The Effect of Carbon Dissection of Waste Plastics on Inhibiting the Adhesion of Fine Iron Ore Particles during Hydrogen Reduction
Abstract
:1. Introduction
2. Experiment
2.1. Experimental Materials
2.2. Experimental Device
2.3. Experimental Programs and Methods
3. Results and Discussion
3.1. Results and Analysis of the Pre-Experiment
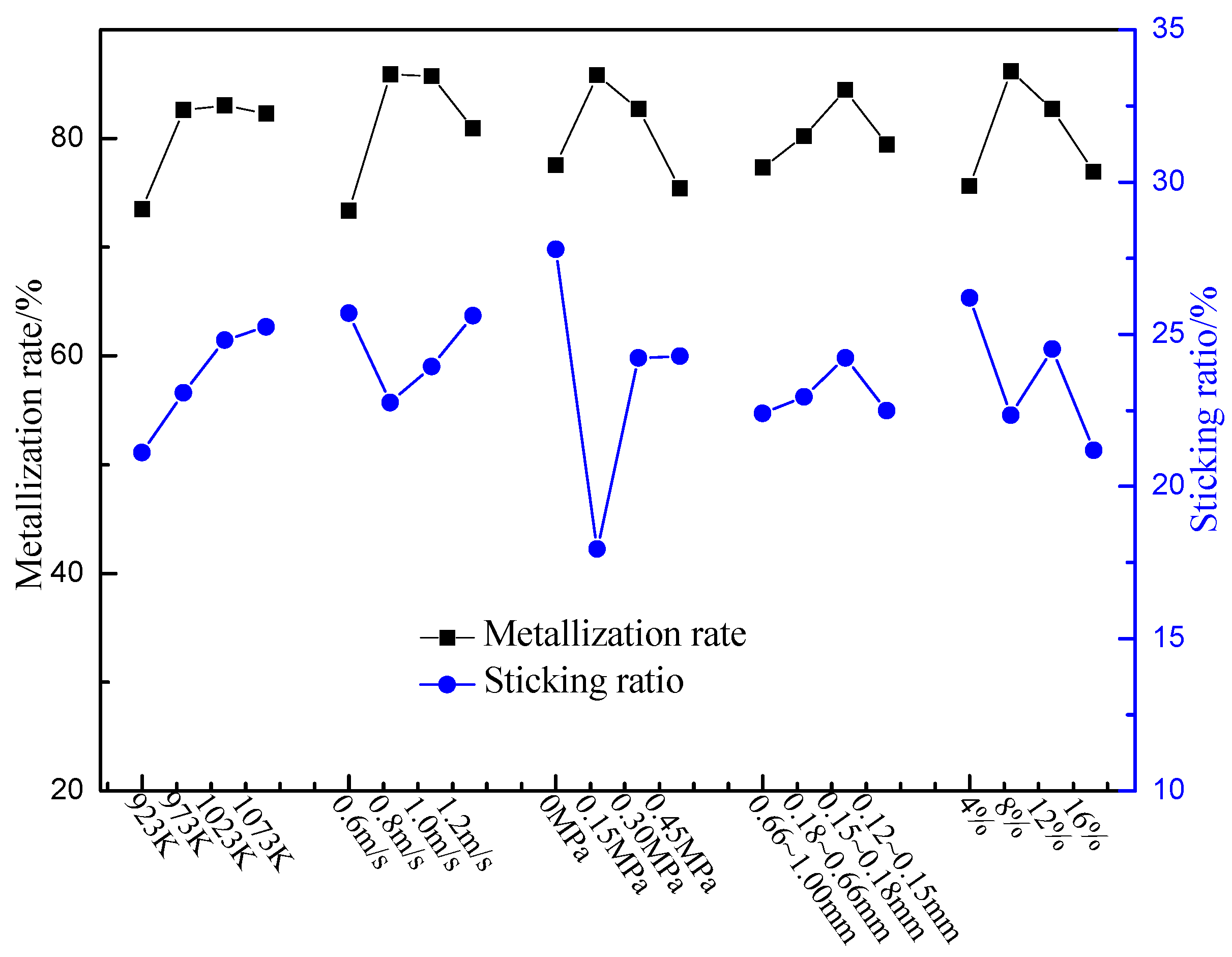
3.2. Optimization of Operating Parameters
4. Mechanism of Carbon Precipitation in Inhibiting Sticking of Iron Ore Particles
4.1. Sticking Mechanism of Iron Ore
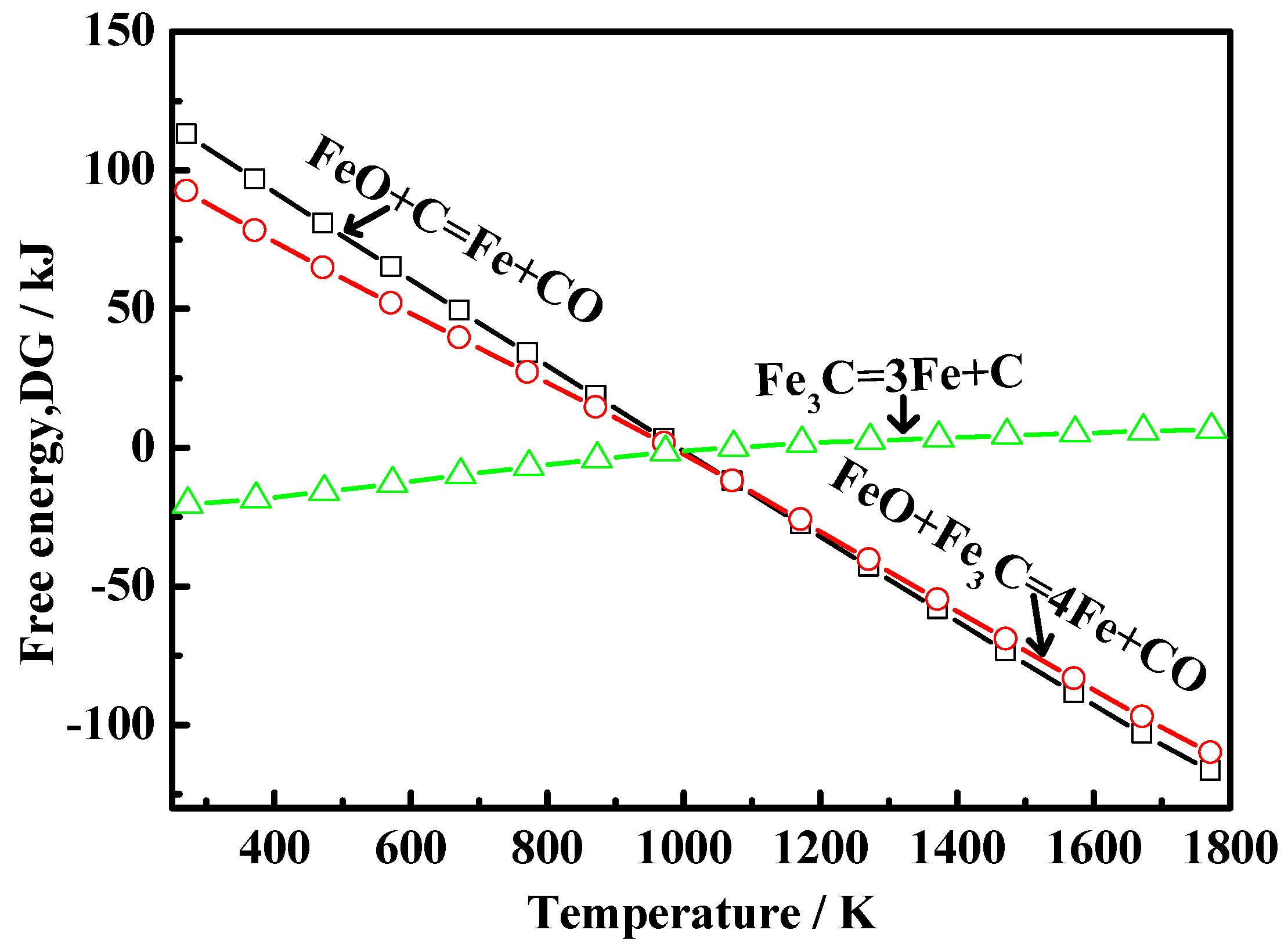
4.2. Reaction Mechanism of Carbon Precipitation from Energetic Waste Plastics
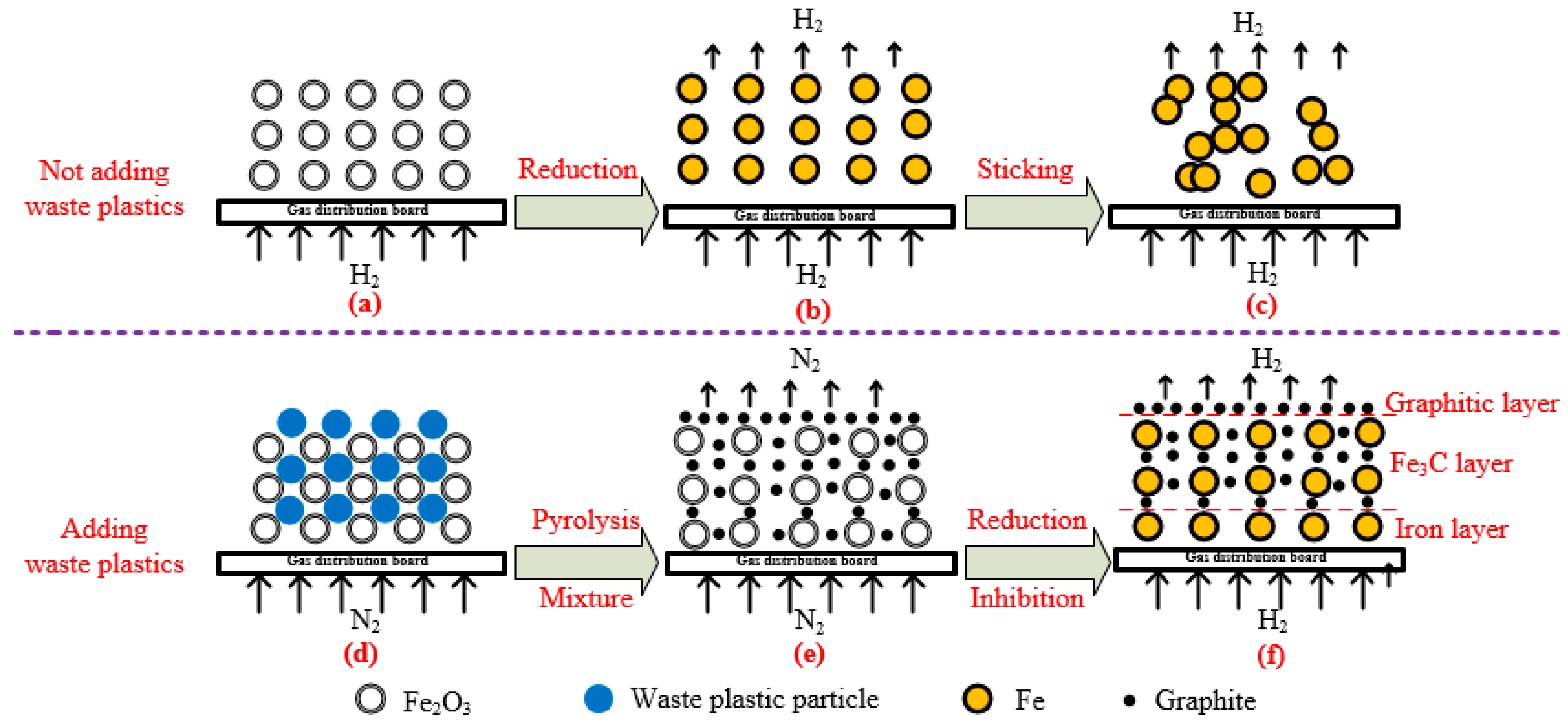
4.3. Mechanism of Carbon Precipitation in Inhibiting Sticking
5. Conclusions
- Graphite which is pyrolyzed by waste plastics can effectively inhibit the sticking between fine iron particles.
- The primary and secondary influencing factors of the metallization rate during fluidized reduction are linear velocity, mass content of energetic waste plastics, reduction pressure, reduction temperature, and particle size of energetic waste plastics. Meanwhile, the primary and secondary influencing factors of the sticking ratio during fluidized reduction are reduction pressure, mass content of energetic waste plastics, reduction temperature, linear velocity, and particle size of energetic waste plastics.
- The optimal operating parameters are reduction temperature in the range of 923–973 K, linear velocity of 0.8 m/s, reduction pressure of 0.15 MPa, reduction pressure of 0.15 MPa, size of energetic waste plastics in the range of 0.18–0.66 mm, and mass content of energetic waste plastics of 8%.
- Sticking is caused by the mutual reunion of metal atoms.
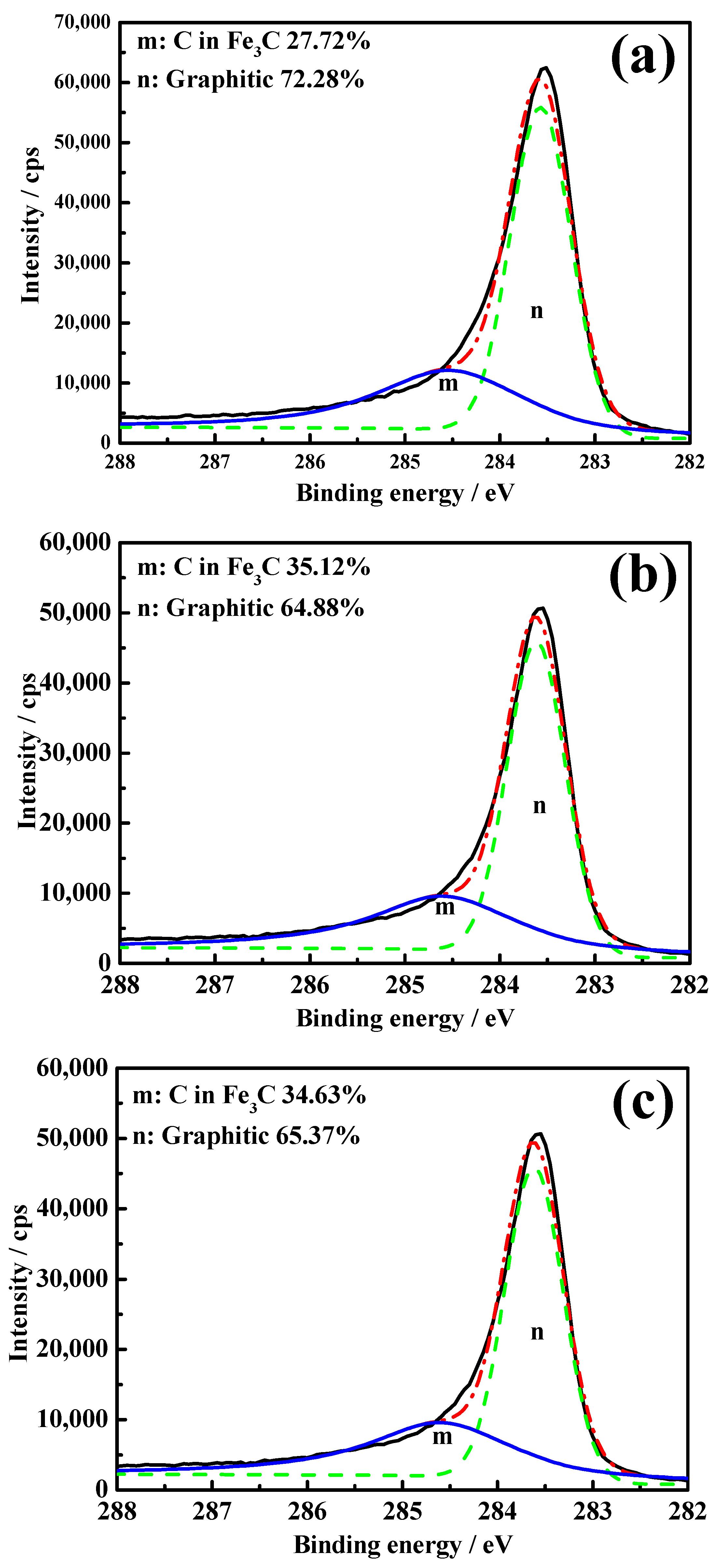
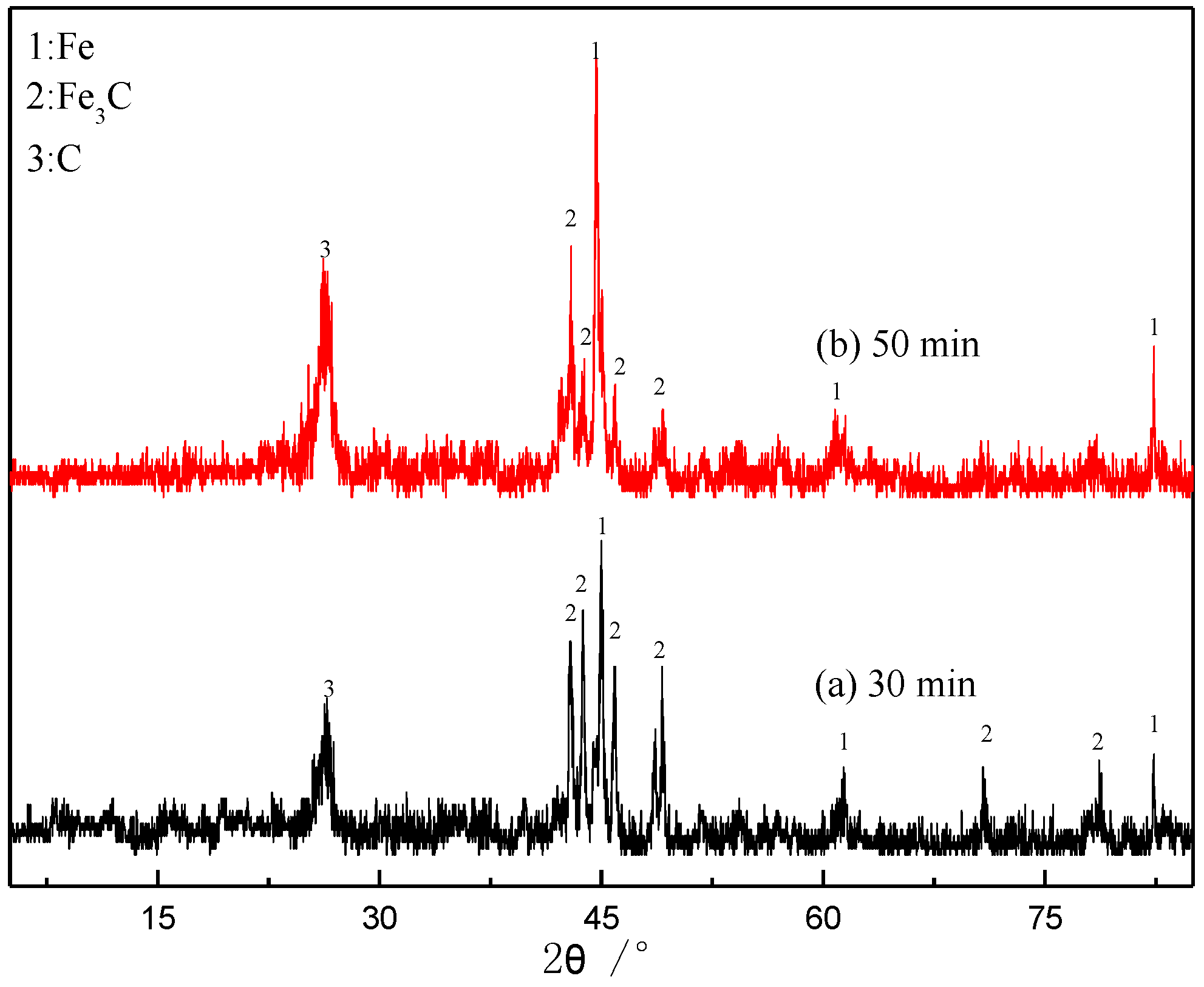
- The occurrence states of carbons deposited from energetic waste plastics can be divided into two types: graphite and carbon from Fe3C. Carbon from Fe3C slows down the sticking, while the graphite hinders the contact among particles, thereby effectively inhibiting the sticking.
Author Contributions
Funding
Conflicts of Interest
References
- Cui, L.W.; Xia, H.D.; Wang, C.; Yi, J.J.; Kong, L.H.; Chen, J. Current Status of Iron-Ore Resources in China and Screening of Object Iron-Ore Geological Data. Geol. Explor. 2012, 48, 1–5. [Google Scholar]
- Yang, M.; Xiao, W.D.; Zhang, P. Processing mineralogy study on lead and zinc oxide ore in Sichuan. Metals 2016, 6, 93. [Google Scholar] [CrossRef]
- Yang, T.J.; Zhang, J.L.; Zuo, H.B. Energy-Saving and Emission-Reducing of Blast Furnace Ironmaking Production in China. J. Iron Steel Res. Int. 2009, 16, 591–596. [Google Scholar]
- Liu, D.H.; Wang, X.Z.; Zhang, J.L.; Liu, Z.J.; Jiao, K.X.; Liu, X.L.; Wang, R.R. Study on the controlling steps and reduction kinetics of iron oxide briquettes with CO-H2 mixtures. Metall. Res. Technol. 2017, 114, 611. [Google Scholar] [CrossRef]
- Tang, J.; Chu, M.S.; Ying, Z.W.; Li, F.; Feng, C.; Liu, Z.G. Non-Isothermal gas-based direct reduction behavior of high chromium vanadium-titanium magnetite pellets and the melting separation of metallized pellets. Metals 2017, 7, 153. [Google Scholar] [CrossRef]
- Gransden, J.F.; Sheasby, J.S. Sticking of iron ore during reduction by hydrogen in a fluidized bed. Can. Metall. Q. 1974, 13, 649–657. [Google Scholar] [CrossRef]
- Komatina, M.; Gudenau, H.W. The Sticking Problem during Direct Reduction of Fine Iron Ore in the Fluidized Bed. Metalurgija 2004, 10, 309–328. [Google Scholar]
- Xue, Q.; Ma, Y.J.; Lei, J.F.; Yang, R.; Wang, C. Evolution of microstructure and phase composition of Ti-3Al-5Mo-4.5V alloy with varied β phase stability. J. Mater. Sci. Technol. 2018. [Google Scholar] [CrossRef]
- Bizhanov, A.; Malysheva, T. Metallization of extruded briquettes (BREX) in midrex process. Metals 2017, 7, 259. [Google Scholar]
- Lu, W.K. Observations of whisker growth during iron ore reduction. Scand. J. Metall. 1973, 2, 65–67. [Google Scholar]
- Gong, Q.M.; Huang, Q.Z.; Huang, B.Y.; Wu, F.Q.; Chen, T.F.; Zhang, F.Q. Effect of carbonization pressure on morphology of pitch derived carbon and densification efficiency of C/C composites used for aircraft brakes. New Carbon Mater. 2002, 17, 23–28. [Google Scholar]
- Shi, Y.J.; Liu, S.J.; Yao, J.Z.; Li, G.L. Carbon adhesion and reduction of iron ore powder in a fluidized bed. Iron Steel 1993, 28, 1–7. [Google Scholar]
- Mikami, T.; Kamiya, H.; Horio, M. The mechanism of defluidization of iron particles in a fluidized bed. Powder Technol. 1996, 89, 231–238. [Google Scholar] [CrossRef]
- Zhong, Y.W.; Gong, X.Z.; Wang, Z.; Guo, Z.C. Effect of iron surfaces with nano/micro structures on the sticking of Fe2O3 in fluidized bed reduction. J. Univ. Sci. Technol. Beijing 2011, 33, 406–412. [Google Scholar]
- Wang, Q.; Zou, X.; Matsuura, H.; Wang, C. Evolution of Inclusions During the 1473 K (1200 °C) Heating Process of EH36 Shipbuilding Steel. Metall. Mater. Trans. B 2018, 49, 18–22. [Google Scholar] [CrossRef]
- Krishnan, S.H.; Sharma, R.; Dash, P.S.; Haldar, S.K.; Biswas, B. Use of waste plastics in cokemaking at Tata Steel. Ironmak. Steelmak. 2013, 33, 288–292. [Google Scholar] [CrossRef]
- Shao, J.H.; Guo, Z.C.; Tang, H.Q. Influence of reducing atmosphere on the sticking during reduction of iron ore fines in a fluidized bed. J. Univ. Sci. Technol. Beijing 2013, 35, 273–281. [Google Scholar]
- Guo, L.; Yu, J.T.; Tang, J.K.; Lin, Y.H.; Guo, Z.C. Influence of coating MgO on sticking and functional mechanism during fluidized bed reduction of vanadium titano-magnetite. J. Iron Steel Res. Int. 2015, 22, 464–472. [Google Scholar] [CrossRef]
- Langston, B.G.; Stephens, F.M. Self-agglomerating fluidized bed reduction. J. Met. 1960, 12, 312–316. [Google Scholar] [CrossRef]
- Piotrowski, K.; Mondal, K.; Lorethova, H.; Stonawski, L.; Szymański, T.; Wiltowski, T. Effect of gas composition on the kinetics of iron oxide reduction in a hydrogen production process. Int. J. Hydrog. Energy 2005, 30, 1543–1554. [Google Scholar] [CrossRef]
- Wang, Q.H.; Shao, J.H.; Lin, Y.H.; Guo, Z.C.; Tang, H.Q. An experimental study on the kinetics of iron ore fine reduced by CO in micro fluidized bed. J. Iron Steel Res. 2012, 24, 6–9. [Google Scholar] [CrossRef]
- Golunski, S.E. Why use platinum in catalytic converters? Platin. Met. Rev. 2007, 51, 162. [Google Scholar] [CrossRef]
- Xu, Q.Y.; Wang, H.C.; Fu, Y.K.; Wang, J.J. Parameters optimization, sticking mechanism and kinetics analysis of fine iron ore in fluidized-reduction process. ISIJ Int. 2016, 56, 1929–1937. [Google Scholar] [CrossRef]
- Liu, M.H.; Ding, Z.W.; Zhang, T.W.; Hou, S.D. Analysis of pressure fuctuation signals in a pressured fluidized bed. Comput. Appl. Chem. 2011, 28, 1281–1284. [Google Scholar]
- Park, E.; Ostrovski, O.; Zhang, J.Q.; Thomson, S.; Howe, R. Characterization of phases formed in the iron carbide process by X-ray diffraction, mossbauer, X-ray photoelectron spectroscopy, and Raman spectroscopy analyses. Metall. Mater. Trans. B 2001, 32, 839–845. [Google Scholar] [CrossRef]
- Dong, X.L.; Zhang, Z.D.; Xiao, Q.F.; Zhao, X.G.; Chuang, Y.C.; Jin, S.R.; Sun, W.M.; Li, Z.J.; Zheng, Z.X.; Yang, H. Characterization of ultrafine γ-Fe(C), α-Fe(C) and Fe3C particles synthesized by arc-discharge in methane. J. Mater. Sci. 1998, 33, 1915–1919. [Google Scholar] [CrossRef]
- Hui, Y.A.; Wang, D.Y.; Jiang, M.F. Phase analysis and production mechanism of iron carbide. J. Northeast. Univ. 2003, 24, 828–831. [Google Scholar]
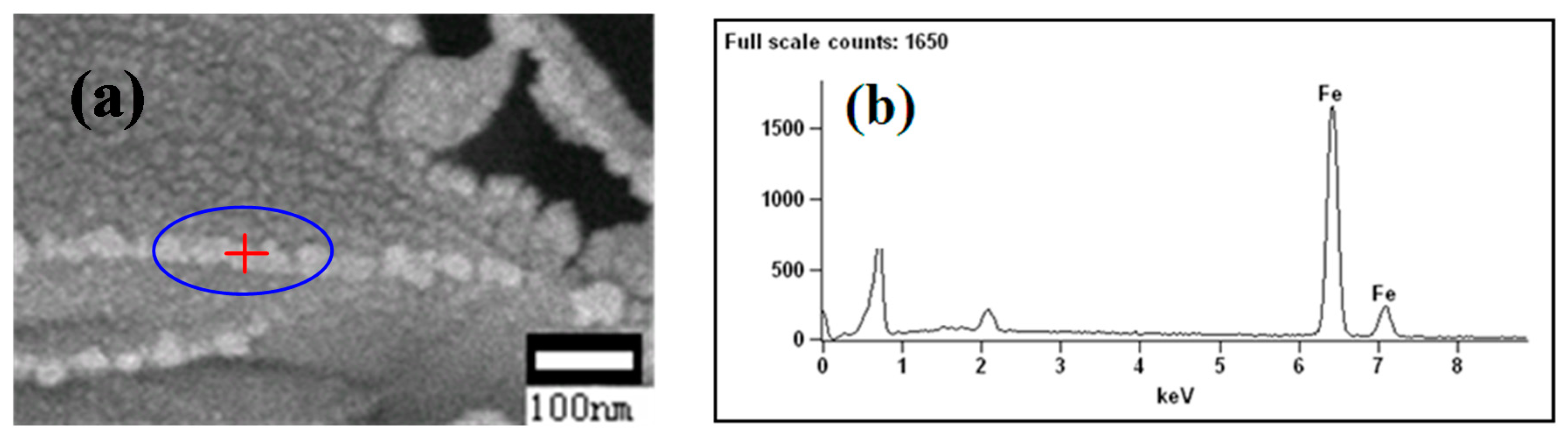
- Zhang, B.; Wang, Z.; Gong, X.Z.; Guo, Z.C. Characterization of precipitated carbon by XPS and its prevention mechanism of sticking during reduction of Fe2O3 particles in the fluidized bed. ISIJ Int. 2013, 53, 411–418. [Google Scholar] [CrossRef]
- Liu, J.H.; Zhang, J.Y.; Zhou, T.P. Influence of foreign oxides on reduction kinetics of iron oxides. J. Iron Steel Res. 2000, 12, 55–58. [Google Scholar]
- Hou, B.L.; Zhang, H.Y.; Li, H.Z.; Zhu, Q.S. Study on kinetics of iron oxide reduction by hydrogen. Chin. J. Chem. Eng. 2012, 20, 10–17. [Google Scholar] [CrossRef]



| Compositions | Al2O3 | CaO | FeO | MgO | MnO | P | S | SiO2 | TFe |
|---|---|---|---|---|---|---|---|---|---|
| Mass % | 2.61 | 0.035 | 0.15 | 0.05 | 1.055 | 0.076 | 0.031 | 4.70 | 57.59 |
| Factor | A Temperature/K | B Linear Velocity/m/s | C The Reducing Pressure/MPa | D Waste Plastic Particle Size/mm | E Waste Plastic Mass Percentage | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Level | A1 | A2 | A3 | A4 | B1 | B2 | B3 | B4 | C1 | C2 | C3 | C4 | D1 | D2 | D3 | D4 | E1 | E2 | E3 | E4 |
| 923 | 973 | 1023 | 1073 | 0.6 | 0.8 | 1.0 | 1.2 | 0 | 0.15 | 0.30 | 0.45 | 0.66–1.00 | 018–0.66 | 0.15–0.18 | 0.12–0.15 | 4% | 8% | 12% | 16% | |
| Condition | Temperature/K | Linear Velocity/m/s | The Reducing Pressure/MPa | Particle Size/mm | Hydrogen Volume Scale | |
|---|---|---|---|---|---|---|
| NO. | ||||||
| 1 | 923 | 0.6 | 0 | 0.66–1.00 | 4% | |
| 2 | 923 | 0.8 | 0.15 | 0.18–0.66 | 8% | |
| 3 | 923 | 1.0 | 0.30 | 0.15–0.18 | 12% | |
| 4 | 923 | 1.2 | 0.45 | 0.12–0.15 | 16% | |
| 5 | 973 | 0.6 | 0.15 | 0.12–0.15 | 16% | |
| 6 | 973 | 0.8 | 0 | 0.15–0.18 | 12% | |
| 7 | 973 | 1.0 | 0.45 | 0.18–0.66 | 8% | |
| 8 | 973 | 1.2 | 0.30 | 0.66–1.00 | 4% | |
| 9 | 1023 | 0.6 | 0.30 | 0.18–0.66 | 8% | |
| 10 | 1023 | 0.8 | 0.45 | 0.66–1.00 | 4% | |
| 11 | 1023 | 1.0 | 0 | 0.12–0.15 | 16% | |
| 12 | 1023 | 1.2 | 0.15 | 0.15–0.18 | 12% | |
| 13 | 1073 | 0.6 | 0.45 | 0.15–0.18 | 12% | |
| 14 | 1073 | 0.8 | 0.30 | 0.12–0.15 | 16% | |
| 15 | 1073 | 1.0 | 0.15 | 0.66–1.00 | 4% | |
| 16 | 1073 | 1.2 | 0 | 0.18–0.66 | 8% | |
| Scheme | Temperature/K | Linear Velocity/m/s | Pressure/MPa | Particle Size of Wasted Plastics/mm | Mass Content of Wasted Plastics/% | Metallization Rate/% | Sticking Ratio/% |
|---|---|---|---|---|---|---|---|
| 1 | 973 | 0.8 | 0.3 | 0 | 0 | 76.44 | 17.1 |
| 2 | 973 | 0.8 | 0.3 | 0.18~0.66 | 4 | 80.63 | 15.1 |
| 3 | 973 | 0.8 | 0 | 0 | 0 | 62.44 | 22.1 |
| 4 | 973 | 0.8 | 0 | 0.18~0.66 | 4 | 70.28 | 18.2 |
| Result | A | B | C | D | E | Metallization Rate/% | Bonding Mass Percentage/% | |
|---|---|---|---|---|---|---|---|---|
| NO. | ||||||||
| 1 | A1 | B1 | C1 | D1 | E1 | 53.19 | 25.21 | |
| 2 | A1 | B2 | C2 | D2 | E2 | 90.18 | 12.86 | |
| 3 | A1 | B3 | C3 | D3 | E3 | 83.15 | 22.20 | |
| 4 | A1 | B4 | C4 | D4 | E4 | 67.44 | 24.21 | |
| 5 | A2 | B1 | C2 | D3 | E4 | 81.77 | 20.06 | |
| 6 | A2 | B2 | C1 | D4 | E3 | 84.03 | 22.64 | |
| 7 | A2 | B3 | C4 | D1 | E2 | 84.09 | 21.8 | |
| 8 | A2 | B4 | C3 | D2 | E1 | 80.63 | 27.82 | |
| 9 | A3 | B1 | C3 | D4 | E2 | 83.30 | 25.31 | |
| 10 | A3 | B2 | C1 | D3 | E1 | 85.71 | 33.93 | |
| 11 | A3 | B3 | C4 | D2 | E4 | 74.80 | 18.93 | |
| 12 | A3 | B4 | C2 | D1 | E3 | 88.35 | 21.02 | |
| 13 | A4 | B1 | C4 | D2 | E3 | 75.25 | 32.19 | |
| 14 | A4 | B2 | C3 | D1 | E4 | 83.67 | 21.56 | |
| 15 | A4 | B3 | C2 | D4 | E1 | 82.95 | 17.83 | |
| 16 | A4 | B4 | C1 | D3 | E2 | 87.28 | 29.39 | |
| Factor | Indicators | A | B | C | D | E |
|---|---|---|---|---|---|---|
| Metallization rate/% | K1 | 293.96 | 293.51 | 310.21 | 309.30 | 302.48 |
| K2 | 330.52 | 343.59 | 343.25 | 320.86 | 344.85 | |
| K3 | 332.16 | 324.99 | 330.75 | 337.91 | 330.78 | |
| K4 | 329.15 | 323.70 | 301.58 | 317.72 | 307.68 | |
| k1 | 73.49 | 73.38 | 77.55 | 77.33 | 75.62 | |
| k2 | 82.63 | 85.90 | 85.81 | 80.22 | 86.21 | |
| k3 | 83.04 | 85.75 | 82.69 | 84.48 | 82.70 | |
| k4 | 82.29 | 80.93 | 75.40 | 79.43 | 76.92 | |
| R | 9.55 | 12.52 | 10.41 | 7.15 | 10.59 | |
| Primary and secondary factors | BECAD | |||||
| Optimization scheme | B2E2C2A3D3 | |||||
| Bonding mass percentage/% | K1 | 84.48 | 102.77 | 111.17 | 89.59 | 104.79 |
| K2 | 92.32 | 90.99 | 71.77 | 91.80 | 89.36 | |
| K3 | 99.19 | 95.76 | 96.89 | 96.89 | 98.05 | |
| K4 | 100.97 | 102.44 | 97.13 | 89.99 | 84.76 | |
| k1 | 21.12 | 25.69 | 27.79 | 22.40 | 26.20 | |
| k2 | 23.08 | 22.75 | 17.94 | 22.95 | 22.34 | |
| k3 | 24.80 | 23.94 | 24.22 | 24.22 | 24.51 | |
| k4 | 25.24 | 25.61 | 24.28 | 22.50 | 21.19 | |
| R | 4.12 | 2.94 | 9.85 | 1.82 | 5.01 | |
| Primary and secondary factors | CEABD | |||||
| Optimization scheme | C2E4A1B2D1 | |||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Liu, Z.; Li, Z.; Wang, J.; Zhou, L. The Effect of Carbon Dissection of Waste Plastics on Inhibiting the Adhesion of Fine Iron Ore Particles during Hydrogen Reduction. Metals 2018, 8, 523. https://doi.org/10.3390/met8070523
Xu Q, Liu Z, Li Z, Wang J, Zhou L. The Effect of Carbon Dissection of Waste Plastics on Inhibiting the Adhesion of Fine Iron Ore Particles during Hydrogen Reduction. Metals. 2018; 8(7):523. https://doi.org/10.3390/met8070523
Chicago/Turabian StyleXu, Qiyan, Zhuangzhuang Liu, Zhiping Li, Jianjun Wang, and Li Zhou. 2018. "The Effect of Carbon Dissection of Waste Plastics on Inhibiting the Adhesion of Fine Iron Ore Particles during Hydrogen Reduction" Metals 8, no. 7: 523. https://doi.org/10.3390/met8070523




