Substrate-Induced Liquid Layering: A New Insight into the Heterogeneous Nucleation of Liquid Metals
Abstract
:1. Introduction
2. Materials and Methods
- Atomic density profile along the z direction , as follows:where is the number of atoms in a bin between and ; denotes the ensemble average, which was calculated by the time average in our simulations; is the volume of a bin with being its cross-sectional area and being the width. This parameter is used to analyze the out-of-plane features of the substrate-induced liquid layers.
- Orientational order parameter [47], as follows:where i, j, and k are three atoms in a bin between and ; atom j and k are the nearest neighbors of atom i; (i,j,k) denotes the xy-plane-projected angle between ij and ik; is the average number of added in one time step; n is an integer; and we used n = 6 in this work as has been shown to clearly distinguish a close-packed layer from a disordered region [48].
- Two-dimensional (2D) structure factor [49,50], as follows:is the scattering vector, which is a vector in the reciprocal space; N is the number of atoms in a bin from to ; and denotes the position of atom i. In this work, was calculated within the substrate-induced liquid layers to show the in-plane structural features.
- Diffusion coefficient D(z), as follows:
3. Results
3.1. Structural Features and Dynamical Properties of the Liquid Al Layers Induced by Sapphire and TiB2
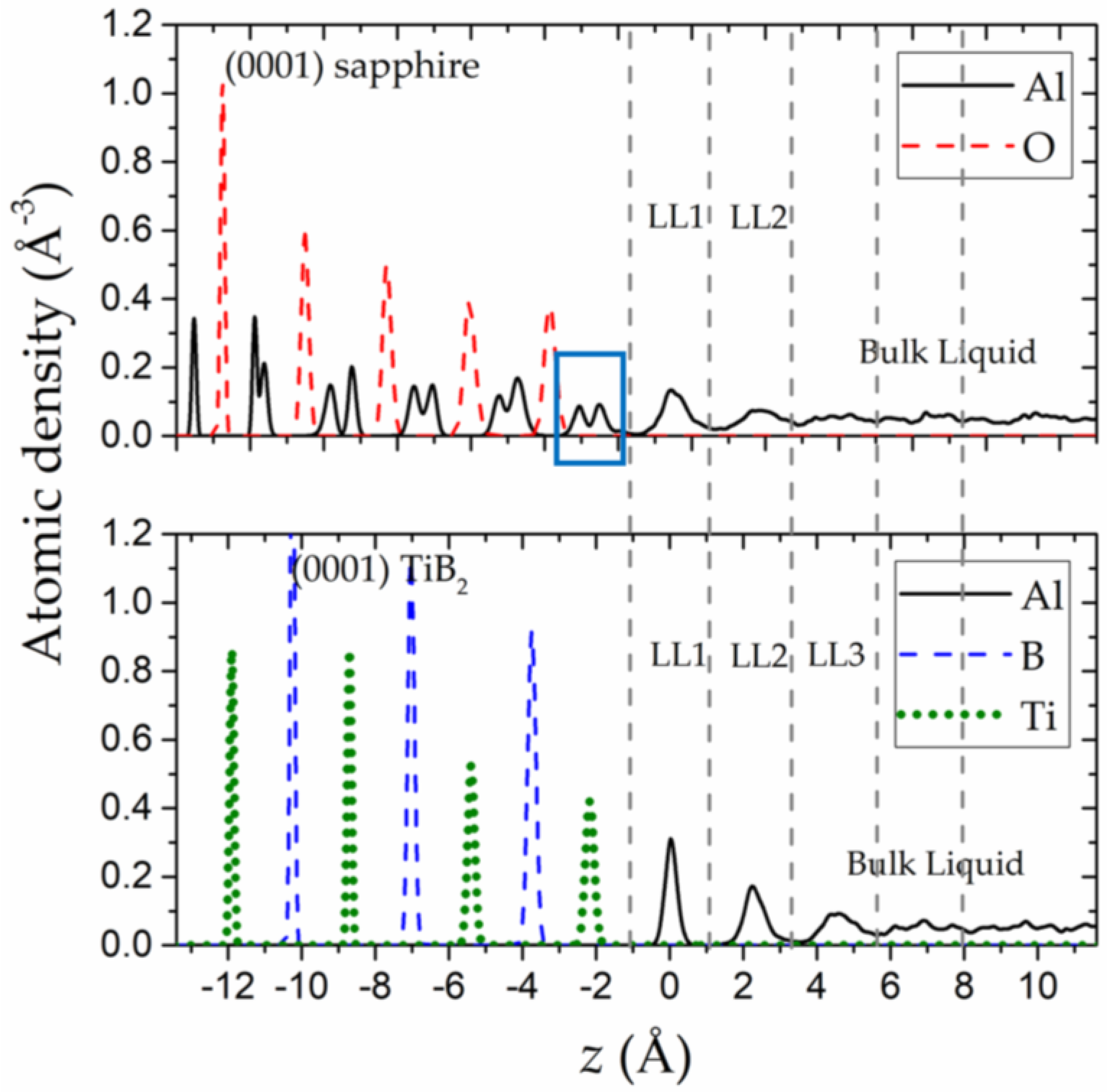
3.1.1. Out-Of-Plane Ordering
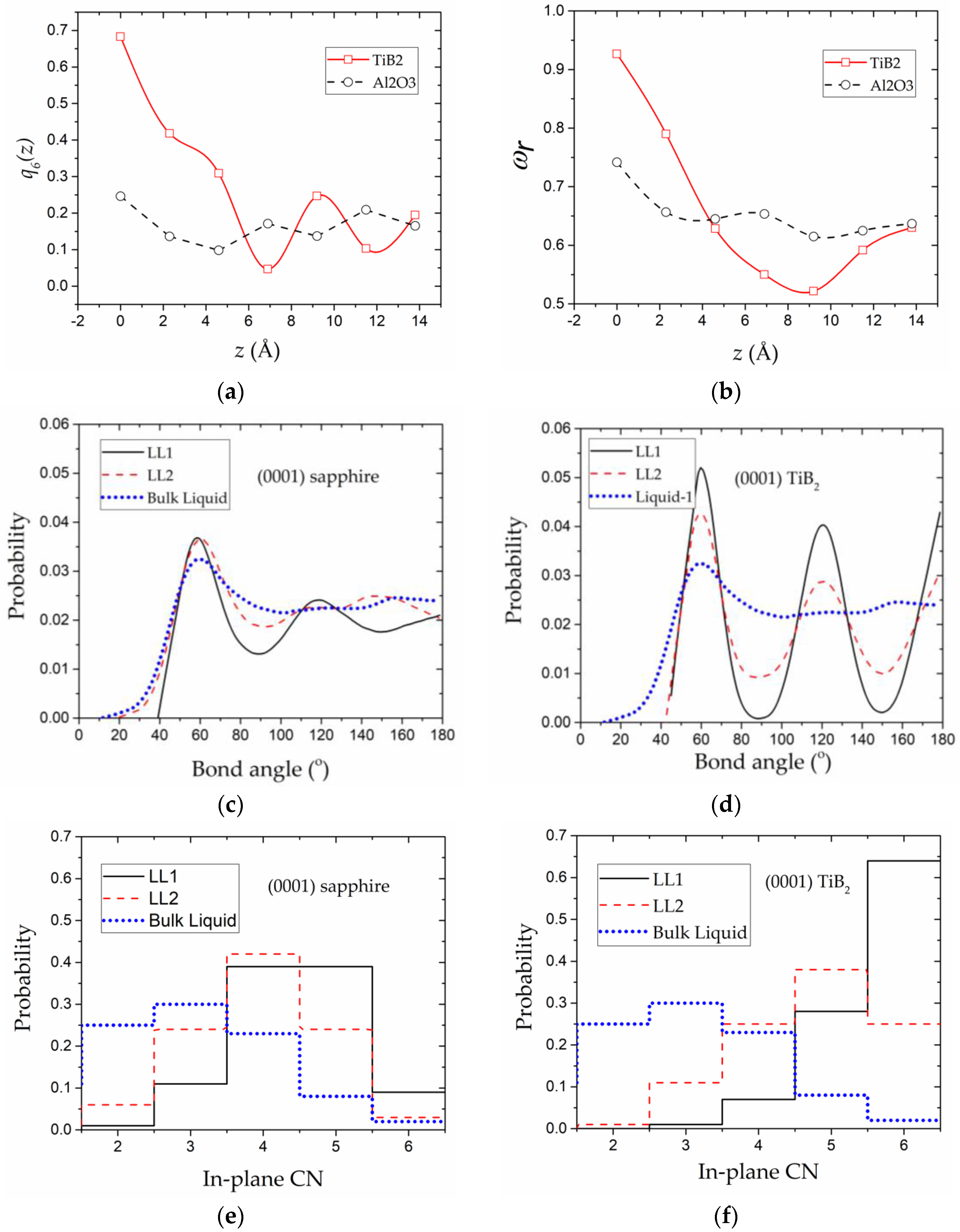
3.1.2. In-Plane Atomic Arrangement
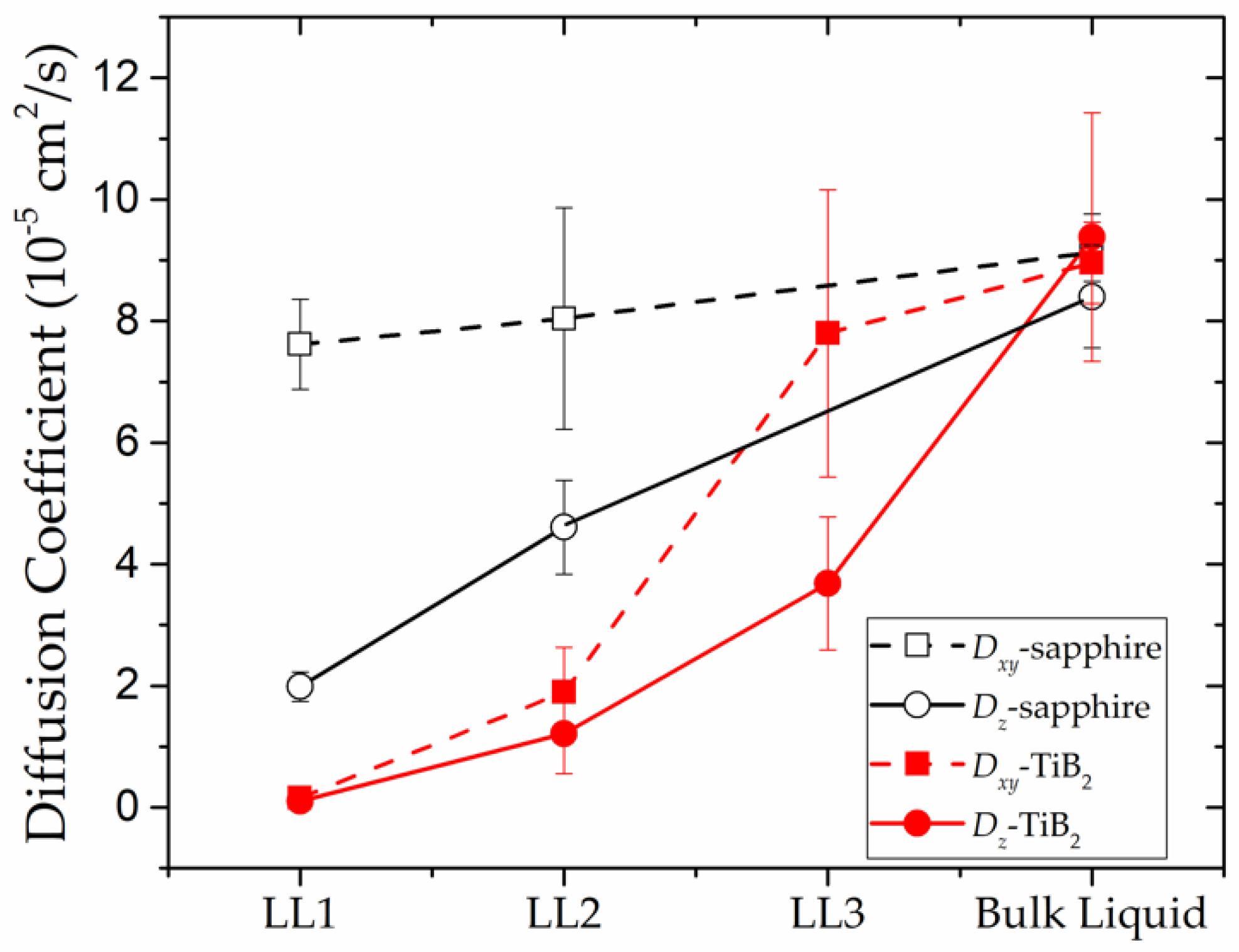
3.1.3. Dynamical Properties of the Substrate-Induced Liquid Al Layers
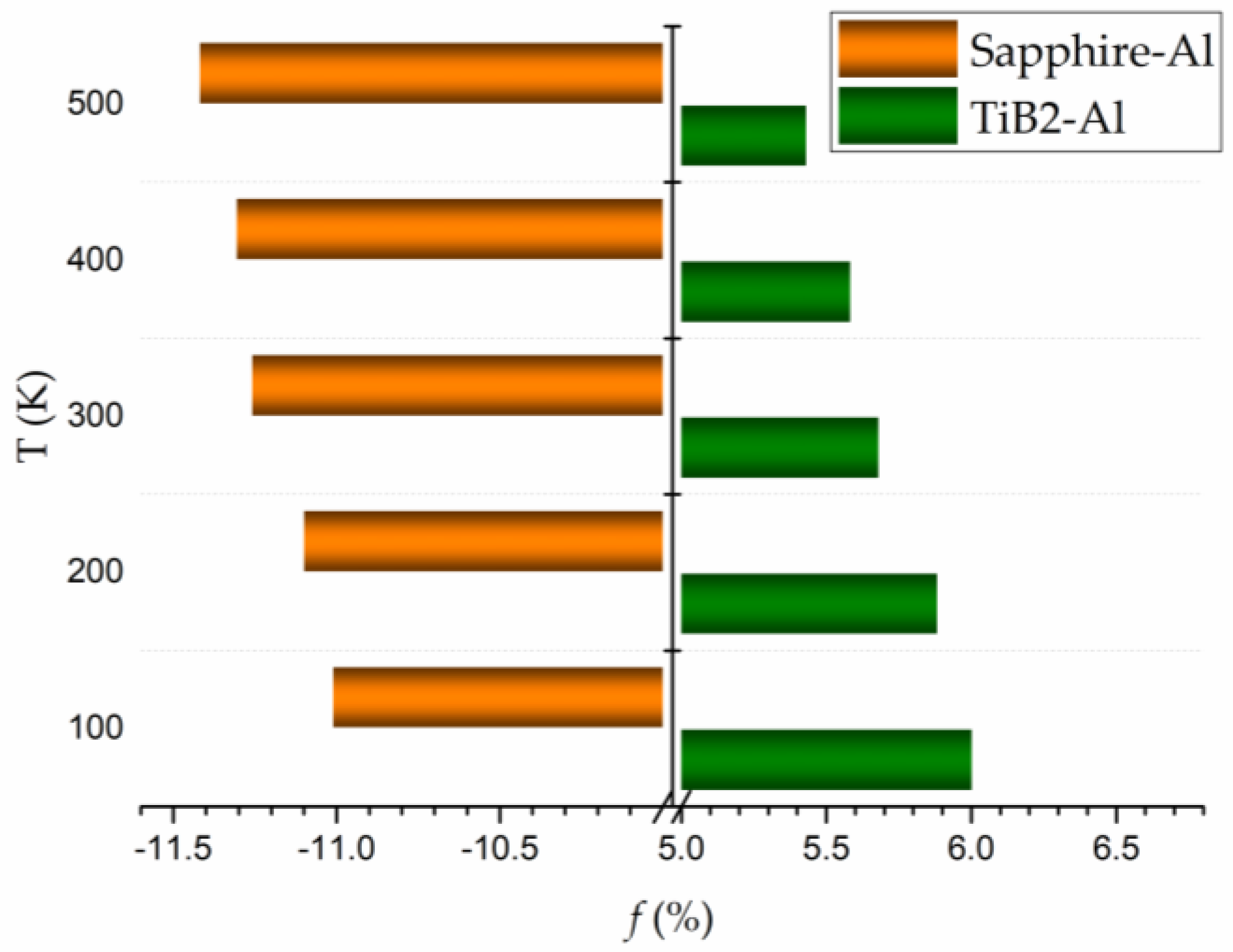
3.2. Factors Influencing Substrate-Induced Liquid Al Layering
4. Discussion
5. Conclusions
- For the (0001) TiB2–liquid Al interface, around three liquid Al layers are induced by the substrate. The first liquid layer (nearest to the substrate) is close-packed with almost perfect six-fold symmetry and translational symmetry. The second liquid layer also shows the close-packed order, while the degree of in-plane ordering is much weaker compared with the first liquid layer. The in-plane structure of the third liquid layer is almost disordered, which is similar to the bulk liquid Al.
- For the (0001) sapphire–liquid Al interface, approximately two liquid Al layers are induced near the substrate. Both of the two liquid layers show low 6-fold symmetry and translational symmetry, indicating a poor in-plane ordering. The first liquid layer induced by the (0001) sapphire exhibits a similar degree of ordering to the third liquid layer near the (0001) TiB2, which demonstrates that, compared with the (0001) TiB2, the (0001) sapphire shows a much poorer capability in triggering liquid Al layers.
- Two factors, the adsorption strength (or reduction of interfacial energy) and interfacial lattice mismatch, determine the structural features of the substrate-induced liquid layers. The adsorption strength is the intrinsic driving force for the liquid layering, while the interfacial lattice mismatch between the substrate and the liquid layers is the barrier. The interfacial lattice mismatch may cause compressive or tensile stress in the liquid layers, and the compressive stress makes the liquid more difficult to become ordered than the tensile stress, by impeding the increase of the liquid atomic density.
- The substrate-induced liquid layers play a critical role in the heterogeneous nucleation, and the differences in the structural features and the dynamical properties of the liquid Al layers, induced by the above two substrates, change their heterogeneous nucleation behaviors.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kelton, K.; Greer, A.L. Nucleation in Condensed Matter: Applications in Materials and Biology; Elsevier: Amsterdam, The Netherlands, 2010; Volume 15. [Google Scholar]
- Zhu, X.; Jiang, W.; Li, M.; Qiao, H.; Wu, Y.; Qin, J.; Liu, X. The effect of Mg adding order on the liquid structure and solidified microstructure of the Al-Si-Mg-P alloy: An experiment and ab initio study. Metals 2014, 5, 40–51. [Google Scholar] [CrossRef]
- Kurtuldu, G.; Jarry, P.; Rappaz, M. Influence of Cr on the nucleation of primary Al and formation of twinned dendrites in Al–Zn–Cr alloys: Can icosahedral solid clusters play a role? Acta Mater. 2013, 61, 7098–7108. [Google Scholar] [CrossRef] [Green Version]
- Mei, H.; Liu, Q.W.; Liu, L.S.; Lai, X.; She, W.C.; Zhai, P.C. Molecular dynamics simulations of the microstructure of the aluminum/alumina interfacial layer. Appl. Surf. Sci. 2015, 324, 538–546. [Google Scholar] [CrossRef]
- Kaplan, W.D.; Chatain, D.; Wynblatt, P.; Carter, W.C. A review of wetting versus adsorption, complexions, and related phenomena: The rosetta stone of wetting. J. Mater. Sci. 2013, 48, 5681–5717. [Google Scholar] [CrossRef]
- Samyn, P. Wetting and hydrophobic modification of cellulose surfaces for paper applications. J. Mater. Sci. 2013, 48, 6455–6498. [Google Scholar] [CrossRef]
- Schulli, T.U.; Daudin, R.; Renaud, G.; Vaysset, A.; Geaymond, O.; Pasturel, A. Substrate-enhanced supercooling in AuSi eutectic droplets. Nature 2010, 464, 1174–1177. [Google Scholar] [CrossRef] [PubMed]
- Comtet, J.; Nigues, A.; Kaiser, V.; Coasne, B.; Bocquet, L.; Siria, A. Nanoscale capillary freezing of ionic liquids confined between metallic interfaces and the role of electronic screening. Nat. Mater. 2017, 16, 634–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandman, M.; Kauffmann, Y.; Koch, C.T.; Kaplan, W.D. Direct Quantification of Ordering at a Solid-Liquid Interface Using Aberration Corrected Transmission Electron Microscopy. Phys. Rev. Lett. 2013, 110, 086106. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Kauffmann, Y.; Scheu, C.; Kaplan, W.D.; Ruhle, M. Ordered liquid aluminum at the interface with sapphire. Science 2005, 310, 661–663. [Google Scholar] [CrossRef] [PubMed]
- Haghayeghi, R.; Qian, M. Initial crystallisation or nucleation in a liquid aluminium alloy containing spinel seeds. Mater. Lett. 2017, 196, 358–360. [Google Scholar] [CrossRef]
- Wang, J.; Horsfield, A.; Schwingenschlögl, U.; Lee, P.D. Heterogeneous nucleation of solid Al from the melt by TiB 2 and Al 3 Ti: An ab initio molecular dynamics study. Phys. Rev. B 2010, 82, 184203. [Google Scholar] [CrossRef]
- Zhang, H.L.; Han, Y.F.; Zhou, W.; Dai, Y.B.; Wang, J.; Sun, B.D. Atomic study on the ordered structure in Al melts induced by liquid/substrate interface with Ti solute. Appl. Phys. Lett. 2015, 106, 041606. [Google Scholar]
- Kang, J.; Zhu, J.; Curtis, C.; Blake, D.; Glatzmaier, G.; Kim, Y.-H.; Wei, S.-H. Atomically Abrupt Liquid-Oxide Interface Stabilized by Self-Regulated Interfacial Defects: The Case of Al/Al2O3 Interfaces. Phys. Rev. Lett. 2012, 108, 226105. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, L.; Zhang, D.; Xia, M.; Wang, Y.; Li, J.G. The Role of Lattice Misfit on Heterogeneous Nucleation of Pure Aluminum. Metall. Mater. Trans. A 2016, 47, 5012–5022. [Google Scholar] [CrossRef]
- Men, H.; Fan, Z. Prenucleation Induced by Crystalline Substrates. Metall. Mater. Trans. A 2018, 49, 2766–2777. [Google Scholar] [CrossRef]
- Greer, A.L. Liquid metals: Supercool order. Nat. Mater. 2006, 5, 13–14. [Google Scholar] [CrossRef]
- Easton, M.A.; Qian, M.; Prasad, A.; StJohn, D.H. Recent advances in grain refinement of light metals and alloys. Curr. Opin. Solid State Mater. Sci. 2016, 20, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Cantor, B. Heterogeneous nucleation and adsorption. Philos. Trans. R. Soc. Lond. A Math. Phys. Eng. Sci. 2003, 361, 409–417. [Google Scholar] [CrossRef]
- Kim, W.T.; Cantor, B. An adsorption model of the heterogeneous nucleation of solidification. Acta Metall. Mater. 1994, 42, 3115–3127. [Google Scholar] [CrossRef]
- Greer, A.; Bunn, A.; Tronche, A.; Evans, P.; Bristow, D. Modelling of inoculation of metallic melts: Application to grain refinement of aluminium by Al–Ti–B. Acta Mater. 2000, 48, 2823–2835. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, Y.; Zhang, Y.; Qin, T.; Zhou, X.; Thompson, G.; Pennycook, T.; Hashimoto, T. Grain refining mechanism in the Al/Al–Ti–B system. Acta Mater. 2015, 84, 292–304. [Google Scholar] [CrossRef]
- Murty, B.S.; Kori, S.A.; Chakraborty, M. Grain refinement of aluminium and its alloys by heterogeneous nucleation and alloying. Int. Mater. Rev. 2002, 47, 3–29. [Google Scholar] [CrossRef]
- Quested, T.; Greer, A. The effect of the size distribution of inoculant particles on as-cast grain size in aluminium alloys. Acta Mater. 2004, 52, 3859–3868. [Google Scholar] [CrossRef]
- Liotti, E.; Arteta, C.; Zisserman, A.; Lui, A.; Lempitsky, V.; Grant, P.S. Crystal nucleation in metallic alloys using x-ray radiography and machine learning. Sci. Adv. 2018, 4, eaar4004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Lu, W.Q.; Hu, Q.D.; Xia, M.X.; Wang, Y.; Li, J.G. Interfacial tuning for the nucleation of liquid AlCu alloy. Acta Mater. 2017, 139, 75–85. [Google Scholar] [CrossRef]
- Brown, A.J.; Dong, H.B.; Howes, P.B.; Nicklin, C.L. In situ observation of the orientation relationship at the interface plane between substrate and nucleus using X-ray scattering techniques. Scr. Mater. 2014, 77, 60–63. [Google Scholar] [CrossRef]
- Han, Y.; Dai, Y.; Shu, D.; Wang, J.; Sun, B. First-principles calculations on the stability of Al/TiB2 interface. Appl. Phys. Lett. 2006, 89, 144107. [Google Scholar] [CrossRef]
- Zhang, H.L.; Han, Y.F.; Dai, Y.B.; Wang, J.; Sun, B.D. An ab initio molecular dynamics study: Liquid-Al/solid-TiB2 interfacial structure during heterogeneous nucleation. J. Phys. D Appl. Phys. 2012, 45, 455307. [Google Scholar] [CrossRef]
- Liang, H.T.; Laird, B.B.; Asta, M.; Yang, Y. In-plane characterization of structural and thermodynamic properties for steps at faceted chemically heterogeneous solid/liquid interfaces. Acta Mater. 2018, 143, 329–337. [Google Scholar] [CrossRef]
- Yang, Y.; Olmsted, D.L.; Asta, M.; Laird, B.B. Atomistic characterization of the chemically heterogeneous Al–Pb solid–liquid interface. Acta Mater. 2012, 60, 4960–4971. [Google Scholar] [CrossRef]
- Guo, C.; Wang, J.; Li, J.; Wang, Z.; Tang, S. Kinetic Pathways and Mechanisms of Two-Step Nucleation in Crystallization. J. Phys. Chem. Lett. 2016, 7, 5008–5014. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, C.; Zhang, Q.; Tang, S.; Li, J.; Wang, Z. Recent Progresses in Modeling of Nucleation During Solidification on the Atomic Scale. Acta Metall. Sin. 2017, 54, 204–216. [Google Scholar]
- Qiao, J.; Zhang, L.; Hui, X.; Lin, J. Kinetic and thermodynamic properties of liquid zinc: An ab initio molecular dynamics study. Comput. Mater. Sci. 2018, 141, 180–184. [Google Scholar] [CrossRef]
- Debela, T.T.; Wang, X.D.; Cao, Q.P.; Lu, Y.H.; Zhang, D.X.; Fecht, H.J.; Tanaka, H.; Jiang, J.Z. Nucleation driven by orientational order in supercooled niobium as seen via ab initio molecular dynamics. Phys. Rev. B 2014, 89, 104205. [Google Scholar] [CrossRef]
- Wang, W.Y.; Han, J.J.; Fang, H.Z.; Wang, J.; Liang, Y.F.; Shang, S.L.; Wang, Y.; Liu, X.J.; Kecskes, L.J.; Mathaudhu, S.N.; et al. Anomalous structural dynamics in liquid Al80Cu20: An ab initio molecular dynamics study. Acta Mater. 2015, 97, 75–85. [Google Scholar] [CrossRef]
- Weber, H.; Schumacher, M.; Jovari, P.; Tsuchiya, Y.; Skrotzki, W.; Mazzarello, R.; Kaban, I. Experimental and ab initio molecular dynamics study of the structure and physical properties of liquid GeTe. Phys. Rev. B 2017, 96, 054204. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular-Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Mishin, Y.; Farkas, D.; Mehl, M.J.; Papaconstantopoulos, D.A. Interatomic potentials for monoatomic metals from experimental data and ab initio calculations. Phys. Rev. B 1999, 59, 3393–3407. [Google Scholar] [CrossRef]
- Alfe, D. First-principles simulations of direct coexistence of solid and liquid aluminum. Phys. Rev. B 2003, 68, 064423. [Google Scholar] [CrossRef]
- Bouchet, J.; Bottin, F.; Jomard, G.; Zerah, G. Melting curve of aluminum up to 300 GPa obtained through ab initio molecular dynamics simulations. Phys. Rev. B 2009, 80, 094102. [Google Scholar] [CrossRef]
- Davidchack, R.L.; Laird, B.B. Simulation of the hard-sphere crystal–melt interface. J. Chem. Phys. 1998, 108, 9452–9462. [Google Scholar] [CrossRef] [Green Version]
- Palafox-Hernandez, J.P.; Laird, B.B. Orientation dependence of heterogeneous nucleation at the Cu–Pb solid-liquid interface. J. Chem. Phys. 2016, 145, 211914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buta, D.; Asta, M.; Hoyt, J.J. Atomistic simulation study of the structure and dynamics of a faceted crystal-melt interface. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2008, 78, 031605. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K. On the Concept of Static Structure Factor. arXiv, 2016; arXiv:1606.03610. [Google Scholar]
- Ma, S.; Brown, A.J.; Yan, R.; Davidchack, R.L.; Howes, P.B.; Nicklin, C.; Zhai, Q.; Jing, T.; Dong, H. Atomistics of adsorption-induced pre-nucleation layering of liquid metals at the interface with poor nucleant substrates. Unpublished work. 2018. [Google Scholar]
- Russo, J.; Tanaka, H. Crystal nucleation as the ordering of multiple order parameters. J. Chem. Phys. 2016, 145, 211801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.H.; Chang, B.; Oyola-Reynoso, S.; Wang, Z.J.; Thuo, M. Quantifying Gauche Defects and Phase Evolution in Self-Assembled Monolayers through Sessile Drops. ACS Omega 2017, 2, 2072–2084. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Oyola-Reynoso, S.; Thuo, M.M. Properties of Self-Assembled Monolayers Revealed via Inverse Tensiometry. Langmuir 2017, 33, 13451–13467. [Google Scholar] [CrossRef] [PubMed]
- Hinnemann, B.; Carter, E.A. Adsorption of Al, O, Hf, Y, Pt, and S atoms on α-Al2O3 (0001). J. Phys. Chem. C 2007, 111, 7105–7126. [Google Scholar] [CrossRef]
- Siegel, D.J.; Hector Jr, L.G.; Adams, J.B. Adhesion, atomic structure, and bonding at the Al (111)/α−Al2O3 (0001) interface: A first principles study. Phys. Rev. B 2002, 65, 085415. [Google Scholar] [CrossRef]
- Xiao, L.; Schneider, W.F. Surface termination effects on metal atom adsorption on α-alumina. Surf. Sci. 2008, 602, 3445–3453. [Google Scholar] [CrossRef]
- Duan, Y.; Li, J.; Li, T.; Zhang, X.; Wang, Z.; Li, H. Density dependent structural phase transition for confined copper: Origin of the layering. Phys. Chem. Chem. Phys. 2018, 20, 9337–9342. [Google Scholar] [CrossRef] [PubMed]
- Alert, R.; Casademunt, J.; Tierno, P. Landscape-inversion phase transition in dipolar colloids: Tuning the structure and dynamics of 2D crystals. Phys. Rev. Lett. 2014, 113, 198301. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.Y. An Epitaxial Model for Heterogeneous Nucleation on Potent Substrates. Metall. Mater. Trans. A 2013, 44a, 1409–1418. [Google Scholar] [CrossRef]



| Interfacial Systems | Out-of-Plane Structural Features | In-Plane Structural Features | ||||
|---|---|---|---|---|---|---|
| 1 (Å) | (Å) | (Å−3) | In-Plane CN | |||
| Sapphire–liquid Al | LL1 | 2.19 | 0.47 | 0.14 | 4.45 | 0.25 |
| LL2 | 2.30 | 0.62 | 0.08 | 3.94 | 0.14 | |
| TiB2–liquid Al | LL1 | 2.22 | 0.15 | 0.34 | 5.56 | 0.68 |
| LL2 | 2.33 | 0.31 | 0.18 | 4.74 | 0.42 | |
| LL3 | 2.40 | 0.49 | 0.09 | 3.77 | 0.31 | |
| Substrates | W (eV/atom) | Vibrational Frequencies (cm−1) Involving Significant Adsorbate Motion | |
|---|---|---|---|
| In-Plane | Out-Of-Plane | ||
| Sapphire | −2.05 | 171.69; 204.20 | 219.28 |
| TiB2 | −3.36 | 113.98; 115.41 | 185.95 |
| Temperature (K) | Sapphire (Å) 1 | TiB2 (Å) 2 | Al (Å) |
|---|---|---|---|
| 100 | 4.813 | 3.041 | 4.057 |
| 200 | 4.817 | 3.043 | 4.064 |
| 300 | 4.820 | 3.045 | 4.075 |
| 400 | 4.826 | 3.047 | 4.082 |
| 500 | 4.831 | 3.049 | 4.091 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, S.; Yan, R.; Jing, T.; Dong, H. Substrate-Induced Liquid Layering: A New Insight into the Heterogeneous Nucleation of Liquid Metals. Metals 2018, 8, 521. https://doi.org/10.3390/met8070521
Ma S, Yan R, Jing T, Dong H. Substrate-Induced Liquid Layering: A New Insight into the Heterogeneous Nucleation of Liquid Metals. Metals. 2018; 8(7):521. https://doi.org/10.3390/met8070521
Chicago/Turabian StyleMa, Sida, Rui Yan, Tao Jing, and Hongbiao Dong. 2018. "Substrate-Induced Liquid Layering: A New Insight into the Heterogeneous Nucleation of Liquid Metals" Metals 8, no. 7: 521. https://doi.org/10.3390/met8070521




