Corrosion, Erosion and Wear Behavior of Complex Concentrated Alloys: A Review
Abstract
:1. Introduction
2. Evaluation of Surface Degradation Mechanisms
2.1. Corrosion Characterization
2.2. Wear Testing
2.3. Erosion and Erosion Corrosion Characterization
3. Corrosion Behavior of Complex Concentrated Alloys
4. Erosion and Erosion Corrosion of CCAs
5. Wear Behavior of CCAs
6. Conclusions
- Several CCA compositions showed high corrosion resistance in terms of corrosion current density, corrosion potential and pitting resistance. This was primarily attributed to the high wt% of passivating elements, such as Co, Cr, and Ni (cumulatively as high as 40%) in several of the alloys studied.
- Precipitation of secondary phases by either addition of elements or heat treatment deteriorated the corrosion behavior of multi-phase complex concentrated alloys compared to single-phase ones. On the other hand, heat treatment and secondary phase precipitation resulted in surface hardening and improved the wear resistance and erosion characteristics of the alloys.
- Alloying elements that contributed to the precipitation of secondary phases such as B, Cu, Ti, Mo, and Al deteriorated corrosion resistance. The secondary phase precipitates resulted in galvanic corrosion and promoted materials’ degradation.
- Erosion and erosion-corrosion resistance of CCAs was superior when compared to stainless steel grades, owing to their strong passivation and relatively higher hardness.
- When compared to conventional alloys, CCAs/HEAs in many cases showed better overall corrosion and erosion resistance in different media. However, there are significant knowledge gaps with respect to surface passivation mechanisms and synergy between the different degradation routes.
- The wear resistance of some CCA compositions was significantly higher than state of the art steels, such as the SJ grades. The wear resistance varied between 0.8–2.0 m/mm3 as a function of Vanadium, Boron and Aluminum content.
- Two-phase BCC + FCC alloys and single-phase BCC alloys showed orders of magnitude higher wear resistance (~5500 m/mm3 wear resistance) when compared to single-phase FCC alloys (~1.0 m/mm3 wear resistance).
- In addition to as-cast and heat treated alloys, thermally sprayed and annealed CCA coatings showed better wear resistance with minimal weight loss when compared to structural steels.
- Certain CCA compositions demonstrated excellent marine corrosion resistance. The wear volume loss was an order of magnitude lower than mild steels.
7. Future Opportunities and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Murty, B.S.; Yeh, J.; Ranganathan, S. High-Entropy Alloys; Butterworth-Heinemann: Oxford, UK, 2014. [Google Scholar]
- Yeh, J.; Chen, S.; Lin, S.; Gan, J.; Chin, T.; Shun, T.; Tsau, C.; Chang, S. Nanostructured high entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Yeh, J.-W. Recent progress in high entropy alloys. Ann. Chim. Sci. Mater. 2006, 31, 633–648. [Google Scholar] [CrossRef]
- Senkov, O.; Wilks, G.; Miracle, D.; Chuang, C.; Liaw, P. Refractory high-entropy alloys. Intermetallics 2010, 18, 1758–1765. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, Y.L.; Lin, S.J.; Chen, S.K. High-entropy alloys—A new era of exploitation. In Materials Science Forum; Trans Tech Publications: Zürich, Switzerland, 2007; Volume 560, pp. 1–9. [Google Scholar]
- Cantor, B. Multicomponent and high entropy alloys. Entropy 2014, 16, 4749–4768. [Google Scholar] [CrossRef]
- Chen, S.Y.; Yang, X.; Dahmen, K.A.; Liaw, P.K.; Zhang, Y. Microstructures and crackling noise of AlxNbTiMoV high entropy alloys. Entropy 2014, 16, 870–884. [Google Scholar] [CrossRef]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A fracture-resistant high-entropy alloy for cryogenic applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, R.; Kumar, N.; Komarasamy, M. Lattice strain framework for plastic deformation in complex concentrated alloys including high entropy alloys. Mater. Sci. Technol. 2015, 31, 1259–1263. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.; Knight, P.; Vincent, A. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375, 213–218. [Google Scholar] [CrossRef]
- Hemphill, M.A.; Yuan, T.; Wang, G.; Yeh, J.; Tsai, C.; Chuang, A.; Liaw, P. Fatigue behavior of Al0. 5CoCrCuFeNi high entropy alloys. Acta Mater. 2012, 60, 5723–5734. [Google Scholar] [CrossRef]
- Huang, P.; Yeh, J.; Shun, T.; Chen, S. Multi-principal-element alloys with improved oxidation and wear resistance for thermal spray coating. Adv. Eng. Mater. 2004, 6, 74–78. [Google Scholar] [CrossRef]
- Grewal, H.S.; Sanjiv, R.M.; Arora, H.S.; Kumar, R.; Ayyagari, A.; Mukherjee, S.; Singh, H. Activation energy and high temperature oxidation behavior of multi-principal element alloy. Adv. Eng. Mater. 2017, 19. [Google Scholar] [CrossRef]
- Pickering, E.; Jones, N.G. High-entropy alloys: A critical assessment of their founding principles and future prospects. Int. Mater. Rev. 2016, 61, 183–202. [Google Scholar] [CrossRef]
- Miracle, D.; Senkov, O. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Miracle, D.B. High-entropy alloys: A current evaluation of founding ideas and core effects and exploring “nonlinear alloys”. JOM 2017, 69, 2130–2136. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, B.; Liaw, P.K. Corrosion-resistant high-entropy alloys: A review. Metals 2017, 7, 43. [Google Scholar] [CrossRef]
- Chen, Y.; Duval, T.; Hung, U.; Yeh, J.; Shih, H. Microstructure and electrochemical properties of high entropy alloys—A comparison with type-304 stainless steel. Corros. Sci. 2005, 47, 2257–2279. [Google Scholar] [CrossRef]
- Levy, A.V. Solid Particle Erosion and Erosion-Corrosion of Materials; ASM International: Almere, The Netherlands, 1995. [Google Scholar]
- Hutchings, I. Tribology: Friction and Wear of Engineering Materials, 1st ed.; Elsevier Butterworth-Heinemann: Oxford, UK, 1992. [Google Scholar]
- Stachowiak, G.W.; Batchelor, A.W. Corrosive and oxidative wear. In Engineering Tribology, 3rd ed.; Elsevier Butterworth-Heinemann: Amsterdam, The Netherlands, 2005; pp. 649–651. [Google Scholar]
- Arndt, R.E. Cavitation in fluid machinery and hydraulic structures. Ann. Rev. Fluid Mech. 1981, 13, 273–326. [Google Scholar] [CrossRef]
- Wood, R.J. Marine wear and tribocorrosion. Wear 2017, 376, 893–910. [Google Scholar] [CrossRef]
- Finnie, I. Some reflections on the past and future of erosion. Wear 1995, 186, 1–10. [Google Scholar] [CrossRef]
- ASTM International. Standard Guide for Determining Synergism between Wear and Corrosion; ASTM G119-93; ASTM International: West Conshohocken, PA, USA, 1994; pp. 507–512. [Google Scholar]
- Nair, R.; Selvam, K.; Arora, H.; Mukherjee, S.; Singh, H.; Grewal, H. Slurry erosion behavior of high entropy alloys. Wear 2017, 386, 230–238. [Google Scholar] [CrossRef]
- Zhao, J.; Ji, X.; Shan, Y.; Fu, Y.; Yao, Z. On the microstructure and erosion-corrosion resistance of AlCrFeCoNiCu high-entropy alloy via annealing treatment. Mater. Sci. Technol. 2016, 32, 1271–1275. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, S.; Zhang, C.; Zhang, H.; Dong, S. Phase evolution and cavitation erosion-corrosion behavior of FeCoCrAlNiTix high entropy alloy coatings on 304 stainless steel by laser surface alloying. J. Alloy. Compd. 2017, 698, 761–770. [Google Scholar] [CrossRef]
- Hsu, Y.; Chiang, W.; Wu, J. Corrosion behavior of FeCoNiCrCux high-entropy alloys in 3.5% sodium chloride solution. Mater. Chem. Phys. 2005, 92, 112–117. [Google Scholar] [CrossRef]
- Liaw, P.; Egami, T.; Zhang, C.; Zhang, F.; Zhang, Y. Radiation Behavior of High-Entropy Alloys for Advanced Reactors, Technical Report. US Department of Energy, University of Tennessee/Oak Ridge Natonal Laboratory. 2008. Available online: http://www.iaea.org/inis/collection/NCLCollectionStore/_Public/46/119/46119545.pdf (accessed on July 28 2018).
- Ayyagari, A.; Barthelemy, C.; Gwalani, B.; Banerjee, R.; Scharf, T.W.; Mukherjee, S. Reciprocating sliding wear behavior of high entropy alloys in dry and marine environments. Mater. Chem. Phys. 2018, 210, 162–169. [Google Scholar] [CrossRef]
- Kumar, N.; Fusco, M.; Komarasamy, M.; Mishra, R.; Bourham, M.; Murty, K. Understanding effect of 3.5 wt. % NaCl on the corrosion of Al0.1CoCrFeNi high-entropy alloy. J. Nucl. Mater. 2017, 495, 154–163. [Google Scholar] [CrossRef]
- Kao, Y.; Lee, T.; Chen, S.; Chang, Y. Electrochemical passive properties of AlxCoCrFeNi (x = 0, 0.25, 0.50, 1.00) alloys in sulfuric acids. Corros. Sci. 2010, 52, 1026–1034. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, B.; Xie, X.; Brechtl, J.; Dahmen, K.A.; Liaw, P.K. Corrosion of AlxCoCrFeNi high-entropy alloys: Al-content and potential scan-rate dependent pitting behavior. Corros. Sci. 2017, 119, 33–45. [Google Scholar] [CrossRef]
- Lin, C.; Tsai, H. Evolution of microstructure, hardness, and corrosion properties of high-entropy Al0. 5CoCrFeNi alloy. Intermetallics 2011, 19, 288–294. [Google Scholar] [CrossRef]
- Soare, V.; Mitrica, D.; Constantin, I.; Badilita, V.; Stoiciu, F.; Popescu, A.; Carcea, I. Influence of remelting on microstructure, hardness and corrosion behaviour of AlCoCrFeNiTi high entropy alloy. Mater. Sci. Technol. 2015, 31, 1194–1200. [Google Scholar] [CrossRef]
- Qiu, X. Microstructure and properties of AlCrFeNiCoCu high entropy alloy prepared by powder metallurgy. J. Alloy. Compd. 2013, 555, 246–249. [Google Scholar] [CrossRef]
- Hsu, C.; Yeh, J.; Chen, S.; Shun, T. Wear resistance and high-temperature compression strength of Fcc CuCoNiCrAl0.5Fe alloy with boron addition. Metall. Mater. Trans. A 2004, 35, 1465–1469. [Google Scholar] [CrossRef]
- Lee, C.; Chen, Y.; Hsu, C.; Yeh, J.; Shih, H. The effect of boron on the corrosion resistance of the high entropy alloys Al0.5CoCrCuFeNiB x. J. Electrochem. Soc. 2007, 154, C424–C430. [Google Scholar] [CrossRef]
- Xiao, D.; Zhou, P.; Wu, W.; Diao, H.; Gao, M.; Song, M.; Liaw, P. Microstructure, mechanical and corrosion behaviors of AlCoCuFeNi-(Cr,Ti) high entropy alloys. Mater. Des. 2017, 116, 438–447. [Google Scholar] [CrossRef]
- Li, B.; Kun, P.; Hu, A.; Zhou, L.; Zhu, J.; Li, D. Structure and properties of FeCoNiCrCu0.5Alx high-entropy alloy. Trans. Nonferr. Met. Soc. China 2013, 23, 735–741. [Google Scholar] [CrossRef]
- Chou, Y.; Yeh, J.; Shih, H. The effect of molybdenum on the corrosion behaviour of the high-entropy alloys Co1.5CrFeNi1.5Ti0.5Mox in aqueous environments. Corros. Sci. 2010, 52, 2571–2581. [Google Scholar] [CrossRef]
- Chou, Y.; Wang, Y.; Yeh, J.; Shih, H. Pitting corrosion of the high-entropy alloy Co1.5CrFeNi1.5Ti0.5Mo0.1 in chloride-containing sulphate solutions. Corros. Sci. 2010, 52, 3481–3491. [Google Scholar] [CrossRef]
- Ren, B.; Liu, Z.; Li, D.; Shi, L.; Cai, B.; Wang, M. Corrosion behavior of CuCrFeNiMn high entropy alloy system in 1 M sulfuric acid solution. Mater. Corros. 2012, 63, 828–834. [Google Scholar] [CrossRef]
- Cheng, J.; Liang, X.; Wang, Z.; Xu, B. Formation and mechanical properties of CoNiCuFeCr high-entropy alloys coatings prepared by plasma transferred arc cladding process. Plasma Chem. Plasma Process. 2013, 33, 979–992. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, J.; Hou, C.; Li, J.; Jiang, Q. Dense and smooth amorphous films of multicomponent FeCoNiCuVZrAl high-entropy alloy deposited by direct current magnetron sputtering. Mater. Des. 2013, 46, 675–679. [Google Scholar] [CrossRef]
- Li, X.; Zheng, Z.; Dou, D.; Li, J. Microstructure and properties of coating of FeAlCuCrCoMn high entropy alloy deposited by direct current magnetron sputtering. Mater. Res. 2016, 19, 802–806. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, Y.; He, Y. Synthesis and characterization of FeCoNiCrCu high-entropy alloy coating by laser cladding. Mater. Des. 2011, 32, 1910–1915. [Google Scholar] [CrossRef]
- Qiu, X.; Zhang, Y.; He, L.; Liu, C. Microstructure and corrosion resistance of AlCrFeCuCo high entropy alloy. J. Alloy. Compd. 2013, 549, 195–199. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, C.; Zhang, C.; Guan, M.; Tan, J. Laser surface alloying of FeCoCrAlNi high-entropy alloy on 304 stainless steel to enhance corrosion and cavitation erosion resistance. Opt. Laser Technol. 2016, 84, 23–31. [Google Scholar] [CrossRef]
- Qiu, X.; Zhang, Y.; Liu, C. Effect of Ti content on structure and properties of Al2CrFeNiCoCuTix high-entropy alloy coatings. J. Alloy. Compd. 2014, 585, 282–286. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, Y.; He, Y.; Jiao, H. Microstructure and properties of 6FeNiCoSiCrAlTi high-entropy alloy coating prepared by laser cladding. Appl. Surf. Sci. 2011, 257, 2259–2263. [Google Scholar] [CrossRef]
- Shon, Y.; Joshi, S.S.; Katakam, S.; Rajamure, R.S.; Dahotre, N.B. Laser additive synthesis of high entropy alloy coating on aluminum: Corrosion behavior. Mater. Lett. 2015, 142, 122–125. [Google Scholar] [CrossRef]
- Marcus, P. On some fundamental factors in the effect of alloying elements on passivation of alloys. Corros. Sci. 1994, 36, 2155–2158. [Google Scholar] [CrossRef]
- Cheng, J.; Liang, X.; Xu, B. Effect of Nb addition on the structure and mechanical behaviors of CoCrCuFeNi high-entropy alloy coatings. Surf. Coat. Technol. 2014, 240, 184–190. [Google Scholar] [CrossRef]
- Ye, X.; Ma, M.; Cao, Y.; Liu, W.; Ye, X.; Gu, Y. The property research on high-entropy alloy AlxFeCoNiCuCr coating by laser cladding. Phys. Procedia 2011, 12, 303–312. [Google Scholar] [CrossRef]
- Li, W.; Liu, G.; Guo, J. Microstructure and electrochemical properties of AlxFeCoNiCrTi high-entropy alloys. Foundry 2009, 58, 431–435. [Google Scholar]
- Qiu, X.; Liu, C. Microstructure and properties of Al2CrFeCoCuTiNix high-entropy alloys prepared by laser cladding. J. Alloy. Compd. 2013, 553, 216–220. [Google Scholar] [CrossRef]
- Qiu, X.; Wu, M.; Liu, C.; Zhang, Y.; Huang, C. Corrosion performance of Al2CrFeCoxCuNiTi high-entropy alloy coatings in acid liquids. J. Alloy. Compd. 2017, 708, 353–357. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, S.; Zhang, C.; Chen, J.; Dong, S. Phase evolution characteristics and corrosion behavior of FeCoCrAlCu-X0.5 coatings on cp Cu by laser high-entropy alloying. Opt. Laser Technol. 2017, 94, 68–71. [Google Scholar] [CrossRef]
- Ren, B.; Zhao, R.; Liu, Z.; Guan, S.; Zhang, H. Microstructure and properties of Al0.3CrFe1.5MnNi0.5Tix and Al0.3CrFe1.5MnNi0.5Six high-entropy alloys. Rare Met. 2014, 33, 149–154. [Google Scholar] [CrossRef]
- Li, Q.; Yue, T.; Guo, Z.; Lin, X. Microstructure and corrosion properties of AlCoCrFeNi high entropy alloy coatings deposited on AISI 1045 steel by the electrospark process. Metall. Mater. Trans. A 2013, 44, 1767–1778. [Google Scholar] [CrossRef]
- Soare, V.; Mitrica, D.; Constantin, I.; Popescu, G.; Csaki, I.; Tarcolea, M.; Carcea, I. The mechanical and corrosion behaviors of as-cast and re-melted AlCrCuFeMnNi multi-component high-entropy alloy. Metall. Mater. Trans. A 2015, 46, 1468–1473. [Google Scholar] [CrossRef]
- Argade, G.R.; Joshi, S.S.; Ayyagari, A.V.; Mukherjee, S.; Mishra, R.S.; Dahotre, N.B. Tribocorrosion Performance of Laser Additively Processed High Entropy Alloy Coatings on Aluminum. Appl. Surf. Sci. under review.
- Yuan, Y.; Faqin, X.; Tiebang, Z.; Hongchao, K.; Rui, H.; Jinshan, L. Microstructure control and corrosion properties of AlCoCrFeNiTi0.5 high-entropy alloy. Rare Met. Mater. Eng. 2012, 5, 025. [Google Scholar]
- Lee, C.; Chang, C.; Chen, Y.; Yeh, J.; Shih, H. Effect of the aluminium content of AlxCrFe1.5MnNi0.5 high-entropy alloys on the corrosion behaviour in aqueous environments. Corros. Sci. 2008, 50, 2053–2060. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, G.; Dai, P. Evolution of the microstructure and properties of laser-clad FeCrNiCoBx high-entropy alloy coatings. Mater. Sci. Technol. 2016, 32, 1666–1672. [Google Scholar] [CrossRef]
- Lin, C.; Tsai, H.; Bor, H. Effect of aging treatment on microstructure and properties of high-entropy Cu0.5CoCrFeNi alloy. Intermetallics 2010, 18, 1244–1250. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, X.; Zhang, C.; Li, J. Microstructure and corrosion behaviour of FeCoNiCuSnx high entropy alloys. Mater. Sci. Technol. 2015, 31, 1148–1152. [Google Scholar] [CrossRef]
- Li, J.; Yang, X.; Zhu, R.; Zhang, Y. Corrosion and serration behaviors of TiZr0.5NbCr0.5VxMoy high entropy alloys in aqueous environments. Metals 2014, 4, 597–608. [Google Scholar] [CrossRef]
- Ji, X.; Duan, H.; Zhang, H.; Ma, J. Slurry erosion resistance of laser clad NiCoCrFeAl3 high-entropy alloy coatings. Tribol. Trans. 2015, 58, 1119–1123. [Google Scholar] [CrossRef]
- Zhang, L.S.; Ma, G.L.; Fu, L.C.; Tian, J.Y. Recent progress in high-entropy alloys. Eur. J. Control 2013, 631, 227–232. [Google Scholar] [CrossRef]
- Komarasamy, M.; Kumar, N.; Tang, Z.; Mishra, R.; Liaw, P. Effect of microstructure on the deformation mechanism of friction stir-processed Al0.1CoCrFeNi high entropy alloy. Mater. Res. Lett. 2015, 3, 30–34. [Google Scholar] [CrossRef]
- Liu, J.; Chen, C.; Xu, Y.; Wu, S.; Wang, G.; Wang, H.; Fang, Y.; Meng, L. Deformation twinning behaviors of the low stacking fault energy high-entropy alloy: An in-situ TEM study. Scr. Mater. 2017, 137, 9–12. [Google Scholar] [CrossRef]
- Nair, R.B.; Arora, H.S.; Ayyagari, A.; Mukherjee, S.; Grewal, H.S. High entropy alloys: Prospective materials for tribo-corrosion applications. Adv. Eng. Mater. 2018, 1700946. [Google Scholar] [CrossRef]
- Nair, R.; Arora, H.; Mukherjee, S.; Singh, S.; Singh, H.; Grewal, H. Exceptionally high cavitation erosion and corrosion resistance of a high entropy alloy. Ultrason. Sonochem. 2018, 41, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Grewal, H.; Agrawal, A.; Singh, H. Slurry erosion mechanism of hydroturbine steel: Effect of operating parameters. Tribol. Lett. 2013, 52, 287–303. [Google Scholar] [CrossRef]
- Ang, A.S.M.; Berndt, C.C.; Sesso, M.L.; Anupam, A.; Praveen, S.; Kottada, R.S.; Murty, B. Plasma-sprayed high entropy alloys: Microstructure and properties of AlCoCrFeNi and MnCoCrFeNi. Metall. Mater. Trans. A 2015, 46, 791–800. [Google Scholar] [CrossRef]
- Liao, W.; Lan, S.; Gao, L.; Zhang, H.; Xu, S.; Song, J.; Wang, X.; Lu, Y. Nanocrystalline high-entropy alloy (CoCrFeNiAl0.3) thin-film coating by magnetron sputtering. Thin Solid Films 2017, 638, 383–388. [Google Scholar] [CrossRef]
- Ye, Q.; Feng, K.; Li, Z.; Lu, F.; Li, R.; Huang, J.; Wu, Y. Microstructure and corrosion properties of CrMnFeCoNi high entropy alloy coating. Appl. Surf. Sci. 2017, 396, 1420–1426. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, X.; Yu, X.; Li, J. Synthesis and characterization of refractory TiZrNbWMo high-entropy alloy coating by laser cladding. Surf. Coat. Technol. 2017, 311, 321–329. [Google Scholar] [CrossRef]
- Nair, R.B.; Arora, H.S.; Mandal, P.; Das, S.; Grewal, H.S. High-performance microwave-derived multi-principal element alloy coatings for tribological application. Adv. Eng. Mater. 2018, 1800163. [Google Scholar] [CrossRef]
- Taillon, G.; Pougoum, F.; Lavigne, S.; Ton-That, L.; Schulz, R.; Bousser, E.; Savoie, S.; Martinu, L.; Klemberg-Sapieha, J. Cavitation erosion mechanisms in stainless steels and in composite metal–ceramic HVOF coatings. Wear 2016, 364, 201–210. [Google Scholar] [CrossRef]
- Hong, S.; Wu, Y.; Zhang, J.; Zheng, Y.; Zheng, Y.; Lin, J. Synergistic effect of ultrasonic cavitation erosion and corrosion of WC–CoCr and FeCrSiBMn coatings prepared by HVOF spraying. Ultrason. Sonochem. 2016, 31, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Cuppari, M.D.V.; Souza, R.; Sinatora, A. Effect of hard second phase on cavitation erosion of Fe–Cr–Ni–C alloys. Wear 2005, 258, 596–603. [Google Scholar] [CrossRef]
- Jiang, G.; Zheng, Y.; Yang, Y.; Fang, H. Caviation erosion of bainitic steel. Wear 1998, 215, 46–53. [Google Scholar] [CrossRef]
- Sakamoto, A.; Yamasaki, T.; Matsumura, M. Erosion-corrosion tests on copper alloys for water tap use. Wear 1995, 186, 548–554. [Google Scholar] [CrossRef]
- Man, H.; Kwok, C.; Yue, T. Cavitation erosion and corrosion behaviour of laser surface alloyed MMC of SiC and Si3N4 on Al alloy AA6061. Surf. Coat. Technol. 2000, 132, 11–20. [Google Scholar] [CrossRef]
- Wu, J.; Lin, S.; Yeh, J.; Chen, S.; Huang, Y.; Chen, H. Adhesive wear behavior of AlxCoCrCuFeNi high-entropy alloys as a function of aluminum content. Wear 2006, 261, 513–519. [Google Scholar] [CrossRef]
- Chen, M.; Lin, S.; Yeh, J.; Chuang, M.; Chen, S.; Huang, Y. Effect of vanadium addition on the microstructure, hardness, and wear resistance of Al0.5CoCrCuFeNi high-entropy alloy. Metall. Mater. Trans. A 2006, 37, 1363–1369. [Google Scholar] [CrossRef]
- Chuang, M.; Tsai, M.; Wang, W.; Lin, S.; Yeh, J. Microstructure and wear behavior of AlxCo1.5CrFeNi1.5Tiy high-entropy alloys. Acta Mater. 2011, 59, 6308–6317. [Google Scholar] [CrossRef]
- Gwalani, B.; Ayyagari, A.; Choudhuri, D.; Scharf, T.; Mukherjee, S.; Gibson, M.; Banerjee, R. Microstructure and wear resistance of an intermetallic-based Al0.25Ti0.75CoCrFeNi high entropy alloy. Mater. Chem. Phys. 2018, 210, 197–206. [Google Scholar] [CrossRef]
- Lin, Y.; Cho, Y. Elucidating the microstructure and wear behavior for multicomponent alloy clad layers by in situ synthesis. Surf. Coat. Technol. 2008, 202, 4666–4672. [Google Scholar] [CrossRef]
- Chen, J.; Chen, P.; Lin, C.; Chang, C.; Chang, Y.; Wu, W. Microstructure and wear properties of multicomponent alloy cladding formed by gas tungsten arc welding (GTAW). Surf. Coat. Technol. 2009, 203, 3231–3234. [Google Scholar] [CrossRef]
- Huo, W.; Shi, H.; Ren, X.; Zhang, J. Microstructure and wear behavior of CoCrFeMnNbNi high-entropy alloy coating by TIG cladding. Adv. Mater. Sci. Eng. 2015, 2015. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; He, P.; Lin, T.; Shi, Y. Microstructure and wear properties of CuNiSiTiZr high-entropy alloy coatings on TC11 titanium alloy produced by electrospark—Computer numerical control deposition process. Surf. Coat. Technol. 2015, 283, 156–161. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, Y.; Vilar, R.; Shen, J. Dry sliding wear behavior of laser clad TiVCrAlSi high entropy alloy coatings on Ti–6Al–4V substrate. Mater. Des. 2012, 41, 338–343. [Google Scholar] [CrossRef]
- Tian, L.; Xiong, W.; Liu, C.; Lu, S.; Fu, M. Microstructure and wear behavior of atmospheric plasma-sprayed AlCoCrFeNiTi high-entropy alloy coating. J. Mater. Eng. Perform. 2016, 25, 5513–5521. [Google Scholar] [CrossRef]
- Wang, L.; Chen, C.; Yeh, J.; Ke, S. The microstructure and strengthening mechanism of thermal spray coating NixCo0.6Fe0.2CrySizAlTi0.2 high-entropy alloys. Mater. Chem. Phys. 2011, 126, 880–885. [Google Scholar] [CrossRef]
- Hsu, C.; Sheu, T.; Yeh, J.; Chen, S. Effect of iron content on wear behavior of AlCoCrFexMo0.5Ni high-entropy alloys. Wear 2010, 268, 653–659. [Google Scholar] [CrossRef]
- Ayyagari, A.; Hasannaeimi, V.; Arora, H.; Mukherjee, S. Electrochemical and friction characteristics of metallic glass composites at the microstructural length-scales. Sci. Rep. 2018, 8, 906. [Google Scholar] [CrossRef] [PubMed]
































| Flow Related Parameters | Erodent Related Parameters | Materials Related Parameters |
|---|---|---|
|
|
|
| Complex Concentrated Alloy | Microstructure | Corrosion Environment | Test Procedure/Analysis | Major Finding |
|---|---|---|---|---|
| AlCoCrCu0.5FeNiSi [18] | Two Phase: Dendritic phase: mixture of amorphous and BCC; Inter-dendritic phases: amorphous and nano-scale precipitates | 3.5 wt% NaCl, H2SO4 at 30–70 °C | Anodic polarization | CCA showed overall better corrosion resistance than SS304, but had poor pitting resistance; Corrosion resistance was lower than SS304 at higher temperatures |
| Al0.1CoCrFeNi [31] | Single phase FCC | 3.5 wt% NaCl | Potentiodynamic Polarization | Very high corrosion resistance, passive region as wide as 1 V; Grain boundary corrosion, very low corrosion current density |
| Al0.3CrFe1.5MnNi0.5Tix [61] | BCC with increasing intermetallic with increasing Ti | 3.5 wt% NaCl | Potentiodynamic Polarization | Adding Ti lowered corrosion resistance |
| Al0.3CrFe1.5MnNi0.5Six [61] | BCC with increasing intermetallic with increasing Si | 3.5 wt% NaCl | Potentiodynamic Polarization | Adding Si lowered corrosion resistance |
| Al0.5CoCrCuFeNiBx [39] | FCC + BCC crystal structure | 1 N H2SO4 | Anodic polarization | CCA was nobler than SS304 in terms of corrosion current density, and corrosion potentials; not susceptible to localized corrosion in sulfate solutions |
| Al0.5CoCrFeNi [35] | FCC solid solution matrix with secondary phases rich in Al–Ni | 3.5 wt% NaCl | Potentiodynamic Polarization | Secondary phases rich in Al–Ni were more susceptible to corrosion |
| Al2CrFeNiCoCuTix [51] | x = 0.0 FCC + BCC1 | 0.5 M HNO3 | Potentiodynamic Polarization | Increasing Ti content increased corrosion resistance in terms of corrosion current density |
| x = 0.5 FCC + BCC2 | ||||
| x = 1.0 BCC1 + BCC2 | ||||
| x = 1.5 BCC1 + BCC2 | ||||
| x = 2.0 FCC + BCC2 | ||||
| AlCoCrCuFeNi [37] | FCC + BCC two phase mixture | 1 mol/L NaCl | Potentiodynamic Polarization | Good corrosion resistance despite two phase structure |
| AlCoCrFeNi [62] | BCC | 3.5 wt% NaCl | Potentiodynamic Polarization | Corrosion resistance after processing was three orders of magnitude better than steel and one order of magnitude better than unprocessed HEA |
| AlCoCrFeNiTi [36] | Complex Microstructure: Al, Co, Ni and Ti rich dendritic phase. Fe and Cr rich inter-dendritic phase. Ti and Ni rich third phase. Ordered phase—A2, B2, D03 and A12 | 3.5 wt% NaCl | Polarization | Ti addition improved corrosion resistance of the alloy; Through the re-melting process, the distribution of elements in the alloy improved, improving the corrosion resistance |
| AlCrCuFeMnNi [63] | Complex Microstructure: BCC dendritic phase, interdendritic area with two phases—a eutectic type and FCC solid solution phase | 3.5 wt% NaCl | Potentiodynamic Polarization | CCA was easier to passivate; higher corrosion resistance than SS304L; Galvanic coupling reduced by dissolving Cu during re-melting |
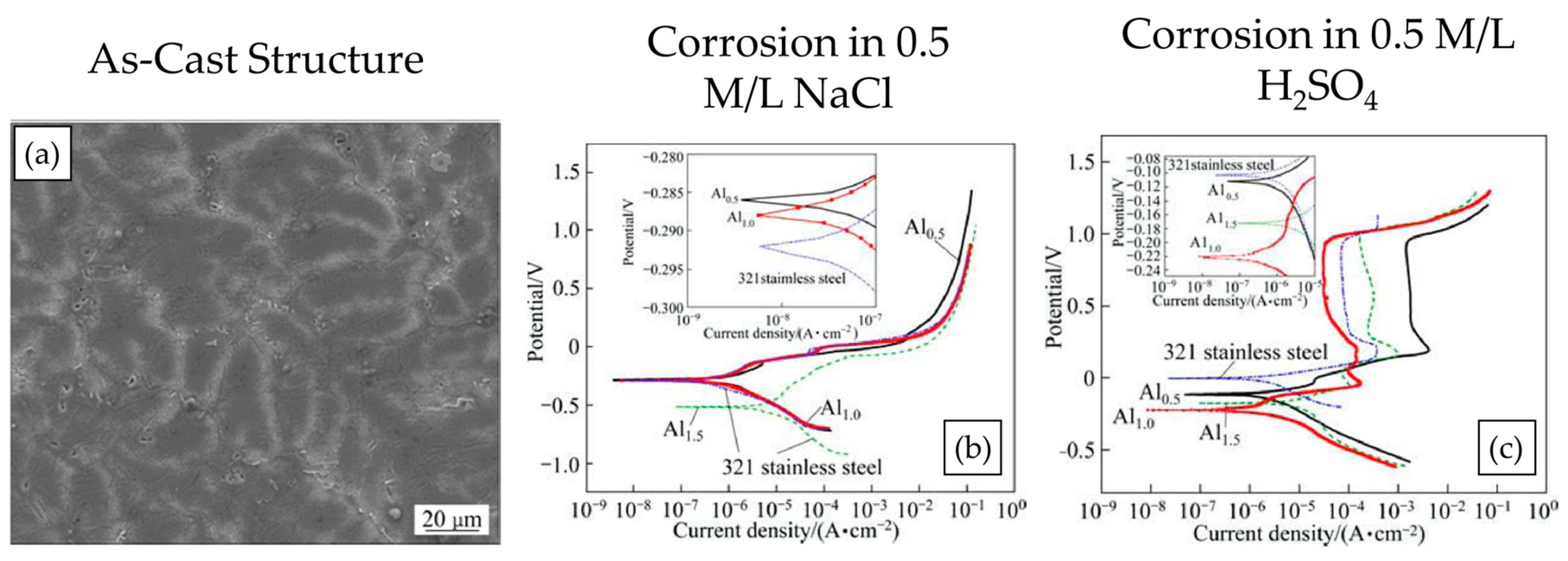
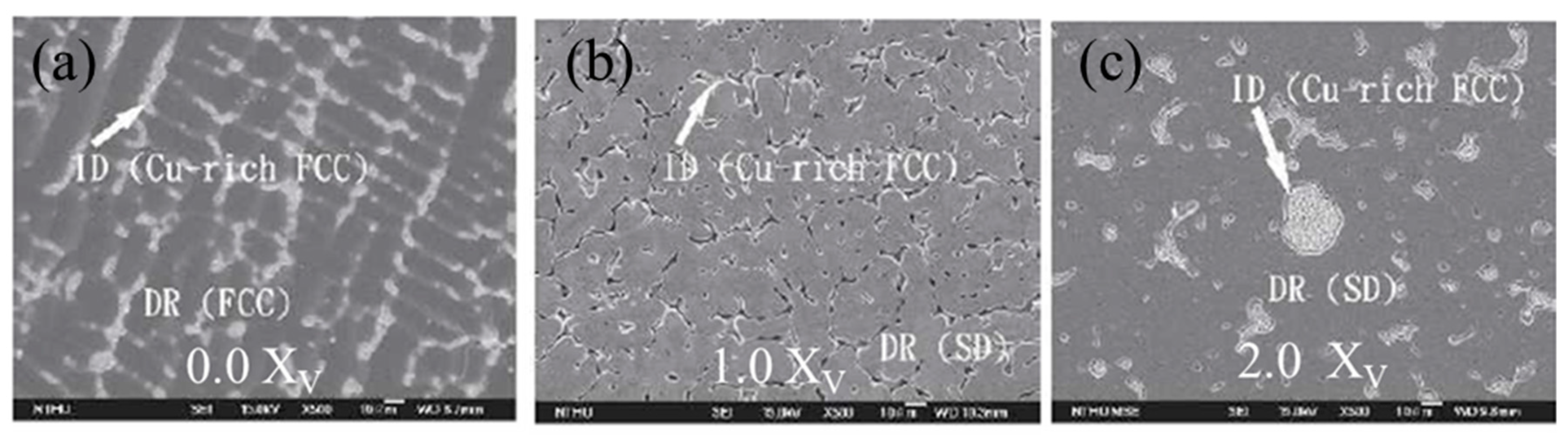
| AlxCoCrCu0.5FeNi [41] | x = 0.5 is FCC | 0.5 mol/L H2SO4 + 0.5 mol/L NaCl solution | Potentiodynamic Polarization | Single-phase alloys had better corrosion resistance that phase mixtures; BCC alloy was comparable with 321 stainless steel |
| x = 1.0 is BCC | ||||
| x = 1.5 FCC + BCC | ||||
| AlCoCrCuFe [49] | FCC and BCC phase mixture | 1 mol/L NaCl and 0.5 mol/L H2SO4 | Potentiodynamic Polarization | Segregation of Cu was seen in the microstructure; CCA performed better in NaCl solution than in acidic solution |
| AlxCoCrFeNi (x = 0, 0.25, 0.50, 1.00) [33] | x = 0 | 0.5 mol/L H2SO4 | Potentiodynamic Polarization | Corrosion current density decreased with Al content at 23 °C; Overall superior corrosion resistance compared to steels |
| x = 0.25 | ||||
| x = 0.50 | ||||
| x = 1.00 | ||||
| AlxCoCrFeNi [34] | x = 0.3 | 3.5 wt% NaCl | Potentiodynamic Polarization | Increasing Al content decreased corrosion resistance by formation of intermetallic phases |
| x = 0.5 | ||||
| x = 0.7 | ||||
| AlxCrFe1.5MnNi0.5 [66] | x = 0.0 FCC | 1 mol/L NaCl + 0.5 mol/L H2SO4 | Potentiodynamic Polarization | Alloys showed extended passive region, greater than 1 V; Increasing Al lowered corrosion resistance in terms of pitting behavior |
| x = 0.3 BCC + FCC | ||||
| x = 0.5 BCC | ||||
| BxCoCrFeNi [67] | x = 0.5 FCC | 3.5 wt% NaCl | Potentiodynamic Polarization | Corrosion resistance improved with increasing B content up to 1%., beyond which corrosion resistance decreased; The CCAs showed superior corrosion resistance than SS304 |
| x = 0.75 FCC | ||||
| x = 1.0 FCC | ||||
| x = 1.25 FCC + M2B | ||||
| Co1.5CrFeNi1.5Ti0.5Mo0.1 [43] | FCC solid-solution structure | 0.001 to 1 M NaCl and sulfate doped 1 M NaCl | Potentiodynamic Polarization | Sulfate ions increased the pitting potential and critical pitting potential of the alloys |
| Co1.5CrFeNi1.5Ti0.5Mox [42] | FCC solid-solution | 0.5 M H2SO4 1 M NaCl and NaOH | Potentiodynamic Polarization | Mo addition lowered the overall corrosion resistance |
| CoCrCu0.5FeNi [68] | Dendritic Structure: Copper lean dendritic phase, copper rich interdendritic phase, aged at different temperatures | 3.5 wt% NaCl | Potentiodynamic Polarization | Corrosion current density lowered while corrosion potential decreased with aging temperature; Corrosion properties worsened when heat treated at 1100–1350 °C. Pitting increased with aging temperature |
| CoCrCuFeNiNb [55] | FCC and Laves phases | 6 M HCl | Potentiodynamic Polarization | Alloying with Nb lowered corrosion current density |
| CoCrFeMnNi [31] | Simple single phase FCC | 3.5 wt% NaCl | Potentiodynamic Polarization | ~500 mV wide passivation region; Corrosion rate as low as one micron per year |
| CoCrCuxFeNi [29] | FCC and Cu rich FCC | 3.5 wt% NaCl | Potentiodynamic Polarization | Addition of Cu deteriorated the corrosion resistance |
| Immersion Test | Galvanic corrosion between inter-dendritic region and dendrite resulting in localized corrosion | |||
| CoCuFeNiSnx [69] | Single phase FCC solid solution when Sn < 0.09, small BCC phase beyond that | 3.5 wt% NaCl and 5% NaOH | Potentiodynamic Polarization | CCAs showed wide passivation range in NaOH and relatively smaller region in NaCl; Better resistance than SS304; FeCoNiCuSn0.04 showed improved corrosion resistance |
| CoCuFeNiSnx [69] x = 0–0.09 | FCC when x < 0.09, small BCC for x > 0.09 | 3.5 wt% NaCl and 5% NaOH | Potentiodynamic Polarization | Better corrosion resistance than SS304 alloy when tested in NaCl, while lower corrosion resistance when tested in NaOH |
| Cr0.5NbTiZr0.5, Cr0.5NbTiVZr0.5 Cr0.5MoNbTiZr0.5 [70] | Dendritic Structure: BCC Disordered Solid Solution phase and Cr2Zr phase | 3.5 wt% NaCl and 0.5 M H2SO4 | Potentiodynamic Polarization | Superior corrosion resistance, with passive region more than 1400 mV; Mo and V addition decreased corrosion resistance but improved pitting resistance in NaCl and H2SO4 |
| CoCrCuxFeNi [29] | FCC crystal structure, Cu rich interdendritic phase | 3.5 wt% NaCl | Potentiodynamic Polarization | Increasing Cu content caused segregation into inter-dendritic phases, and consequent deterioration of corrosion resistance; General corrosion trend was seen as FeCoNiCrCu > FeCoNiCrCu0.5 > FeCoNiCr |
| CuCr2Fe2Ni2Mn2 Cu2CrFe2NiMn2 [44] | FCC | 1 M H2SO4 | Potentiodynamic Polarization | Cr2 alloy showed better corrosion resistance while Cu2 alloy promoted segregation and had lower corrosion resistance |
| FCC + BCC | ||||
| AlCoCuFeNi AlCoCuFeNiCr AlCoCuFeNiTi AlCoCrCuFeNiTi [40] | FCC + A2 + B2 | 0.5 mol/L H2SO4 | Potentiodynamic Polarization | Adding Ti decreased the corrosion resistance of the AlCoCuFeNi alloys, whereas adding Cr improved corrosion resistance |
| System | Alloy | Hardness (HV) | Wear Resistance |
|---|---|---|---|
| Al0.5CoCrCuFeNiVx [90] | Al0.5CoCrCuFeNiV0.2 | 200 | 0.910 |
| Al0.5CoCrCuFeNiV0.4 | 225 | 0.875 | |
| Al0.5CoCrCuFeNiV0.4 | 325 | 0.850 | |
| Al0.5CoCrCuFeNiV0.8 | 450 | 0.900 | |
| Al0.5CoCrCuFeNiV1.0 | 650 | 0.925 | |
| Al0.5CoCrCuFeNiV1.2 | 575 | 1.050 | |
| Al0.5CoCrCuFeNiV1.4 | 578 | 1.100 | |
| Al0.5CoCrCuFeNiV1.6 | 600 | 1.050 | |
| Al0.5CoCrCuFeNiV1.8 | 600 | 1.100 | |
| Al0.5CoCrCuFeNiV2.0 | 598 | 1.110 | |
| AlxCo1.5CrFeNi1.5Tiy [91] | Al0Co1.5CrFeNi1.5Ti0.5 | 501 | 250 |
| Al0.2Co1.5CrFeNi1.5Ti0.5 | 480 | 255 | |
| Al0Co1.5CrFeNi1.5Ti | 650 | 2000 | |
| Al0.2Co1.5CrFeNi1.5Ti | 700 | 5500 | |
| AlxCoCrCuFeNi [89] | Al0.5CoCrCuFeNi | 252 | 0.925 |
| Al1.5CoCrCuFeNi | 350 | 0.825 | |
| Al2.0CoCrCuFeNi | 550 | 0.850 | |
| Al0.5BxCoCrCuFe [38] | Al0.5B0CoCrCuFe | 300 | 0.8 |
| Al0.5B0.2CoCrCuFe | 400 | 0.9 | |
| Al0.5B0.6CoCrCuFe | 500 | 1.0 | |
| Al0.5B1.0CoCrCuFe | 725 | 1.6 | |
| AlCoCrFexMo0.5Ni [100] | AlCoCrFe0.6Mo0.5Ni | 675 | 1600 |
| AlCoCrFe1.0Mo0.5Ni | 700 | 1500 | |
| AlCoCrFe1.5Mo0.5Ni | 525 | 1200 | |
| AlCoCrFe2.0Mo0.5Ni | 425 | 1250 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayyagari, A.; Hasannaeimi, V.; Grewal, H.S.; Arora, H.; Mukherjee, S. Corrosion, Erosion and Wear Behavior of Complex Concentrated Alloys: A Review. Metals 2018, 8, 603. https://doi.org/10.3390/met8080603
Ayyagari A, Hasannaeimi V, Grewal HS, Arora H, Mukherjee S. Corrosion, Erosion and Wear Behavior of Complex Concentrated Alloys: A Review. Metals. 2018; 8(8):603. https://doi.org/10.3390/met8080603
Chicago/Turabian StyleAyyagari, Aditya, Vahid Hasannaeimi, Harpreet Singh Grewal, Harpreet Arora, and Sundeep Mukherjee. 2018. "Corrosion, Erosion and Wear Behavior of Complex Concentrated Alloys: A Review" Metals 8, no. 8: 603. https://doi.org/10.3390/met8080603





