Preparation of Spherical Mo5Si3 Powder by Inductively Coupled Thermal Plasma Treatment
Abstract
:1. Introduction
2. Experimental Procedures
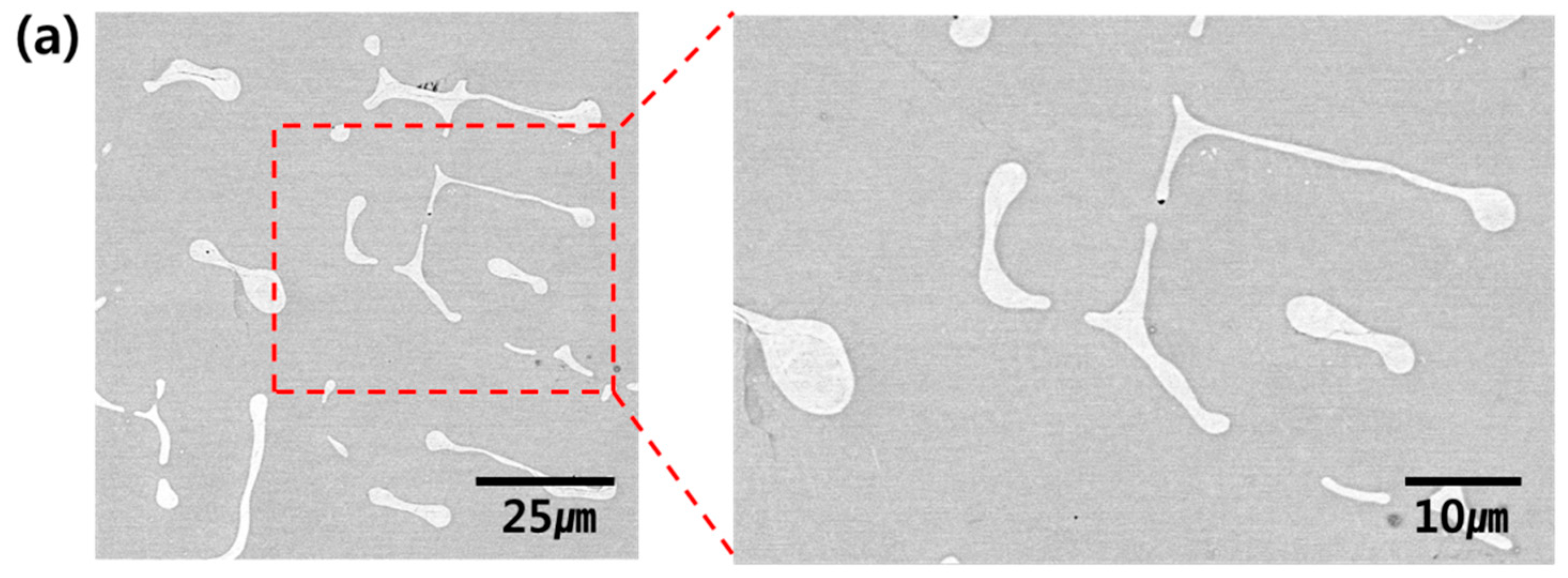
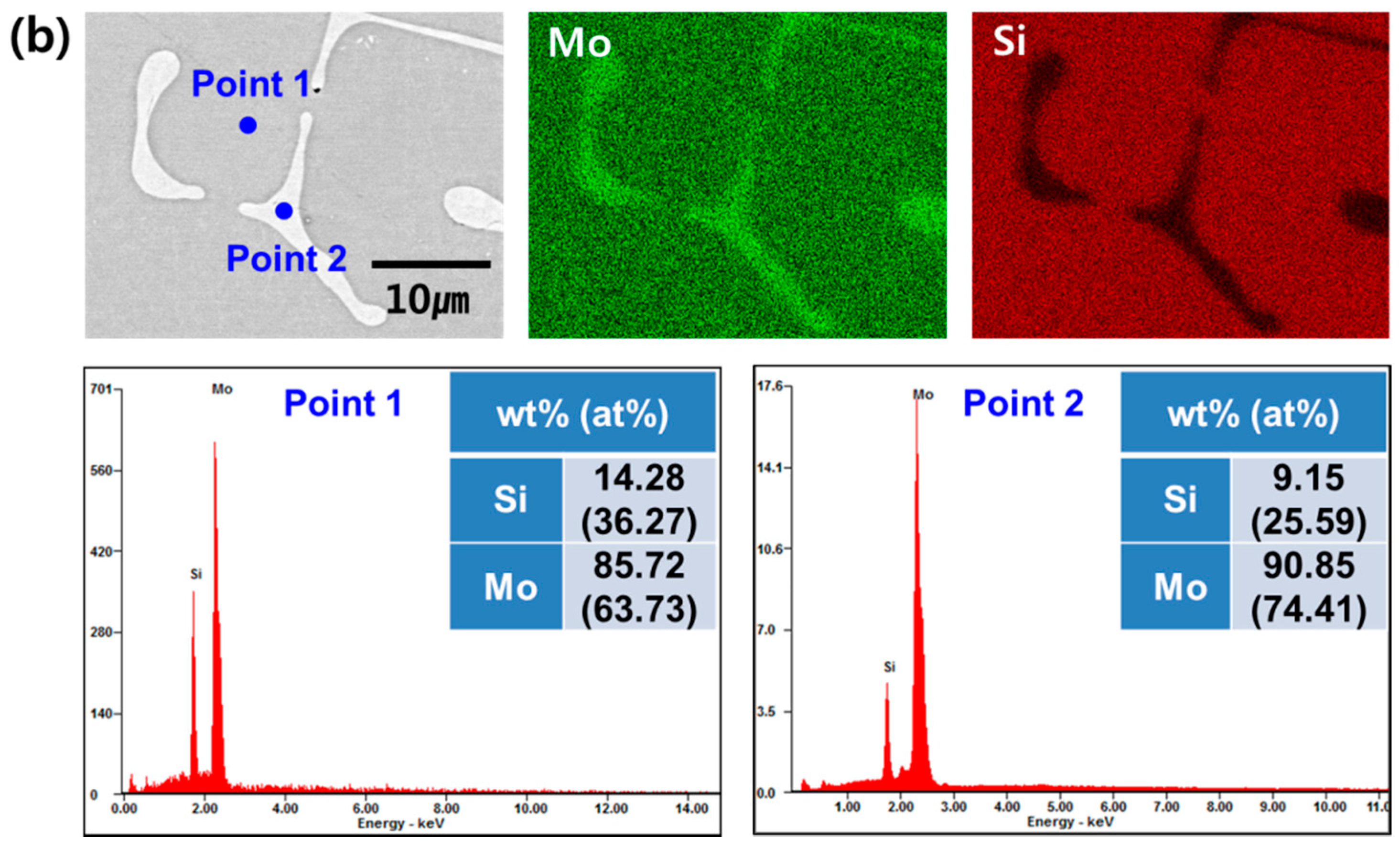
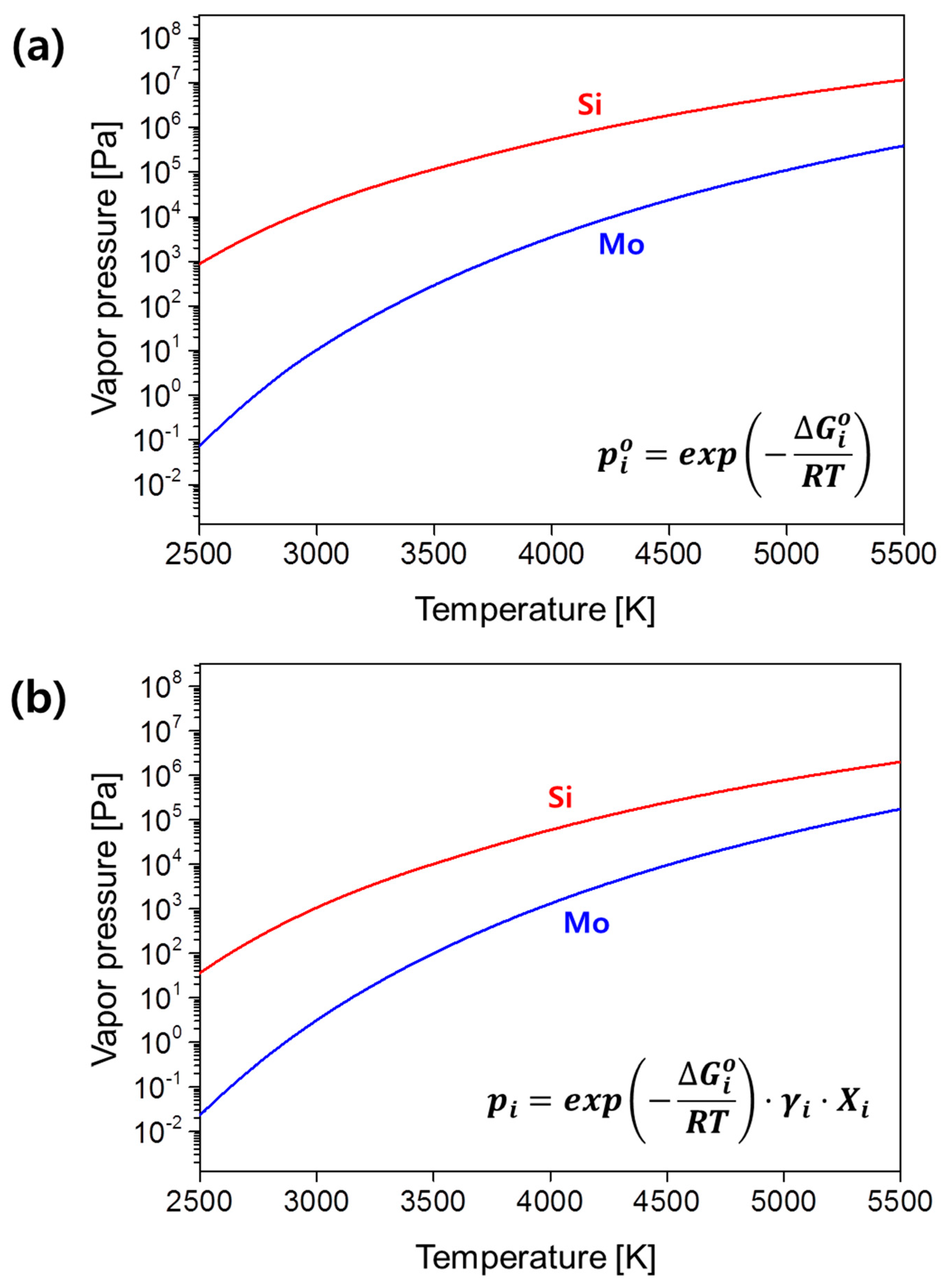
3. Results and Discussion
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Petrovic, J.J. MoSi2-based high-temperature structural silicides. MRS Bull. 1993, 18, 35–41. [Google Scholar] [CrossRef]
- Chu, F.; Thoma, D.J.; McClellan, K.J.; Peralta, P. Mo5Si3 single crystals: Physical properties and mechanical behavior. Mater. Sci. Eng. A 1999, 261, 44–52. [Google Scholar] [CrossRef]
- Yeh, C.L.; Chou, C.C.; Hwang, P.W. Experimental and Numerical Studies on Self-Propagating High-Temperature Synthesis of Ta5Si3 Intermetallics. Metals 2015, 5, 1580–1590. [Google Scholar] [CrossRef]
- Schneibel, J.H.; Liu, C.T.; Easton, D.S.; Carmichael, C.A. Microstructure and mechanical properties of Mo-Mo3Si-Mo5SiB2 silicides. Mater. Sci. Eng. A 1999, 261, 78–83. [Google Scholar] [CrossRef]
- Kwon, N.Y.; Kim, Y.D.; Suk, M.J.; Lee, S.; Oh, S.T. Synthesis of Mo-Si-B intermetallic compounds with continuous α-Mo matrix by pulverization of ingot and hydrogen reduction of MoO3 powders. Int. J. Refract. Met. Hard Mater. 2017, 65, 25–28. [Google Scholar] [CrossRef]
- Bewlay, B.P.; Jackson, M.R.; Zhao, J.C.; Subramanian, P.R. A review of very-high-temperature Nb-silicide-based composites. Metall. Mater. Trans. A 2003, 34, 2043–2052. [Google Scholar] [CrossRef]
- Tang, Y.; Gua, X. High temperature deformation behavior of an optimized Nb-Si based ultrahigh temperature alloy. Scr. Mater. 2016, 116, 16–20. [Google Scholar] [CrossRef]
- Byun, J.M.; Hwang, S.H.; Lee, S.; Kim, Y.D. Research trend of Mo based superalloys. J. Kor. Powd. Met. Inst. 2013, 20, 487–493. [Google Scholar] [CrossRef]
- Kieffer, R.; Benesovsky, F. Recent developments in the field of silicides and borides of the high-melting-point transition metals. Powder Metall. 1958, 1, 145–171. [Google Scholar] [CrossRef]
- Akinc, M.; Meyer, M.K.; Kramer, M.J.; Thom, A.J.; Huebsch, J.J.; Cook, B. Boron-doped molybdenum silicides for structural applications. Mater. Sci. Eng. A 1999, 261, 16–23. [Google Scholar] [CrossRef]
- Choe, H.; Chen, D.; Schneibel, J.H.; Ritchie, R.O. Ambient to high temperature fracture toughness and fatigue-crack propagation behavior in a Mo–12Si–8.5B (at.%) intermetallic. Intermetallics 2001, 9, 319–329. [Google Scholar] [CrossRef]
- Ström, E.; Zhang, J. Mechanical anisotropy and segregation in Mo5Si3 studied by EBSD. Intermetallics 2005, 13, 367–372. [Google Scholar] [CrossRef]
- Mendiratta, M.G.; Dimiduk, D.M. Strength and toughness of a Nb/Nb5Si3 composite. Metall. Mater. Trans. A 1993, 24, 501–504. [Google Scholar] [CrossRef]
- Zamani, S.; Bakhsheshi-Rad, H.R.; Shokuhfar, A.; Vaezi, M.R.; Kadir, M.R.A.; Shafiee, M.R.M. Synthesis and characterization of MoSi2-Mo5Si3 nanocomposite by mechanical alloying and heat treatment. Int. J. Refract. Met. Hard Mater. 2012, 31, 236–241. [Google Scholar] [CrossRef]
- Krüger, M.; Schmelzer, J.; Helmecke, M. Similarities and Differences in Mechanical Alloying Processes of V-Si-B and Mo-Si-B Powders. Metals 2016, 6, 241. [Google Scholar] [CrossRef]
- Kermani, M.; Razavi, M.; Rahimipour, M.R.; Zakeri, M. The effect of mechanical alloying on microstructure and mechanical properties of MoSi2 prepared by spark plasma sintering. J. Alloy. Compd. 2014, 593, 242–249. [Google Scholar] [CrossRef]
- Fang, Z.Z.; Paramore, J.D.; Sun, P.; Chandran, K.S.R.; Zhang, Y.; Xia, Y.; Cao, F.; Koopman, M.; Free, M. Powder metallurgy of titanium—Past, present, and future. Int. Mater. Rev. 2017. [Google Scholar] [CrossRef]
- Danzaki, Y.; Wagatsuma, K.; Syoji, T.; Yoshimi, K. Dissolution of molybdenum-silicon (-boron) alloys using a mixture of sulfuric, nitric and hydrofluoric acids and a sequential correction method for ICP-AES analysis. Fresen J. Anal. Chem. 2001, 369, 184–186. [Google Scholar] [CrossRef]
- Chu, F.; Thoma, D.J.; McClellan, K.; Peralta, P.; He, Y. Synthesis and properties of Mo5Si3 single crystals. Intermetallics 1999, 7, 611–620. [Google Scholar] [CrossRef]
- Boulos, M.I. The role of transport phenomena and modeling in the development of thermal plasma technology. Plasma Chem. Plasma Process. 2016, 36, 3–28. [Google Scholar] [CrossRef]
- Leparoux, M.; Loher, M.; Schreuders, C.; Siegmann, S. Neural network modelling of the inductively coupled RF plasma synthesis of silicon nanoparticles. Powder Technol. 2008, 185, 109–115. [Google Scholar] [CrossRef]
- Tong, J.B.; Lu, X.; Liu, C.C.; Pi, Z.Q.; Zhang, R.J.; Qu, X.H. Numerical simulation and prediction of radio frequency inductively coupled plasma spheroidization. Appl. Therm. Eng. 2016, 100, 1198–1206. [Google Scholar] [CrossRef]
- Chakrabarti, O.; Weisensel, L.; Sieber, H. Reactive melt infiltration processing of biomorphic Si–Mo–C ceramics from wood. J. Am. Ceram. Soc. 2005, 88, 1792–1798. [Google Scholar] [CrossRef]
- Xu, L.; Huang, C.; Liu, H.; Zou, B.; Zhu, H.; Zhao, G.; Wang, J. Study on in-situ synthesis of ZrB2 whiskers in ZrB2–ZrC matrix powder for ceramic cutting tools. Int. J. Refract. Met. Hard Mater. 2013, 37, 98–105. [Google Scholar] [CrossRef]
- Su, Y.; Guo, J.; Jia, J.; Liu, G.; L, Y. Composition control of a TiAl melt during the induction skull melting (ISM) process. J. Alloy. Compd. 2002, 334, 261–266. [Google Scholar]
- Liu, Y.; Shao, G.; Tsakiropoulos, P. Thermodynamic reassessment of the Mo-Si and Al-Mo-Si system. Intermetallics 2000, 8, 953–962. [Google Scholar] [CrossRef]




| Sample | Power (kW) | Mo (wt%) | Si (wt%) |
|---|---|---|---|
| Ingot | - | 85.17 (0.06) | 14.83 (0.04) |
| Powders after Spheroidizing | 3 | 85.70 (0.05) | 14.30 (0.03) |
| 4 | 85.91 (0.04) | 14.09 (0.02) | |
| 5 | 86.54 (0.05) | 13.46 (0.03) | |
| 6 | 87.45 (0.06) | 12.55 (0.04) | |
| 7 | 88.85 (0.05) | 11.15 (0.03) |
| Samples | Oxygen (wt%) |
|---|---|
| Ingot | 0.003 (0.001) |
| Powders after Milling | 0.172 (0.002) |
| Powders after Spheroidizing (6 kW) | 0.016 (0.001) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.-W.; Park, J.M.; Choe, B.H.; Lee, S.; Park, J.H.; Park, K.B.; Kim, H.K.; Na, T.-W.; Seo, B.; Park, H.-K. Preparation of Spherical Mo5Si3 Powder by Inductively Coupled Thermal Plasma Treatment. Metals 2018, 8, 604. https://doi.org/10.3390/met8080604
Kang J-W, Park JM, Choe BH, Lee S, Park JH, Park KB, Kim HK, Na T-W, Seo B, Park H-K. Preparation of Spherical Mo5Si3 Powder by Inductively Coupled Thermal Plasma Treatment. Metals. 2018; 8(8):604. https://doi.org/10.3390/met8080604
Chicago/Turabian StyleKang, Jang-Won, Jong Min Park, Byung Hak Choe, Seong Lee, Jung Hyo Park, Ki Beom Park, Hyo Kyu Kim, Tae-Wook Na, Bosung Seo, and Hyung-Ki Park. 2018. "Preparation of Spherical Mo5Si3 Powder by Inductively Coupled Thermal Plasma Treatment" Metals 8, no. 8: 604. https://doi.org/10.3390/met8080604





