Effect of Negative Current on the Microstructure of Oxide Coatings Prepared by Hybrid Pulse Anodization
Abstract
:1. Introduction
2. Materials and Methods
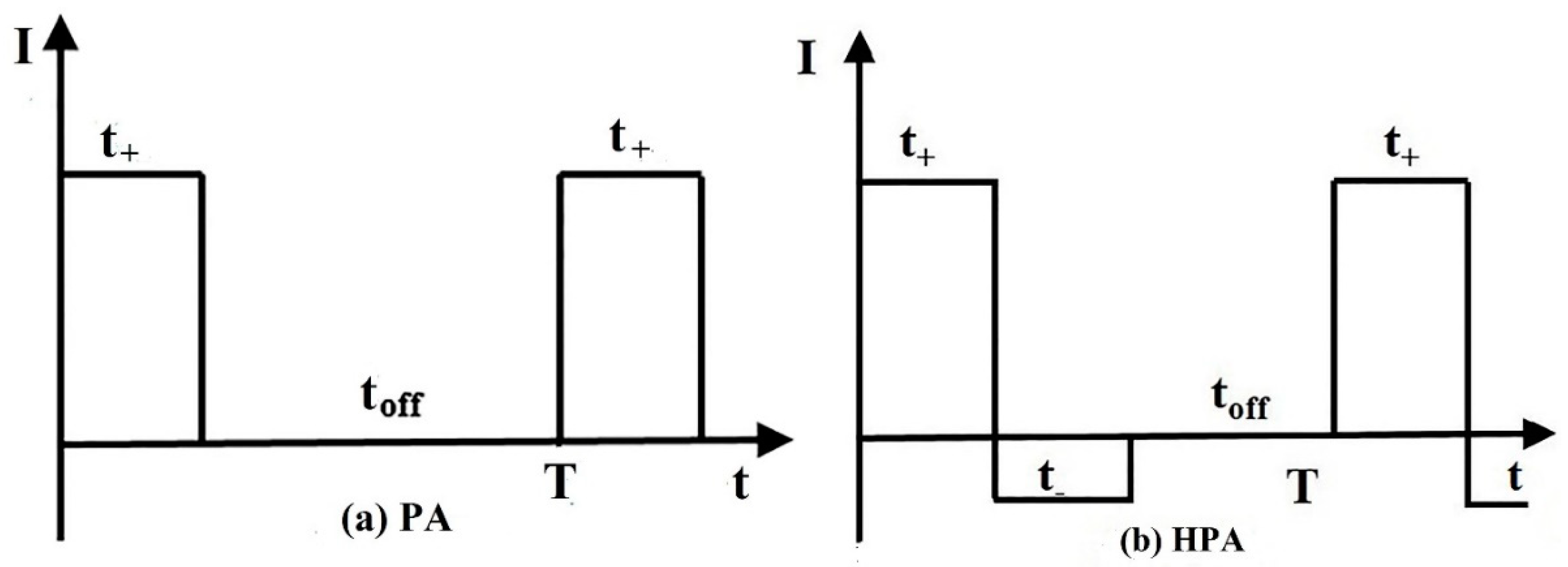
2.1. HPA Process
2.2. Characterization
3. Results
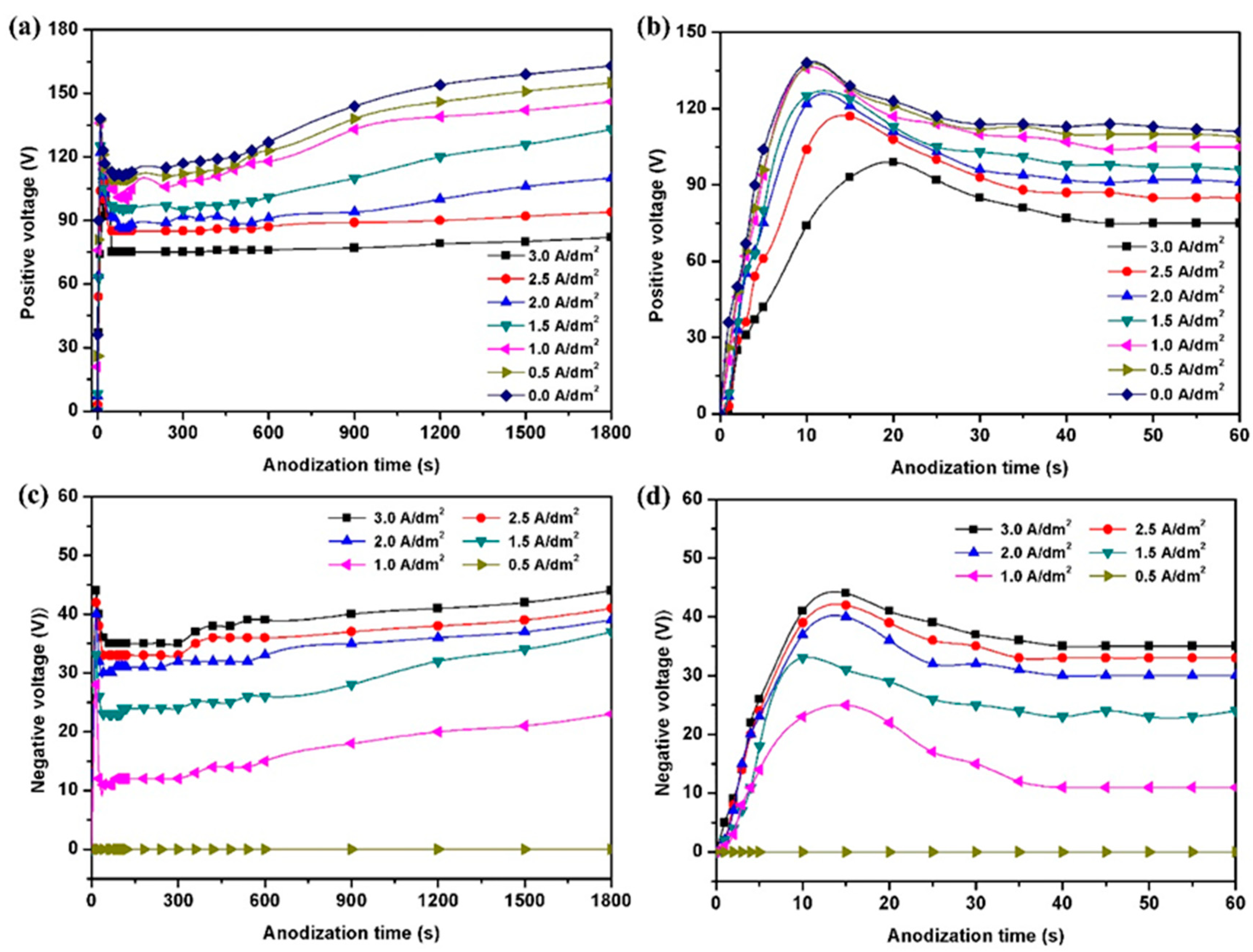
3.1. Effect of Negative Current on Anodization Voltage
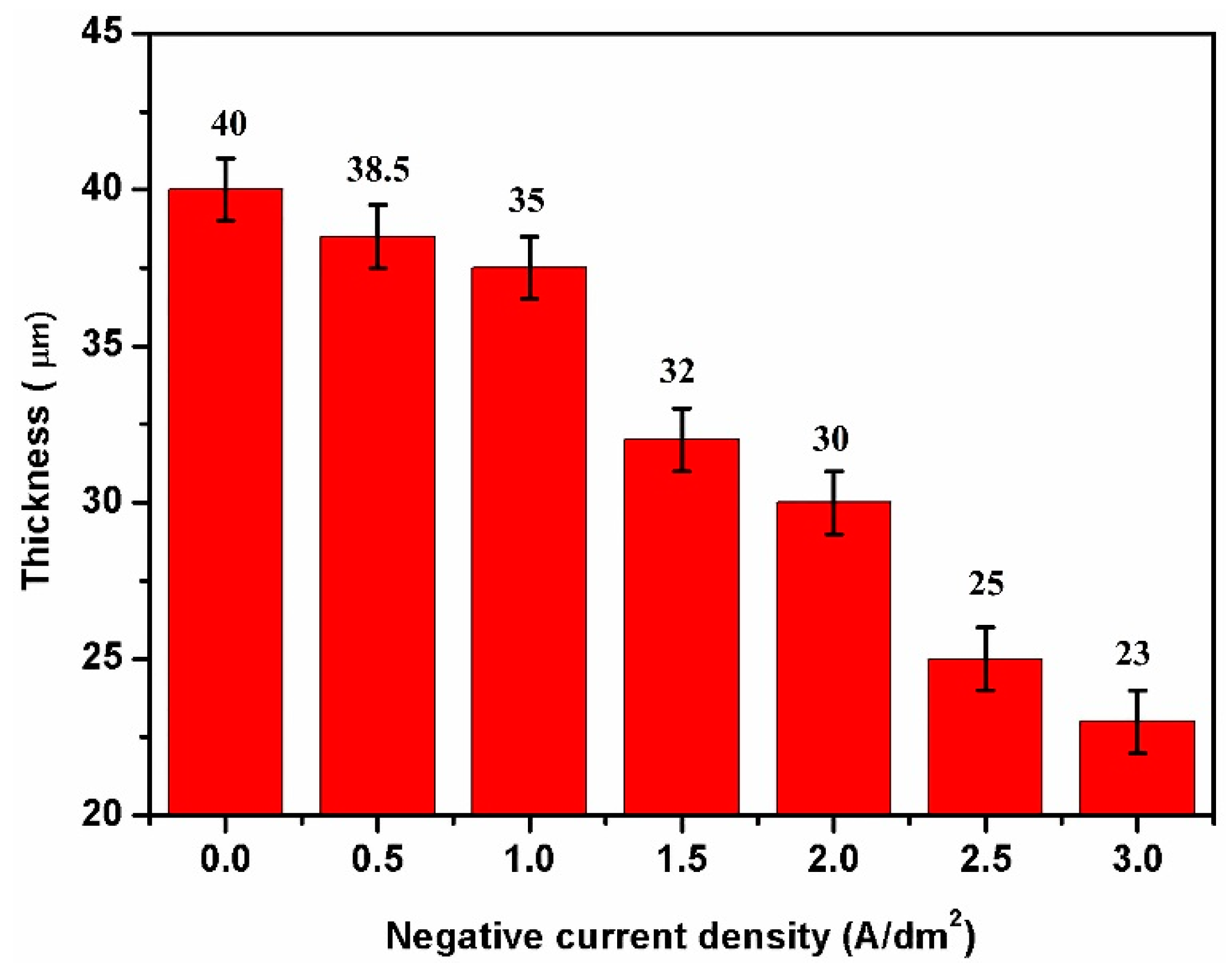
3.2. Effect of Negative Current on Microstructures of HPA Coatings
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, Y.; Lee, S.; Cho, H.; Park, B.; Kim, D.; Hwang, W. Robust superhydrophilic/hydrophobic surface based on self-aggregated Al2O3 nanowires by single-step anodization and self-assembly method. ACS Appl. Mater. Interfaces 2012, 4, 5074–5078. [Google Scholar] [CrossRef] [PubMed]
- Sriram, G.; Patil, P.; Bhat, M.P.; Hegde, R. Current trends in nanoporous anodized alumina platforms for biosensing applications. J. Nanomater. 2016, 2016, 1–24. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, X.; Zhao, M.; Ma, Q.; Chen, J.; Chen, B.; Sindoro, M.; Yang, J.; Han, S.; Lu, Q.; et al. Anodized aluminum oxide templated synthesis of metal-organic frameworks used as membrane reactors. Angew. Chem. Int. Ed. 2017, 56, 578–581. [Google Scholar] [CrossRef]
- Liu, C.; Gillette, E.I.; Chen, X.; Pearse, A.J.; Kozen, A.C.; Schroeder, M.A.; Gregorczyk, K.E.; Lee, S.B.; Rubloff, G.W. An all-in-one nanopore battery array. Nat. Nanotechnol. 2014, 9, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Liao, M.W.; Chung, C.K. Growth of porous anodized alumina on the sputtered aluminum films with 2D–3D morphology for high specific surface area. Appl. Surf. Sci. 2014, 309, 290–294. [Google Scholar] [CrossRef]
- Bensalah, W.; Feki, M.; Wery, M.; Ayedi, H.F. Thick and dense anodic oxide layers formed on aluminum in sulphuric acid bath. J. Mater. Sci. Technol. 2010, 26, 113–118. [Google Scholar] [CrossRef]
- Veys-Renaux, D.; Chahboun, N.; Rocca, E. Anodizing of multiphase aluminium alloys in sulfuric acid: In-situ electrochemical behaviour and oxide properties. Electrochim. Acta 2016, 211, 1056–1065. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, K.G.; Gao, Z.Y.; Junjun, W.U.; Ren, J.Y. A kind of double-sided porous anodic alumina membrane fabricated with the three-step anodic oxidation method. Sci. China Technol. Sci. 2014, 57, 293–297. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Nowak-Stępniowska, A.; Bojar, Z. Quantitative arrangement analysis of anodic alumina formed by short anodizations in oxalic acid. Mater. Charact. 2013, 78, 79–86. [Google Scholar] [CrossRef]
- Zhang, R.; Jiang, K.; Zhu, Y.; Qi, H.; Ding, G. Ultrasound-assisted anodization of aluminum in oxalic acid. Appl. Surf. Sci. 2011, 258, 586–589. [Google Scholar] [CrossRef]
- Sanchez, A.G.; Schreiner, W.; Ballarre, J.; Cisilino, A.; Duffo, G.; Cere, S. Surface modification of titanium by anodic oxidation in phosphoric acid at low potentials. Part 2. In vitro and in vivo study. Surf. Interface Anal. 2013, 45, 1395–1401. [Google Scholar] [CrossRef]
- Elabar, D.; Hashimoto, T.; Qi, J.; Skeldon, P.; Thompson, G.E. Effect of low levels of sulphate on the current density and film morphology during anodizing of aluminium in chromic acid. Electrochim. Acta 2016, 196, 206–222. [Google Scholar] [CrossRef]
- Songjiang, M.; Peng, L.; Haihui, Z.; Chaopeng, F.; Yafei, K. Preparation of anodic films on 2024 aluminum alloy in boric acid-containing mixed electrolyte. Trans. Nonferr. Met. Soc. China 2008, 18, 825–830. [Google Scholar]
- Shih, H.; Tzou, S. Study of anodic oxidation of aluminum in mixed acid using a pulsed current. Surf. Coat. Technol. 2000, 124, 278–285. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Li, Y.; Ma, Y.; Chen, L. Self-organization process of aluminum oxide during hard anodization. Electrochim. Acta 2016, 213, 14–20. [Google Scholar] [CrossRef]
- Pashchanka, M.; Schneider, J.J. Origin of self-organisation in porous anodic alumina films derived from analogy with Rayleigh–Bénard convection cells. J. Mater. Chem. 2011, 21, 18761–18767. [Google Scholar] [CrossRef]
- Bai, A.; Hu, C.; Yang, Y.; Lin, C. Pore diameter control of anodic aluminum oxide with ordered array of nanopores. Electrochim. Acta 2008, 53, 2258–2264. [Google Scholar] [CrossRef]
- Choudhary, R.K.; Mishra, P.; Kain, V.; Singh, K.; Kumar, S.; Chakravartty, J.K. Scratch behavior of aluminum anodized in oxalic acid: Effect of anodizing potential. Surf. Coat. Technol. 2015, 283, 135–147. [Google Scholar] [CrossRef]
- Christoulaki, A.; Dellis, S.; Spiliopoulos, N.; Anastassopoulos, D.L.; Vradis, A.A. Controlling the thickness of electrochemically produced porous alumina membranes: The role of the current density during the anodization. J. Appl. Electrochem. 2014, 44, 701–707. [Google Scholar] [CrossRef]
- Pniowski, W.J.S.; Pniowska, A.N.; Presz, A.; Czujko, T.; Varin, R.A. The effects of time and temperature on the arrangement of anodic aluminum oxide nanopores. Mater. Charact. 2014, 91, 1–9. [Google Scholar] [CrossRef]
- Chung, C.; Liu, T.Y.; Chang, W.T. Effect of oxalic acid concentration on the formation of anodic aluminum oxide using pulse anodization at room temperature. Microsyst. Technol. 2010, 16, 1451–1456. [Google Scholar] [CrossRef]
- Roshani, M.; Sabour Rouhaghdam, A.; Aliofkhazraei, M.; Heydari Astaraee, A. Optimization of mechanical properties for pulsed anodizing of aluminum. Surf. Coat. Technol. 2017, 310, 17–24. [Google Scholar] [CrossRef]
- Bozza, A.; Giovanardi, R.; Manfredini, T.; Mattioli, P. Pulsed current effect on hard anodizing process of 7075-T6 aluminium alloy. Surf. Coat. Technol. 2015, 270, 139–144. [Google Scholar] [CrossRef]
- Chung, C.K.; Chang, W.T.; Liao, M.W.; Chang, H.C.; Lee, C.T. Fabrication of enhanced anodic aluminum oxide performance at room temperatures using hybrid pulse anodization with effective cooling. Elecctrochim. Acta 2011, 56, 6489–6497. [Google Scholar] [CrossRef]
- Chung, C.K.; Chang, W.T.; Liao, M.W.; Chang, H.C. Effect of pulse voltage and aluminum purity on the characteristics of anodic aluminum oxide using hybrid pulse anodization at room temperature. Thin Solid Films 2011, 519, 4754–4758. [Google Scholar] [CrossRef]
- Keller, F.; Hunter, M.S.; Robinson, D.L. Structural features of oxide coatings on aluminum. J. Electrochem. Soc. 1953, 100, 411–419. [Google Scholar] [CrossRef]
- Chung, C.K.; Liao, M.W.; Chang, H.C.; Chang, W.T.; Liu, T.Y. On characteristics of pore size distribution in hybrid pulse anodized high-aspect-ratio aluminum oxide with Taguchi method. Microsyst. Technol. 2013, 19, 387–393. [Google Scholar] [CrossRef]
- Mohammadi, I.; Ahmadi, S.; Afshar, A. Effect of pulse current parameters on the mechanical and corrosion properties of anodized nanoporous aluminum coatings. Mater. Chem. Phys. 2016, 183, 490–498. [Google Scholar] [CrossRef]
- Wang, Y.; Santos, A.; Evdokiou, A.; Losic, D. Rational design of ultra-short anodic alumina nanotubes by short-time pulse anodization. Electrochim. Acta 2015, 154, 379–386. [Google Scholar] [CrossRef]
- Bononi, M.; Giovanardi, R.; Bozza, A. Pulsed current hard anodizing of heat treated aluminum alloys: Frequency and current amplitude influence. Surf. Coat. Technol. 2016, 307, 861–870. [Google Scholar] [CrossRef]
- Mohammadi, I.; Afshar, A. Modification of nanostructured anodized aluminum coatings by pulse current mode. Surf. Coat. Technol. 2015, 278, 48–55. [Google Scholar] [CrossRef]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Jiang, B.; Liu, C.; Shao, Q.; Li, H. Effect of Negative Current on the Microstructure of Oxide Coatings Prepared by Hybrid Pulse Anodization. Metals 2019, 9, 22. https://doi.org/10.3390/met9010022
Huang S, Jiang B, Liu C, Shao Q, Li H. Effect of Negative Current on the Microstructure of Oxide Coatings Prepared by Hybrid Pulse Anodization. Metals. 2019; 9(1):22. https://doi.org/10.3390/met9010022
Chicago/Turabian StyleHuang, Shuo, Bailing Jiang, Cancan Liu, Qingying Shao, and Hongtao Li. 2019. "Effect of Negative Current on the Microstructure of Oxide Coatings Prepared by Hybrid Pulse Anodization" Metals 9, no. 1: 22. https://doi.org/10.3390/met9010022



