Degradation Mechanism of Nickel-Cobalt-Aluminum (NCA) Cathode Material from Spent Lithium-Ion Batteries in Microwave-Assisted Pyrolysis
Abstract
:1. Introduction
1.1. Theory of Pyrolysis
1.2. Thermal Degradation of Organic Materials in Cathode of LIBs
1.3. Theory of Microwave Assisted-Pyrolysis
2. Materials and Methods
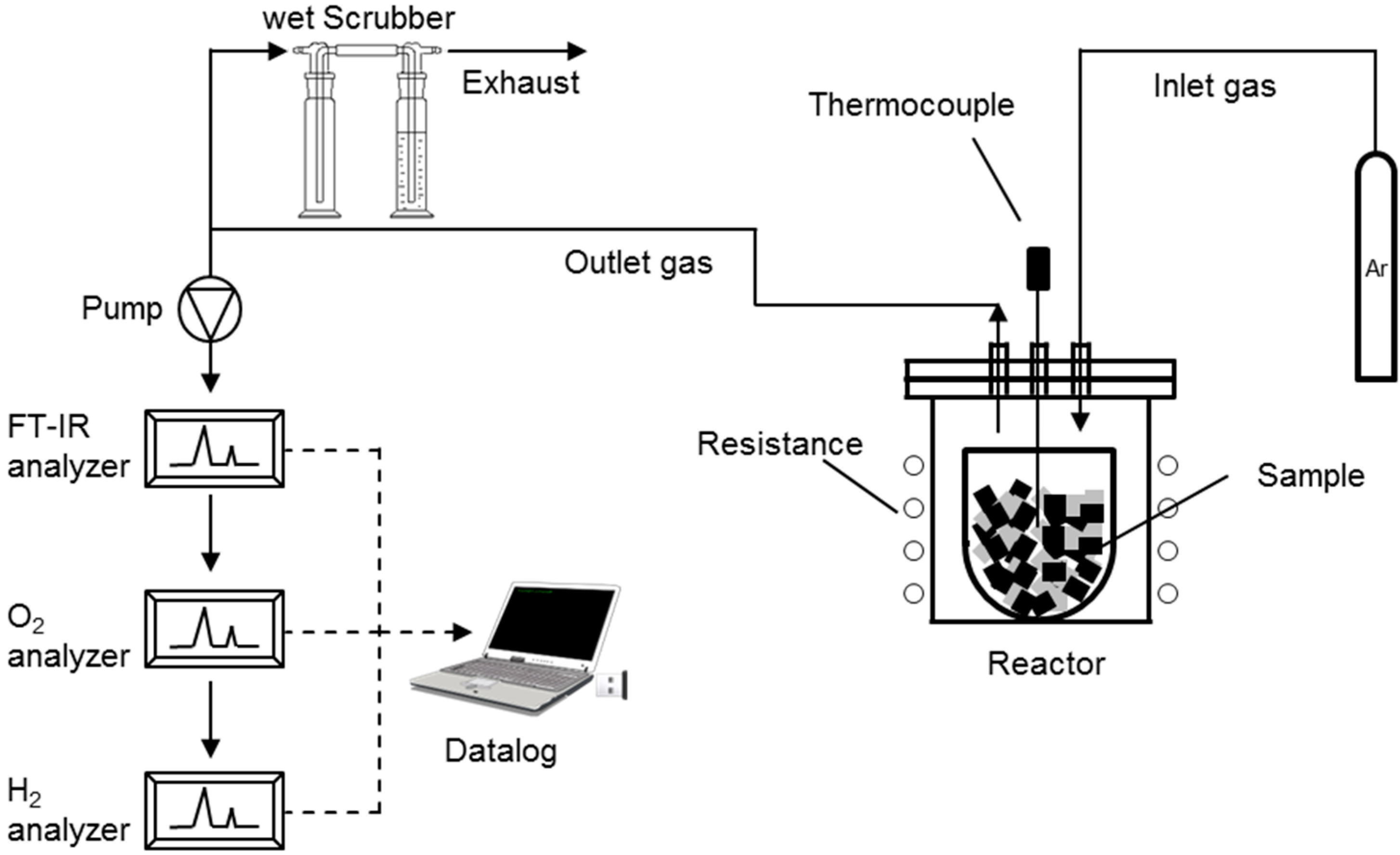
2.1. Experimental Setup for Conventional Pyrolysis
2.2. Experimental Setup for Microwave Pyrolysis
3. Results and Discussion
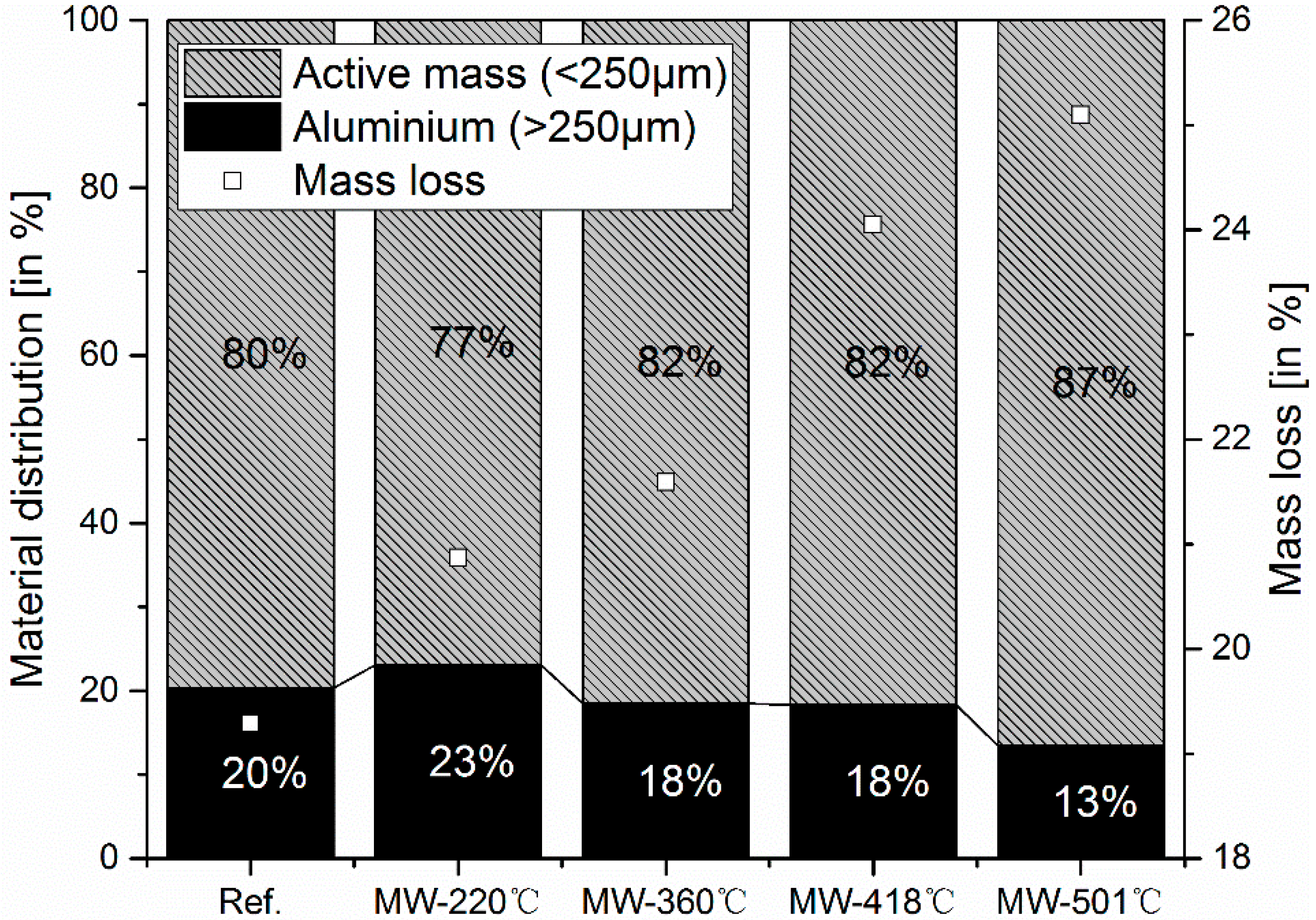
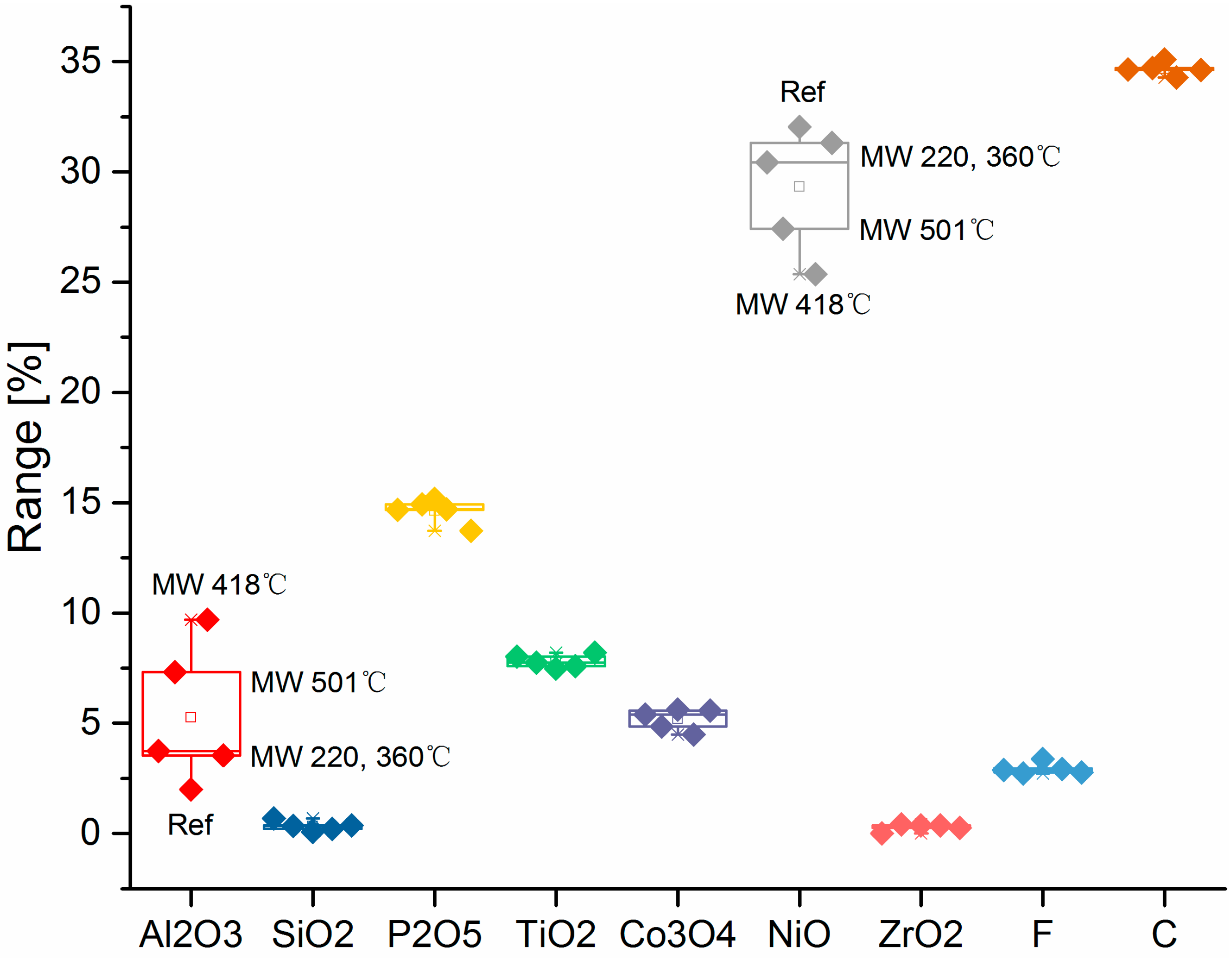
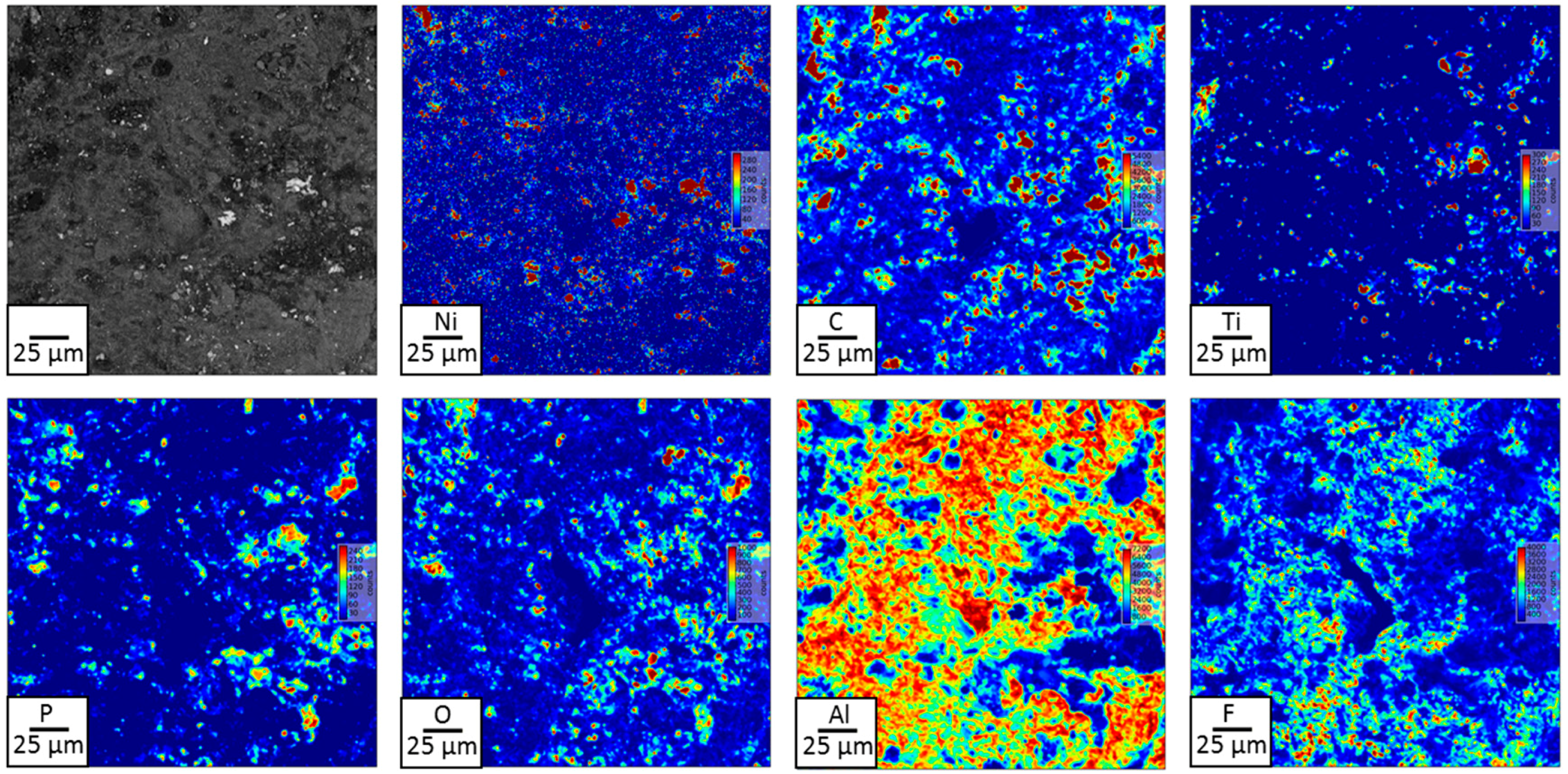
3.1. Solid Products Characterizations
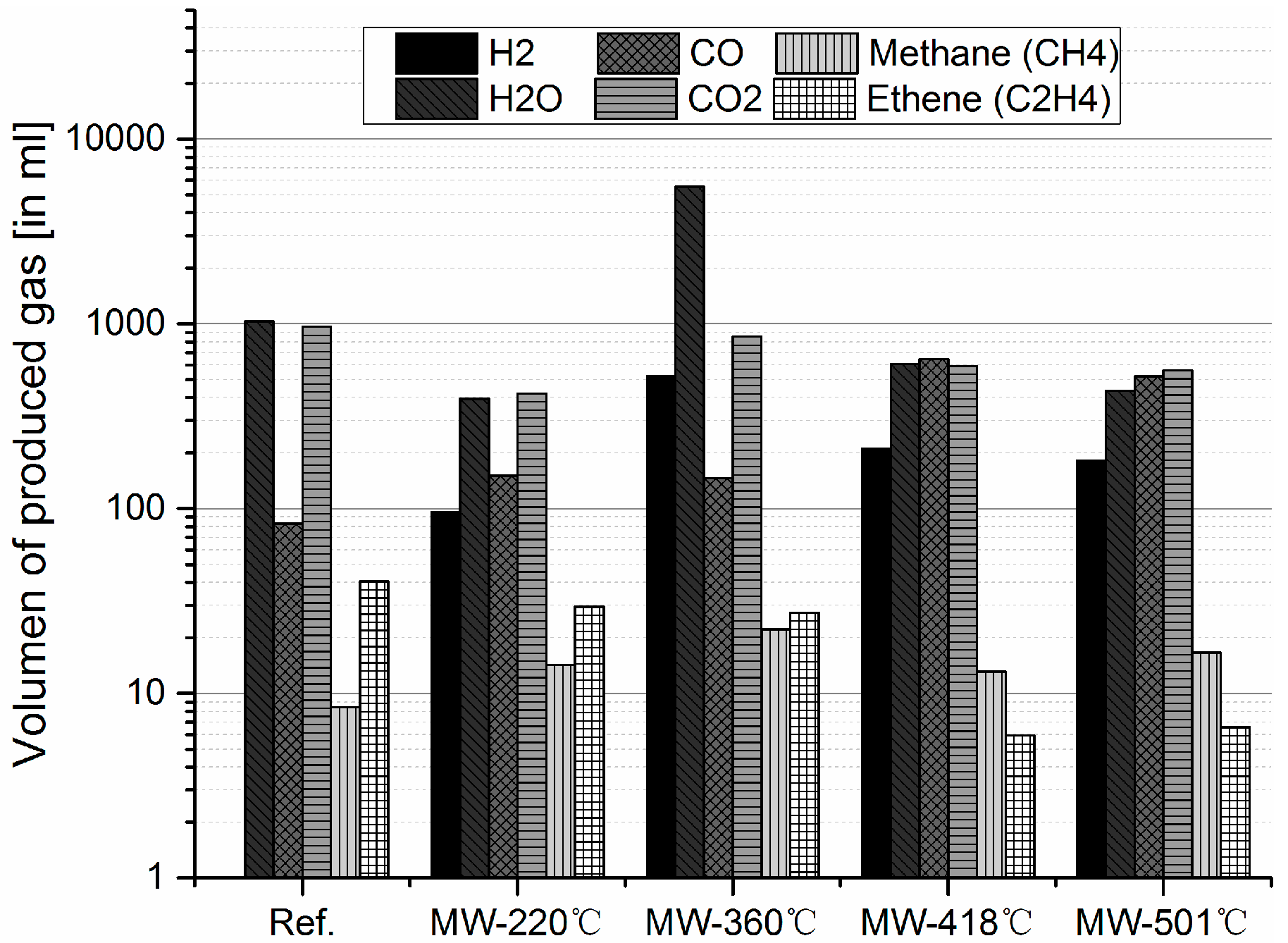
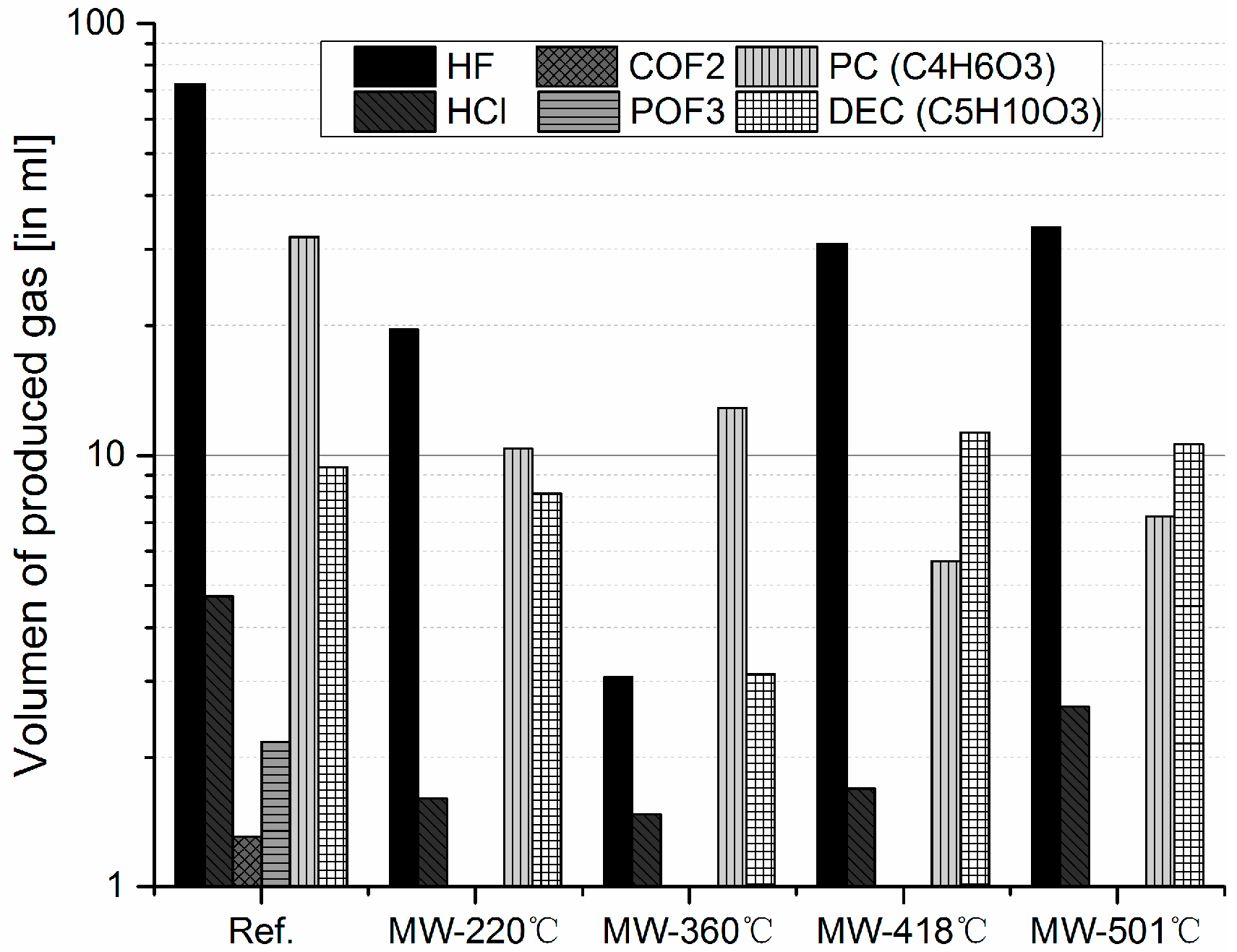
3.2. Gas products Characterization
3.2.1. Degradation during Conventional Pyrolysis of a NCA Cathode Material
3.2.2. Degradation during Microwave Pyrolysis of a NCA Cathode Material
3.2.3. Comparative Study between Conventional and Microwave Pyrolysis on the Formation of Heavy and Light Molecules, and Toxic Compounds
4. Conclusions
- For microwave assisted pyrolysis, the amount of mass loss increases with the temperature. However, excessive exposure of microwave to the cathode material leads to uncontrollable rapid heating of aluminum, which leads to partial melting and eventually the formation of sparks. Like this, the fragmentation of the metal foil takes place and consequently contamination of the black mass cannot be avoided.
- When compared with conventional pyrolysis, microwave-assisted decrease the amount of heavy molecules gases produced. In addition, prolonged exposure to microwave leads to higher temperatures thus even lower amount of heavy molecules are registered.
- Microwave assisted pyrolysis applied to cathodes from LIBs permits catalytic steam and dry reforming reactions, which is evidenced by strong formation of H2 and CO.
- Short chain molecules are more likely to be formed in microwave assisted pyrolysis when compared to conventional pyrolysis. This, due to the rapid heating and breaking of long chain molecules into short molecules. In addition, the process experienced limited secondary reactions in the gas phase due to fast cooling of the produced gas after leaving the sample.
- Microwave pyrolysis at 360 °C is suggested in this study after taking mass loss, active mass yield ratio, heavy and light molecules, and toxic compounds into consideration.
Author Contributions
Funding
Conflicts of Interest
References
- McDowall, J. Understanding lithium-ion technology. In Proceedings of the Battcon, Marco Island, FL, USA, 5–7 May 2008. [Google Scholar]
- Wu, Y. Lithium-Ion Batteries: Fundamentals and Applications; CRC Press: Vancouver, BC, Canada, 2015. [Google Scholar]
- Pillot, C. Lithium Ion Battery Raw Material Supply & Demand 2016–2025. In Proceedings of the Advanced Automotive Battery Conference, Mainz, Germany, 30 January–2 February 2017. [Google Scholar]
- O’Driscoll, M. Industrial mineral recycling in Li-ion batteries: Impact on raw material supply chain? In Proceedings of the Advanced Automotive Battery Conference, Mainz, Germany, 30 January–2 February 2017. [Google Scholar]
- Baylis, R. LIB raw material supply chain bottlenecks: Looking beyond supply/demand/price. In Proceedings of the Advanced Automotive Battery Conference, Mainz, Germany, 30 January–2 February 2017. [Google Scholar]
- Friedrich, B.; Träger, T.; Peters, L. Lithium Ion Battery Recycling and Recent IME Activities. In Proceedings of the Advanced Automotive Battery Conference, Mainz, Germany, 30 January–2 February 2017. [Google Scholar]
- Ordoñez, J.; Gago, E.J.; Girard, A. Processes and technologies for the recycling and recovery of spent lithium-ion batteries. Renew. Sustain. Energy Rev. 2016, 60, 195–205. [Google Scholar] [CrossRef]
- European Li-Ion Battery Advanced Manufacturing for Electric Vehicles (ELIBAMA). Li-ion BATTERIES RECYCLING. The batteries end of life…. 2014. Available online: http://www.webcitation.org/717f6wRdJ (accessed on 23 July 2018).
- Zeng, X.; Li, J.; Singh, N. Recycling of Spent Lithium-Ion Battery: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1129–1165. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Chiueh, P.-T.; Lo, S.-L. A review on microwave pyrolysis of lignocellulosic biomass. Sustain. Environ. Res. 2016, 26, 103–109. [Google Scholar] [CrossRef]
- Sun, J.; Wang, W.; Liu, Z.; Ma, Q.; Zhao, C.; Ma, C. Kinetic Study of the Pyrolysis of Waste Printed Circuit Boards Subject to Conventional and Microwave Heating. Energies 2012, 5, 3295–3306. [Google Scholar] [CrossRef] [Green Version]
- Walkiewicz, J.W.; Kazonich, G.; McGill, S.L. Microwave heating characteristics of selected minerals and compounds. Miner. Metall. Process. 1988, 5, 39–42. [Google Scholar]
- Gupta, M.; Eugene, W.W.L. Microwaves and Metals; John Wiley & Sons (Asia) Pte Ltd.: Singapore, 2007. [Google Scholar]
- Preto, F. Pyrolysis, Char and Energy. Presentation at the Canadian Biochar Initiative, inaugural Meeting, CAnmetEnergy: December 12, 2008, Ste Anne de Bellevue. Available online: http://www.webcitation.org/717hJZ4pw (accessed on 23 July 2018).
- Luyima, A. Recycling of Electronic Waste: Pinted Wiring Board. Ph.D. Thesis, Missouri University of Science and Technology, Rolla, MO, USA, 2013. [Google Scholar]
- Basu, P. Biomass Gasification and Pyrolysis. Practical Design and Theory; Elsevier/AP: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Marto, C. Pyrolysis of Peat: An Experimental Investigation. Master’s Thesis, University of Padua, Padua, Italy, 2014. [Google Scholar]
- Epple, B.; Leithner, R.; Linzer, W.; Walter, H. Simulation von Kraftwerken und Wärmetechnischen Anlagen; Springer: Vienna, Austria, 2009. [Google Scholar]
- Luda, M.P. Waste Electrical and Electronic Equipment (WEEE) Handbook, Handbook: Pyrolysis of WEEE Plastics; Woodhead Publishing: Cambridge, UK, 2012. [Google Scholar]
- Diaz, F.; Flerus, B.; Nagraj, S.; Bokelmann, K.; Stauber, R.; Friedrich, B. Comparative Analysis about Degradation Mechanisms of Printed Circuit Boards (PCBs) in Slow and Fast Pyrolysis: The Influence of Heating Speed. J. Sustain. Metall. 2018, 408, 183. [Google Scholar] [CrossRef]
- Sun, L.; Qiu, K. Vacuum pyrolysis and hydrometallurgical process for the recovery of valuable metals from spent lithium-ion batteries. J. Hazard. Mater. 2011, 194, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Träger, T.; Friedrich, B.; Weyhe, R. Recovery Concept of Value Metals from Automotive Lithium-Ion Batteries. Chem. Ing. Tech. 2015, 87, 1550–1557. [Google Scholar] [CrossRef]
- Guo, H.-J.; Li, X.-H.; Zhang, X.-M.; Wang, Z.-X.; Peng, W.-J.; Zhang, B. Optimizing pyrolysis of resin carbon for anode of lithium ion batteries. J. Cent. South Univ. Technol. 2006, 13, 58–62. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Kawamura, T.; Kimura, A.; Egashira, M.; Okada, S.; Yamaki, J.-I. Thermal stability of alkyl carbonate mixed-solvent electrolytes for lithium ion cells. J. Power Sources 2002, 104, 260–264. [Google Scholar] [CrossRef]
- Spotnitz, R.; Franklin, J. Abuse behavior of high-power, lithium-ion cells. J. Power Sources 2003, 113, 81–100. [Google Scholar] [CrossRef]
- Tobishima, S.-I.; Yamaki, J.-I. A consideration of lithium cell safety. J. Power Sources 1999, 81–82, 882–886. [Google Scholar] [CrossRef]
- Wang, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. Thermal runaway caused fire and explosion of lithium ion battery. J. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, Z.; Lu, X.; Tan, C. Research of explosion mechanism of lithium-ion battery. Prog. Chem. 2006, 18, 823–831. [Google Scholar]
- Sutton, W. Microwave Processing of Ceramic Materials. Am. Ceram. Soc. Bull. 1989, 68, 376–386. [Google Scholar]
- Sun, J.; Wang, W.; Yue, Q. Review on Microwave-Matter Interaction Fundamentals and Efficient Microwave-Associated Heating Strategies. Materials 2016, 9, 231. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, W.; Liu, Z.; Ma, C. Recycling of Waste Printed Circuit Boards by Microwave-Induced Pyrolysis and Featured Mechanical Processing. Ind. Eng. Chem. Res. 2011, 50, 11763–11769. [Google Scholar] [CrossRef]
- Blomgren, G.E. The Development and Future of Lithium Ion Batteries. J. Electrochem. Soc. 2016, 164, A5019–A5025. [Google Scholar] [CrossRef] [Green Version]
- Ehsan, R.; Kerstin, S.; Moritz, V. Kompendium: Li-Ionen-Batterien. Available online: http://www.webcitation.org/717pBmpxj (accessed on 23 July 2018).
- Jackson, W.M.; Conley, R.T. High temperature oxidative degradation of phenol–formaldehyde polycondensates. J. Appl. Polym. Sci. 1964, 8, 2163–2193. [Google Scholar] [CrossRef]
- Gasification. Chemistry, Processes, and Applications; Baker, M.D., Ed.; Nova Science Publishers: New York, NY, USA, 2012. [Google Scholar]
- Gracida-Alvarez, U.R.; Mitchell, M.K.; Sacramento-Rivero, J.C.; Shonnard, D.R. Effect of Temperature and Vapor Residence Time on the Micropyrolysis Products of Waste High Density Polyethylene. Ind. Eng. Chem. Res. 2018, 57, 1912–1923. [Google Scholar] [CrossRef]
- Setiani, P.; Watanabe, N.; Sondari, R.R.; Tsuchiya, N. Mechanisms and kinetic model of hydrogen production in the hydrothermal treatment of waste aluminum. Mater. Renew. Sustain. Energy 2018, 7, 4013. [Google Scholar] [CrossRef]
- Galdámez, J.R.; García, L.; Bilbao, R. Hydrogen Production by Steam Reforming of Bio-Oil Using Coprecipitated Ni−Al Catalysts. Acetic Acid as a Model Compound. Energy Fuels 2005, 19, 1133–1142. [Google Scholar] [CrossRef]
- Wang, D.; Czernik, S.; Chornet, E. Production of Hydrogen from Biomass by Catalytic Steam Reforming of Fast Pyrolysis Oils. Energy Fuels 1998, 12, 19–24. [Google Scholar] [CrossRef]
- Shah, N.; Panjala, D.; Huffman, G.P. Hydrogen Production by Catalytic Decomposition of Methane. Energy Fuels 2001, 15, 1528–1534. [Google Scholar] [CrossRef]







| Type | Reactions | ||
|---|---|---|---|
| Combustion of carbon | C + O2 ↔ CO2 | ∆H = −393.5 kJ/mol | (2) |
| C + 1/2O2 ↔ CO | ∆H = −111.4kJ/mol | (3) | |
| Combustion of hydrocarbons | CxHy + (x + y/4)O2 → xCO2 + (y/2)H2O | (4) | |
| CxHy + (x/2)O2 → xCO + (y/2)H2 | (5) | ||
| Gasification reactions | C + H2O ↔ CO + H2 | ∆H = 131.3 kJ/mol | (6) |
| CO + H2O ↔ CO2 + H2 | ∆H = −41 kJ/mol | (7) | |
| C + 2H2 ↔ CH4 | ∆H = −74 kJ/mol | (8) | |
| Type | Reactions | |
|---|---|---|
| SEI | (CH2OCO2Li)2 → Li2CO3 + C2H4 + CO2 + 1/2O2 | (9) |
| 2Li + (CH2OCO2Li)2 → 2Li2CO3 + C2H4 | (10) | |
| Electrolyte | LiPF6 ⇌ LiF + PF5 | (11) |
| LiPF6 + H2O ⇌ LiF + HF + POF3 | (12) | |
| C2H5OCOOC2H5 + PF5 → C2H5OCOOPF4HF + C2H4 | (13) | |
| C2H5OCOOC2H5 + PF5 → C2H5OCOOPF4 + C2H5F | (14) | |
| C2H5OCOOPF4 → HF + C2H4 + CO2 + POF3 | (15) | |
| C2H5OCOOPF4 → C2H5F + CO2 + POF3 | (16) | |
| C2H5OCOOPF4 + HF → PF4OH + CO2 + C2H5F | (17) | |
| C2H5OH + C2H4 → C2H5OC2H5 | (18) | |
| Decomposition at the cathode | Li0.5CoO2 → 1/2LiCoO2 + 1/6Co3O4 + 1/6O2 | (19) |
| Li(1−x)NiO2 → (1 − 2x)LiNiO2 + xLiNi2O4 (x ≤ 0.5) | (20) | |
| Li(1–x)NiO2 → [Li(1−x)Ni(2x−1)/3] [Ni(4−2x)/3]O(8−4x)/3 + (2x − 1)/3O2 (x > 0.5) | (21) | |
| Li(1−x)NiO2 → (2 − x)Li(1−x)/(2−x)Ni1/(2−x)O + x/2O2 | (22) | |
| PVDF binder | –CH2–CF2– + Li → LiF + –CH=CF– + 0.5H2 | (23) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaz, F.; Wang, Y.; Moorthy, T.; Friedrich, B. Degradation Mechanism of Nickel-Cobalt-Aluminum (NCA) Cathode Material from Spent Lithium-Ion Batteries in Microwave-Assisted Pyrolysis. Metals 2018, 8, 565. https://doi.org/10.3390/met8080565
Diaz F, Wang Y, Moorthy T, Friedrich B. Degradation Mechanism of Nickel-Cobalt-Aluminum (NCA) Cathode Material from Spent Lithium-Ion Batteries in Microwave-Assisted Pyrolysis. Metals. 2018; 8(8):565. https://doi.org/10.3390/met8080565
Chicago/Turabian StyleDiaz, Fabian, Yufengnan Wang, Tamilselvan Moorthy, and Bernd Friedrich. 2018. "Degradation Mechanism of Nickel-Cobalt-Aluminum (NCA) Cathode Material from Spent Lithium-Ion Batteries in Microwave-Assisted Pyrolysis" Metals 8, no. 8: 565. https://doi.org/10.3390/met8080565
APA StyleDiaz, F., Wang, Y., Moorthy, T., & Friedrich, B. (2018). Degradation Mechanism of Nickel-Cobalt-Aluminum (NCA) Cathode Material from Spent Lithium-Ion Batteries in Microwave-Assisted Pyrolysis. Metals, 8(8), 565. https://doi.org/10.3390/met8080565






