubois, S.; Beaufort, M.-F. Reaction Kinetics and Phase Segregation in the 3NiO + 2Al → 3Ni + Al2O3 Thermite System. Int. J. Self Propag. High Temp. Synth. 2003, 12, 261–270. [Google Scholar]


| No. | Charge Composition Ratio, Weight Fractions | Elements Content, atom % | Microhardness, MPa | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NiO | Cr2O3 | MoO3 | WO3 | TiO2 | Al | CaF2 | Ni | Al | Cr | Mo | W | Ti | ||
| 5. | Alloy Ni-Al-Cr-Mo-W | |||||||||||||
| 5.1. | 1 | 0.14 | 0.14 | 0,14 | - | 0.5 | 0.65 | 36.54 | 20.85 | 15.91 | 10.91 | 15.79 | - | 6200 |
| 5.2. | 1 | 0.14 | 0.14 | 0.14 | - | 0.6 | 0.65 | 37.95 | 20.67 | 16.59 | 10.32 | 14.47 | - | 7436 |
| 6. | Alloy Ni-Al-Cr-Mo-W-Ti | |||||||||||||
| 6.1. | 1 | 0.14 | 0.14 | 0.14 | 0.14 | 0.5 | 0.5 | 38.00 | 25.02 | 8.22 | 5.43 | 8.78 | 14.55 | 5584 |
| 6.2. | 1 | 0.14 | 0.14 | 0.14 | 0.14 | 0.5 | 0.65 | 37.00 | 24.42 | 9.16 | 5.71 | 8.3 | 15.41 | 6908 |
| No. | Charge Composition Ratio, Weight Fractions | Elements Content, atom % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NiO | Cr2O3 | V2O3 | MoO3 | Al | CaF2 | Ni | Al | Cr | V | Mo | |
| 7. | Alloy Ni-Al-Cr-V | ||||||||||
| 7.1. | 1 | 0.14 | 0.14 | - | 0.5 | 0.65 | 43.82 | 34.21 | 13.28 | 8.69 | - |
| 7.2. | 1 | 0.14 | 0.14 | - | 0.6 | 0.65 | 44.36 | 34.84 | 13.24 | 7.56 | - |
| 7.3. | 1 | 0.14 | 0.14 | - | 0.65 | 0.65 | 45.11 | 34.09 | 13.41 | 7.39 | - |
| 8. | Alloy Ni-Al-Cr-Mo-V | ||||||||||
| 8.1. | 1 | 0.14 | 0.14 | 0.14 | 0.65 | 0.5 | 35.43 | 29.36 | 10.42 | 11.34 | 13.45 |
| 8.2. | 1 | 0.14 | 0.14 | 0.14 | 0.65 | 0.65 | 35.77 | 29.50 | 10.22 | 10.48 | 14.03 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gostishchev, V.; Ri, E.; Ri, H.; Kim, E.; Ermakov, M.; Khimukhin, S.; Deev, V.; Prusov, E. Synthesis of Complex-Alloyed Nickel Aluminides from Oxide Compounds by Aluminothermic Method. Metals 2018, 8, 439. https://doi.org/10.3390/met8060439
Gostishchev V, Ri E, Ri H, Kim E, Ermakov M, Khimukhin S, Deev V, Prusov E. Synthesis of Complex-Alloyed Nickel Aluminides from Oxide Compounds by Aluminothermic Method. Metals. 2018; 8(6):439. https://doi.org/10.3390/met8060439
Chicago/Turabian StyleGostishchev, Victor, Ernst Ri, Hosen Ri, Evgeniy Kim, Michail Ermakov, Sergey Khimukhin, Vladislav Deev, and Evgeny Prusov. 2018. "Synthesis of Complex-Alloyed Nickel Aluminides from Oxide Compounds by Aluminothermic Method" Metals 8, no. 6: 439. https://doi.org/10.3390/met8060439






