Tantalum and Niobium Selective Extraction by Alkyl-Acetophenone
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Hydrodynamic Properties of 4-MAcPh
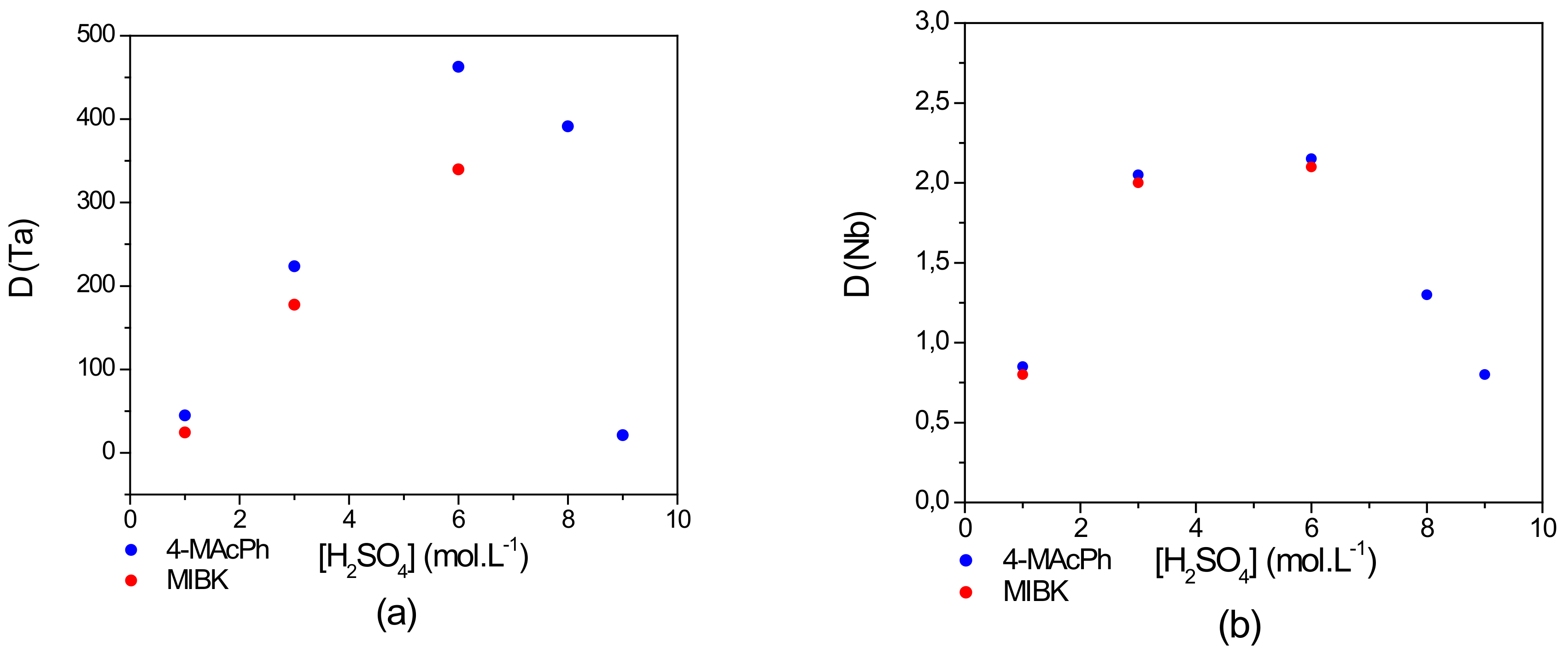
3.2. Grouped and Sequential Extraction of Ta and Nb: Comparison between MAcPh and MIBK
3.3. Extraction Isotherm and McCabe−Thiele Diagram Loading Capacity of 4-MAcPh for Ta
3.4. Stripping of Ta from Loaded 4-MAcPh
3.5. Selective Recovery of Ta from a Model Solution of Capacitor Waste Containing Fe, Mn and Ni as Impurities
4. Discussion and Conclusions
- 4-MAcPh presents adapted intrinsic physicochemical properties for its use in the liquid-liquid extraction process. Indeed, its solubility highlights a low loss in the aqueous phase (0.2 wt%) which is much less than that of the commonly-used MIBK (about 2 wt%). The density of pure 4-MAcPh (0.99997 g∙cm−3) did not cause settler difficulties during our tests due to the significant difference with the sulfuric acid aqueous phase loaded with the metals. Pure 4-MAcPh gives an interfacial tension (IFT) of 21.3 ± 0.4 mN∙m−1 in equilibrium with an aqueous phase composed of 0.4 mol∙L−1 of HF. 6 mol∙L−1 of H2SO4 and 6.6 g∙L−1 of Ta. With these 21.3 mN∙m−1, we need more stirring energy than that for TBP 30% to create an emulsion. The viscosity of pure 4-MAcPh is of the same order of magnitude as those published for TBP and DHOA [27]. It increases slightly when loaded with Ta. This increase has no significant influence on the PST.
- From the results of the comparison between MIBK and MAcPh with respect to Ta and Nb extraction, it was concluded that there is a similar extraction tendency for both Ta and Nb. The D values of Nb remain nearly similar from 1–6 mol∙L−1 of but those of Ta are higher for the 4-MAcPh at 6 mol∙L−1 of . In addition, MIBK is completely solubilized from 6 mol∙L−1 of while only a loss of 0.14–4 wt% is observed with 4-MAcPh between 6 and 9 mol∙L−1 of . In terms of stability and efficiency, 4-MAcPh appears to be more suitable for the extraction of Ta and Nb from a solution composed of 0.06 mol∙L−1 of HF and 1–9 mol∙L−1 of . The decrease of the D value of Ta between 6 and 9 mol∙L−1 of in the case of MAcPh could be due to the carbonyl protonation providing the enolization of the compound as demonstrated by Cox et al. [28].
- From the results of the McCabe−Thiele diagram for Ta extraction, it can be concluded that for a flux composed of a feed containing 7 g∙L−1 of Ta and an organic phase leaving the process with 100 g∙L−1 of Ta, a flow ratio of 14 and two stages are required for a process yield of 99.9%, i.e., a raffinate composed of 0.01 g∙L−1 of Ta.
- From the results of the Ta stripping, it can be concluded that the ammonium oxalate (0.2 or 0.3 mol∙L−1) is adapted to recover Ta from loaded 4-MAcPh quantitatively.
- In regards to MIBK, the price of 4-MAcPh should be a drawback, but due to similar efficiencies without problems concerning safety issues (fire and explosion hazards and readily soluble in aqueous solutions), it appears that 4-MAcPh is a good alternative. Furthermore, the loss in the aqueous phase is low, and considering that the stripping step allows reusing the solvent, this reduces the impact of the price.
- From the results of the selective extraction of Ta from the simulated leaching solution of capacitor waste, it was concluded that the MAcPh is adapted with a separation factor of 120 for Ta with respect to Fe, Ni, Mn and Ag.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Monier, V.; Escalon, V.; Cassowitz, L.; Massari, F.; Deprouw, A. Etude du Potentiel de Recyclage de Certains Métaux Rares; ADEME: Angers, France, 2010. [Google Scholar]
- Polak, C. Métallurgie et Recyclage du Niobium et du Tantale; Techniques de l’ingénieur: Saint-Denis, France, 2012. [Google Scholar]
- Arrachart, G.; Duhamet, J.; Pellet-Rostaing, S.; Toure, M.; Turgis, R. Method for Extracting Tantalum and/or Niobium from an Acidic Aqueous Phase. WO Patent 2015193314A1, 23 December 2015. [Google Scholar]
- Singh, R.P. Processing of Ta2O5 Powders for electronic application. J. Electron. Mater. 2001, 30, 1584–1594. [Google Scholar] [CrossRef]
- Zhu, Z.; Cheng, C.Y. Solvent extraction technology for the separation and purification of niobium and tantalum. A review. Hydrometallurgy 2011, 107, 1–12. [Google Scholar] [CrossRef]
- Gibalo, I.M. Analytical Chemistry of Niobium and Tantalum; Ann Arbor-Humphery Science Publishers: Ann Arbor, MI, USA, 1970; ISBN 9780250399246. [Google Scholar]
- Agulyansky, A. The Chemistry of Tantalum and Niobium Fluoride Compounds; Elsevier: New York, NY, USA, 2004; ISBN 0-444-51604-2. [Google Scholar]
- Varga, L.P.; Wakley, W.D.; Nicholson, L.S.; Madden, M.L.; Patterson, J. Solvent extraction studies of tantalum fluoride complexes with N-Benzoylphenylhydroxylamine tri-n-octylphosphine oxide and methyl isobutyl ketone using computer techniques. Anal. Chem. 1965, 37, 1003–1009. [Google Scholar] [CrossRef]
- Agulyansky, A.; Agulyansky, L.; Travkin, V.F. Liquid-liquid extraction of tantalum with 2-octanol. Chem. Eng. Process. 2004, 43, 1231–1237. [Google Scholar] [CrossRef]
- Niwa, K.; Ichikawa, I. Method of Purifying Tantalum. U.S. Patent 4673554A, 16 June 1987. [Google Scholar]
- Sanda, O.; Taiwo, E. Solvent extraction of tantalum(V) from aqueous sulphate/fluoride solution using trioctyl phosphine oxide in MIBK. Hydrometallurgy 2012, 127, 168–171. [Google Scholar] [CrossRef]
- Agrawal, Y.K. Liquid/liquid extraction separation recovery and transport of tantalum by crown-ether. Talanta 2002, 58, 875–882. [Google Scholar] [CrossRef]
- Gupta, C.K.; Suri, A.K. Niobium and Tantalum Separation Process. Extractive Metallurgy of Niobium; CRC Press Inc.: Boca Raton, FL, USA, 1994; ISBN 0-8493-6071-4. [Google Scholar]
- Werning, J.R.; Higbie, K.B.; Grace, J.T.; Speece, B.F.; Gilbert, H.L. Separation of Tantalum and Niobium by Liquid-Liquid Extraction. Ind. Eng. Chem. 1954, 46, 644–652. [Google Scholar] [CrossRef]
- Ellenburg, J.Y.; Leddicotte, G.W.; Moore, F.L. Separation of Tantalum and Niobium by Liquid-Liquid Extraction. Anal. Chem. 1953, 26, 1045–1047. [Google Scholar] [CrossRef]
- Yang, X.L.; Wang, X.H.; Wei, C.; Zheng, S.L.; Sun, Q.; Wang, D. Extraction kinetics of tantalum by MIBK from pulp using∙Lewis cell. Hydrometallurgy 2013, 131, 34–39. [Google Scholar] [CrossRef]
- Taili, Z.; Xiang, Z.; Rongjun, M.; Zhuoshu, H.; Ming, Q.; Zhonghua, Z. The amide type extractant A101 and its application to the separation of niobium and tantalum. And molybdenum and rhenium. Hydrometallurgy 1982, 8, 379–388. [Google Scholar] [CrossRef]
- Ohmori, H.; Shibata, J.; Nishimura, S.; Sano, M. Extraction of niobium and tantalum with bis-2-ethylhexyl acetamide. Solvent Extr. Ion Exch. 1987, 5, 227–243. [Google Scholar] [CrossRef]
- Bhattacharyya, S.N.; Ganguly, B. Solvent extraction separation of niobium and tantalum. Solvent Extr. Ion Exch. 1984, 2, 699–740. [Google Scholar] [CrossRef]
- Djordjevic, C.; Gorican, H.; Tan, S.L. Solvent extraction of niobium and tantalum: 3. Extraction mechanism in oxalic solutions with long chain tertiary amines. J. Less-Common Met. 1966, 11, 342–350. [Google Scholar] [CrossRef]
- Bludssus, W.; Reichert, K.; Bohmke, U. Process for Removing Antimony from Hydrofluoric Acid Solutions which Contain Ta/Nb. U.S. Patent 5908489A, 1 June 1999. [Google Scholar]
- Babkin, A.G.; Majorov, V.G.; Nikolaev, A.I.; Zolotov, Y.A. Solvent Extraction of Niobium, Tantalum and other Elements from Fluoride Solutions; AN SSSR, Apatity Nauka: Leningrad, Russia, 1988. [Google Scholar]
- Kassikova, N.I.; Kassikova, A.G.; Balabanov, Y.I.; Petrov, V.B.; Kalinnikov, V.T. Niobium, tantalum and titatnium extraction from natural and technogenic raw materials of the kola peninsula by liquid-liquid extraction methods. In Proceedings of the 3rd Balkan Metallurgical Conference (BMC), Ohrid, Republic of Macedonia, 24–27 September 2003; pp. 64–68. [Google Scholar]
- Gibala, I.M.; Albadri, J.S. Niobium and tantalum extraction from hydrochloric acid solution using benzaldehyde and acetophenone. Vestn. Mosk. Univ. Ser. II. Khim. 1969, 2, 98–101. [Google Scholar]
- Freeman, Y. Tantalum and Niobium-Based Capacitors; Springer: Manhattan, NY, USA, 2018; ISBN 978-3-319-67869-6. [Google Scholar]
- FIRADEC. Available online: http://banelec.online.fr/fab/firadec/cg2005.pdf (accessed on 10 July 2018).
- Pathak, P.N.; Kanekar, A.S.; Prabhu, D.R.; Manchanda, V.K. Comparison of Hydrometallurgical Parameters of N,N-Dialkylamides and of Tri-n-Butylphosphate. Solvent Extr. Ion Exch. 2009, 27, 683–694. [Google Scholar] [CrossRef]
- Cox, R.A.; Smith, C.R.; Yates, K. Excess acidity method—Basicities, and rates and mechanisms of enolization, of some acetophenones and acetone, in moderately concentrated sulfuric-acid. Can. J. Chem. 1979, 59, 2952–2958. [Google Scholar] [CrossRef]





| Mineral | General Formula | ||
|---|---|---|---|
| Tantalite | (Fe,Mn)(Ta,Nb)2O6 | 40–80 | 2–30 |
| Columbite | (Fe,Mn)(Ta,Nb)2O6 | 1–40 | 30–75 |
| Wodginite | (Ta,Nb,Sn,Mn,Fe,Ti,Mn)16O32 | 45–70 | 1–15 |
| Microlite | (Ca,Na)2, (Ta,Nb)2(O,OH,F)7 | 50–79 | 1–10 |
| Stueverite | (Fe,Mn)(Ta,Nb,Ti)2O6 | 5–26 | 7–17 |
| Euxenite | (Y,Ca,Ce,U,Th)(Ta,Nb,Ti)2O6 | 2–12 | 22–30 |
| Samarskite | (Fe,Ca,U,Y,Ce)2(Ta,Nb)2O6 | 15–30 | 49–55 |
| Molecular Weight (g∙mol−1) | 134.18 |
| Density at 20 °C (g∙cm−3) | 0.99997 ± 0.00002 |
| Dynamic viscosity at 20 °C (MPa∙s) | 1.735 ± 0.003 |
| Solubility in water (wt%) | 0.197 ± 0.001 |
| Interfacial tension (mN∙m−1) | 21.3 ± 0.4 * |
| A/O | [Ta]aqueous (g∙L−1) | [Ta]organic (g∙L−1) | D |
|---|---|---|---|
| 1 | 0.02 | 13.98 | 570.45 |
| 3 | 0.04 | 20.93 | 572.58 |
| 4 | 0.03 | 27.93 | 950.89 |
| 5 | 0.06 | 34.79 | 627.61 |
| 6 | 0.09 | 41.52 | 445.57 |
| 7 | 0.11 | 48.31 | 436.93 |
| 30 | 2.16 | 142.27 | 65.99 |
| 60 | 4.38 | 151.37 | 34.60 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toure, M.; Arrachart, G.; Duhamet, J.; Pellet-Rostaing, S. Tantalum and Niobium Selective Extraction by Alkyl-Acetophenone. Metals 2018, 8, 654. https://doi.org/10.3390/met8090654
Toure M, Arrachart G, Duhamet J, Pellet-Rostaing S. Tantalum and Niobium Selective Extraction by Alkyl-Acetophenone. Metals. 2018; 8(9):654. https://doi.org/10.3390/met8090654
Chicago/Turabian StyleToure, Moussa, Guilhem Arrachart, Jean Duhamet, and Stephane Pellet-Rostaing. 2018. "Tantalum and Niobium Selective Extraction by Alkyl-Acetophenone" Metals 8, no. 9: 654. https://doi.org/10.3390/met8090654
APA StyleToure, M., Arrachart, G., Duhamet, J., & Pellet-Rostaing, S. (2018). Tantalum and Niobium Selective Extraction by Alkyl-Acetophenone. Metals, 8(9), 654. https://doi.org/10.3390/met8090654






