Microstructure and Wear Resistance of Ti6Al4V Coating Fabricated by Electro-Spark Deposition
Abstract
:1. Introduction
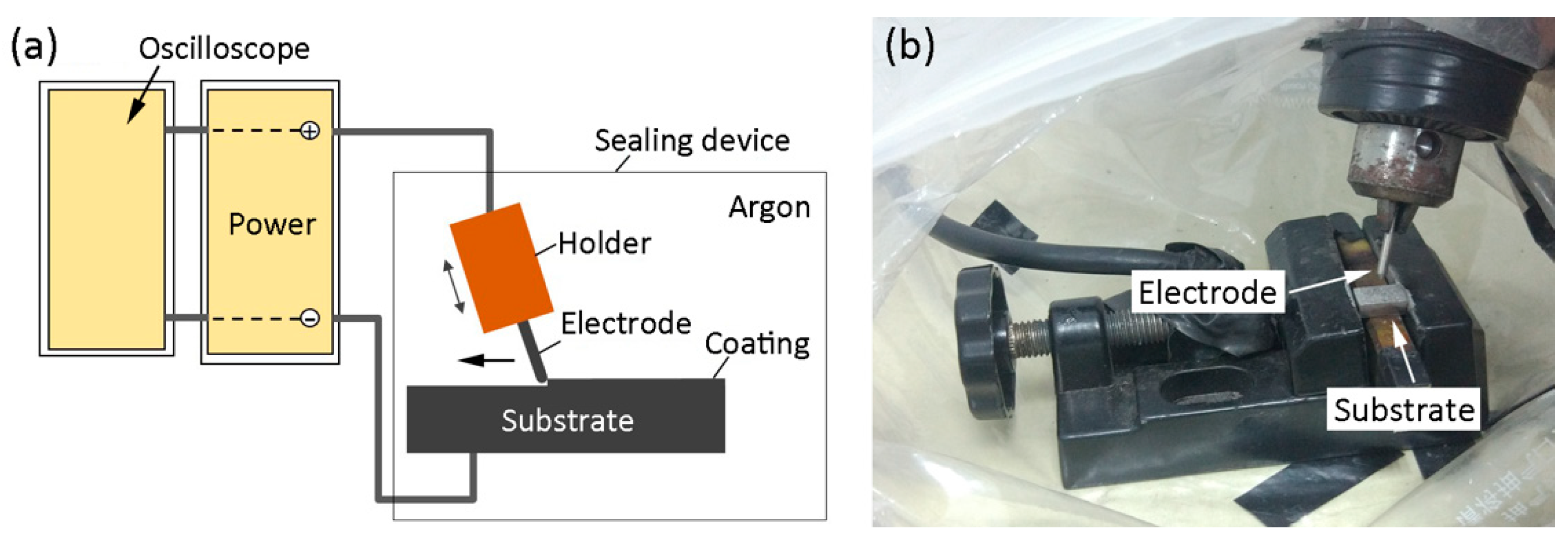
2. Materials and Methods
2.1. Materials
2.2. Microstructural Characterization and Microhardness
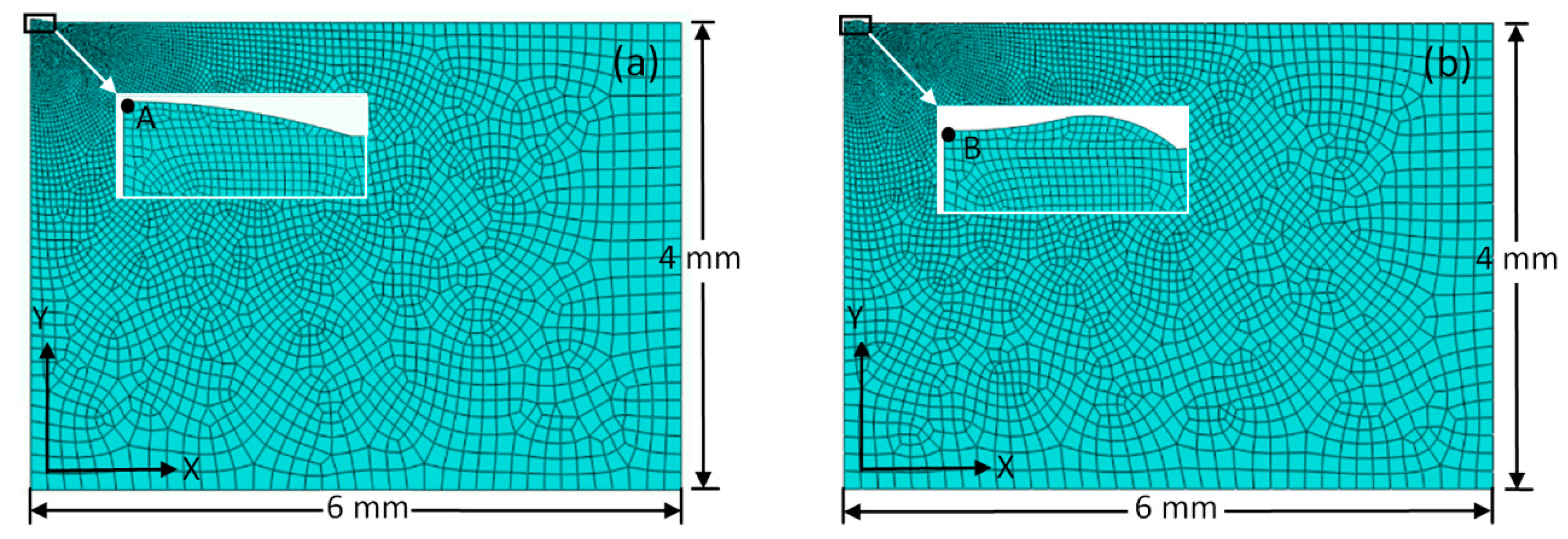
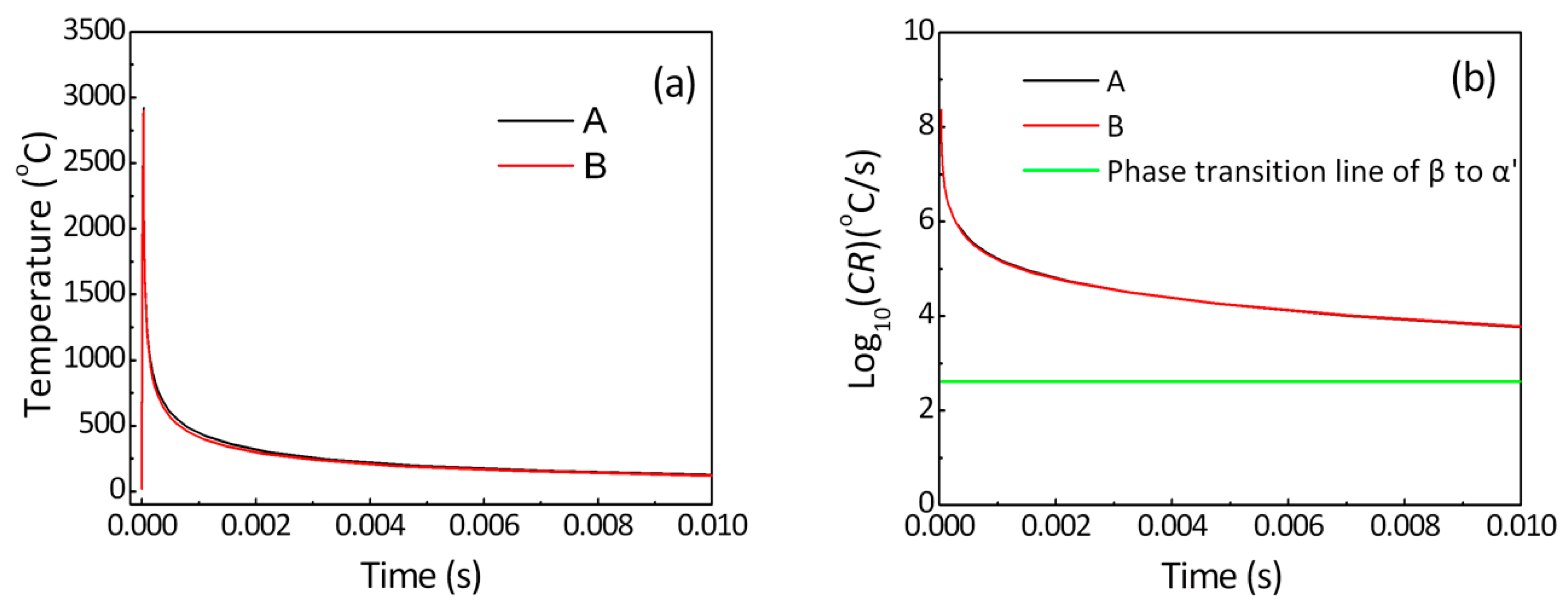
2.3. Simulation
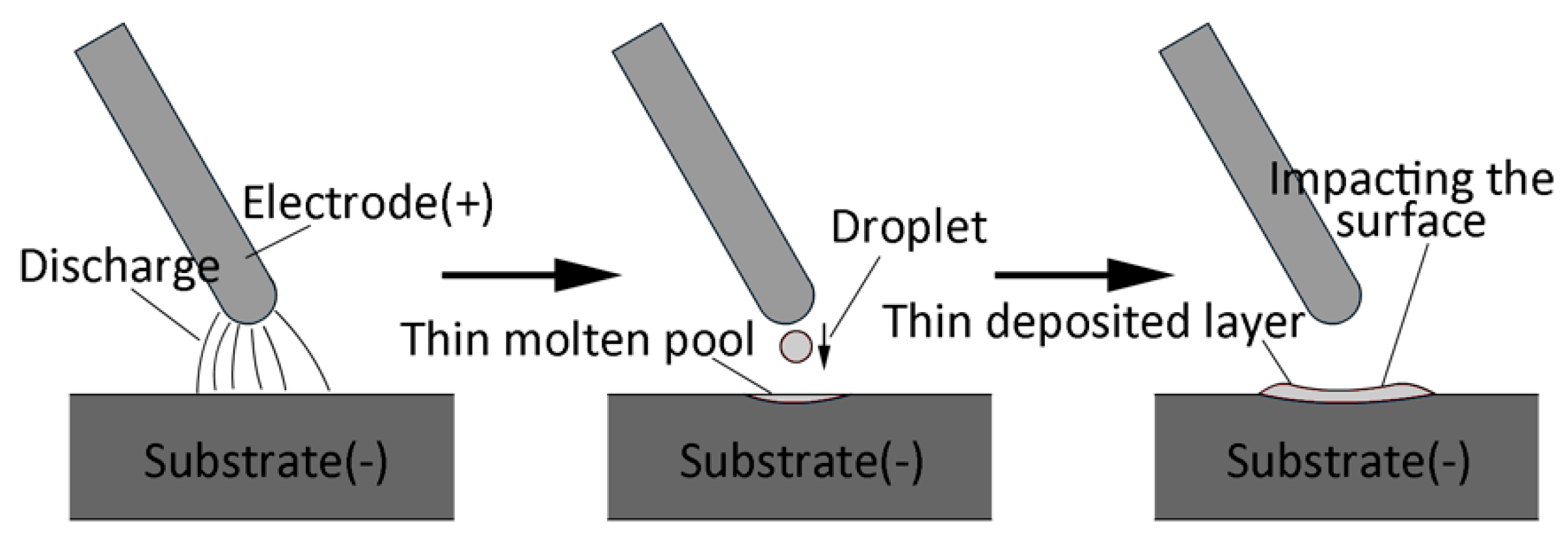
2.3.1. Governing Equation
2.3.2. Initial Conditions and Boundary Conditions
2.3.3. Finite Element Model and Material Properties
- Heat flux of individual electrical discharge had a Gaussian distribution, which was a function of time and space.
- In the heat transfer analysis, heat conduction and convection were considered, while radiation was ignored.
- Material properties were homogeneous on temperature.
2.4. Evaluation of Wear Resistance
3. Results and Discussion
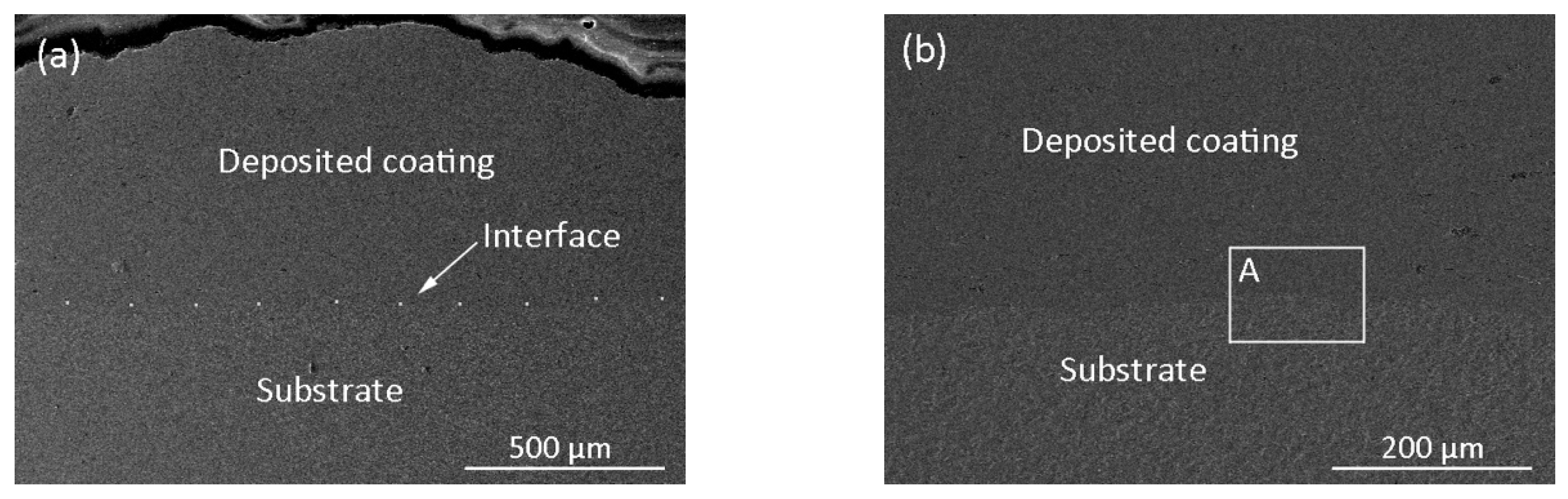
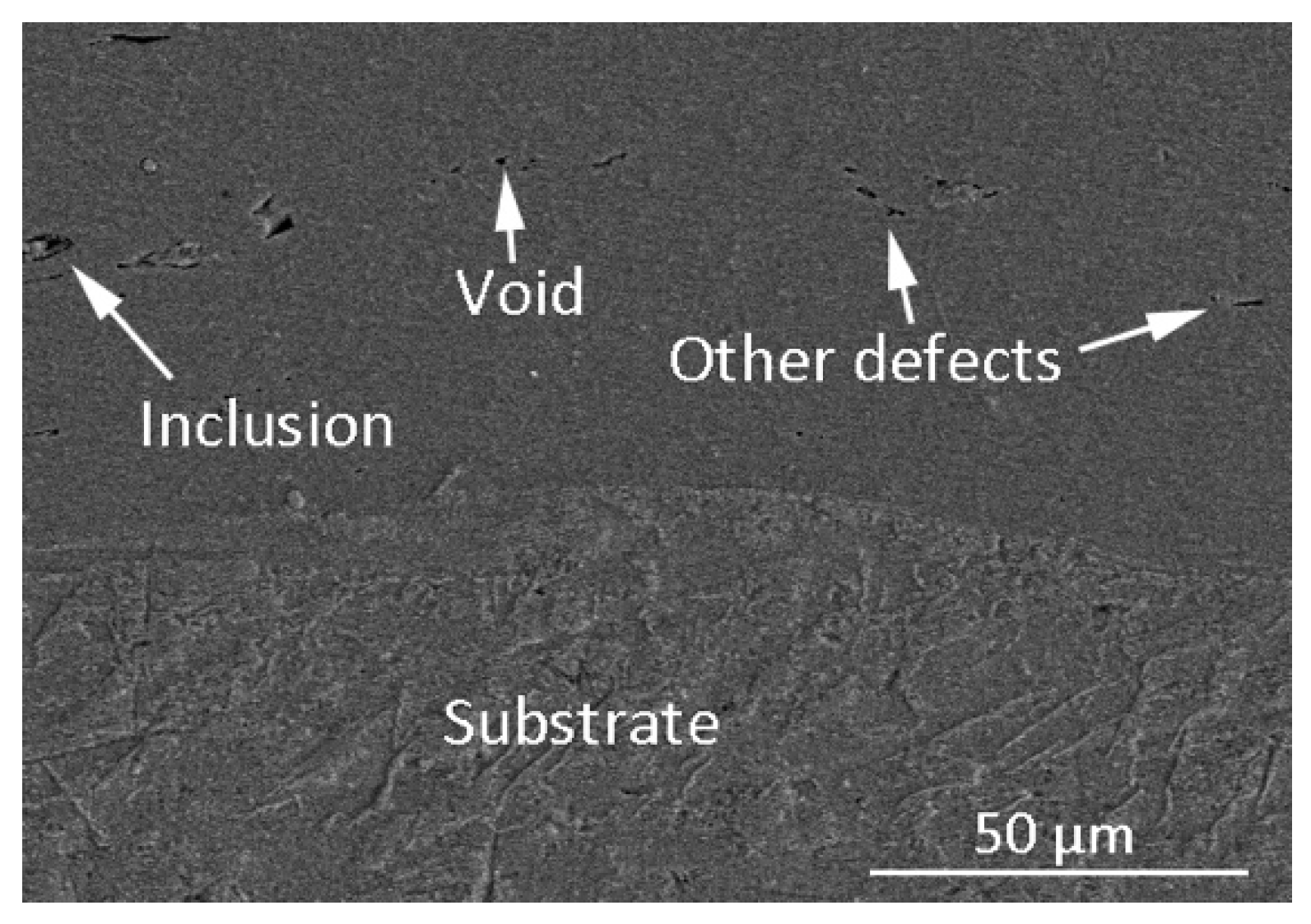
3.1. Cross-Sectional Morphology
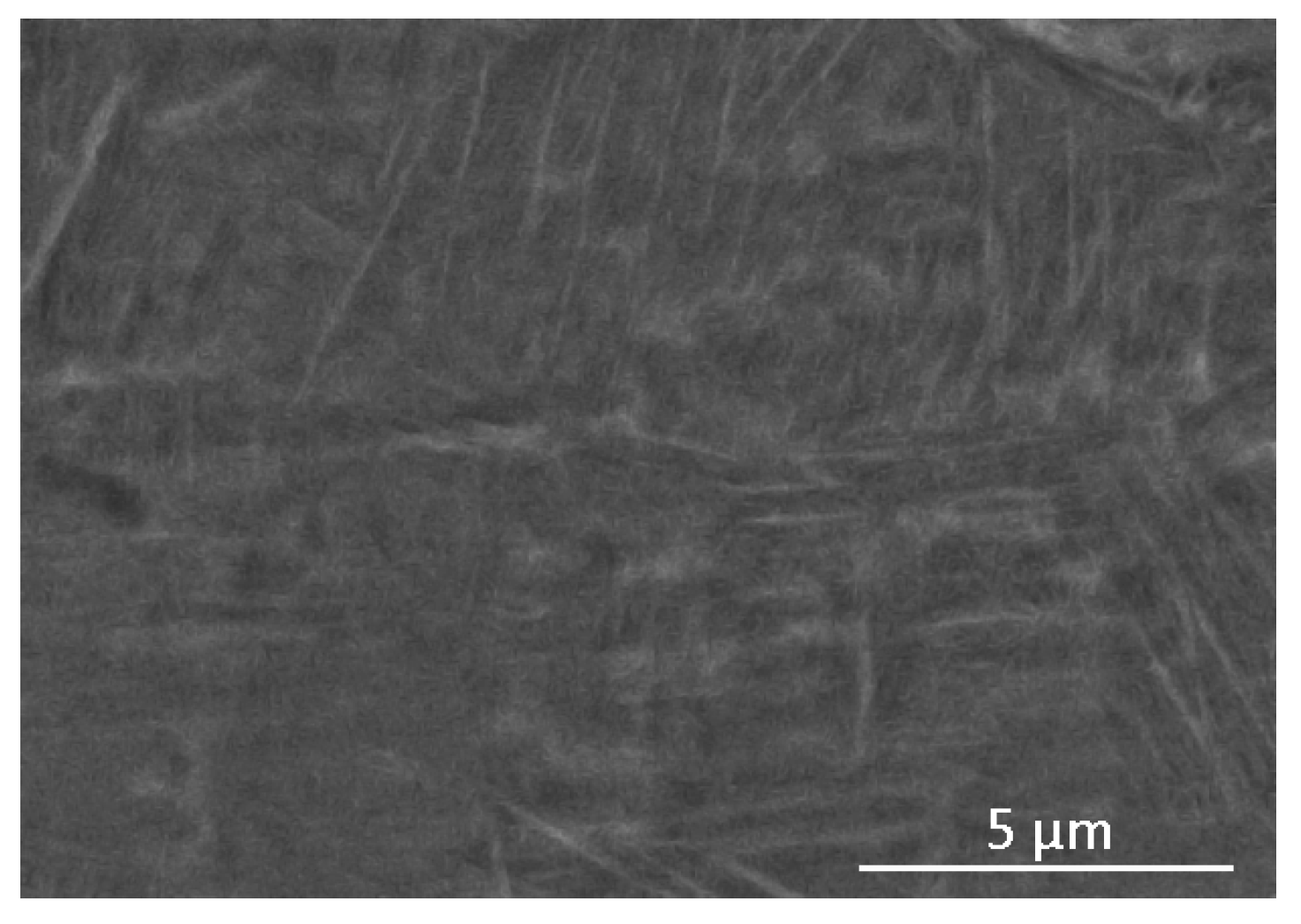
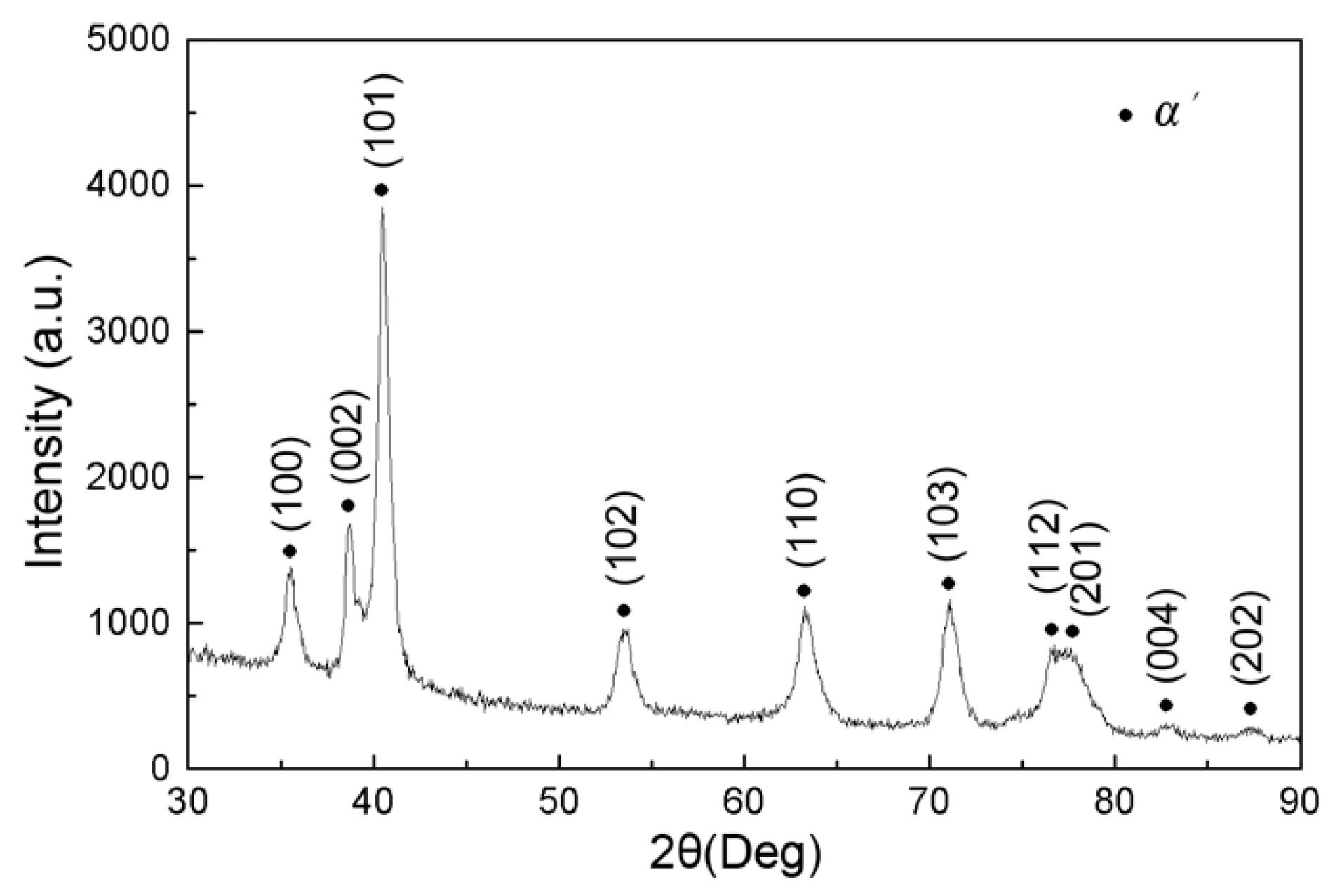
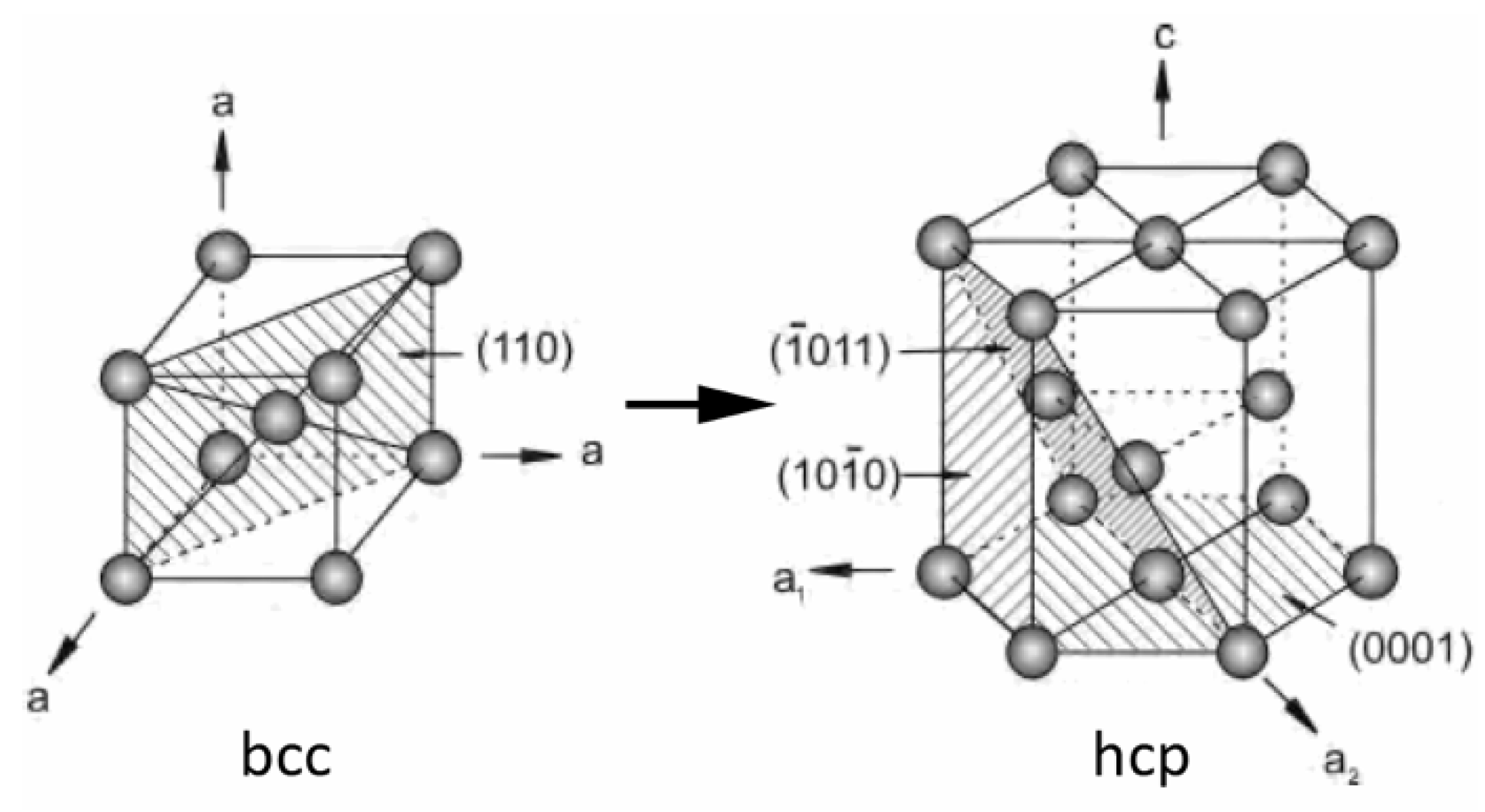
3.2. Microstructure
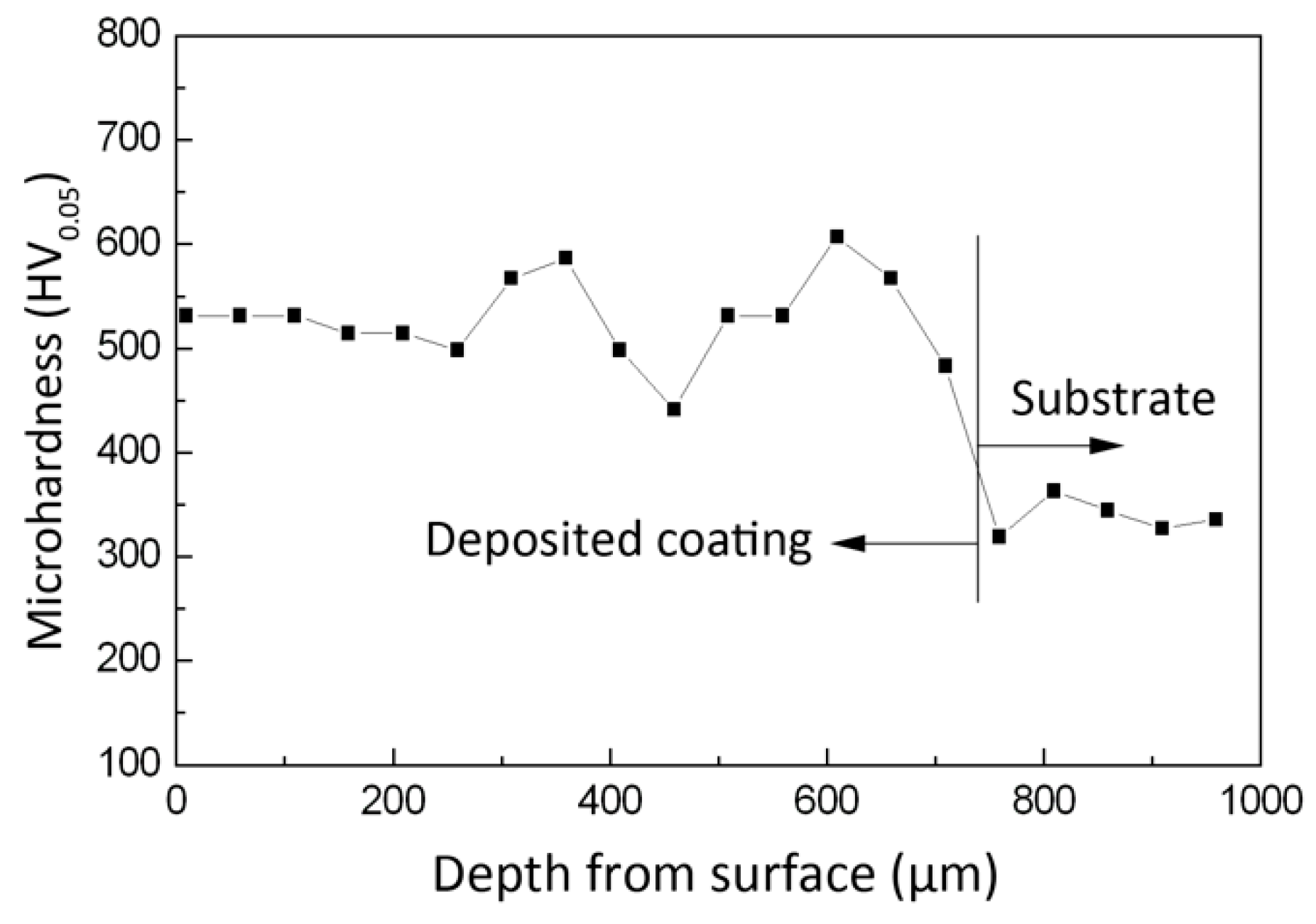
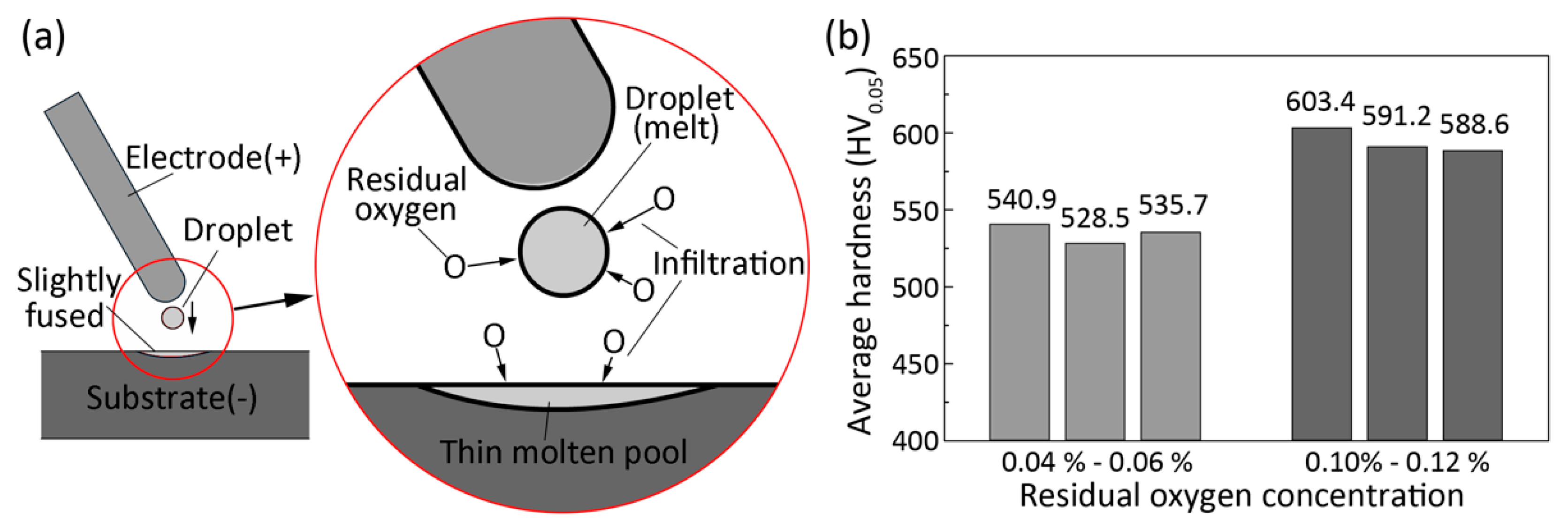
3.3. Microhardness
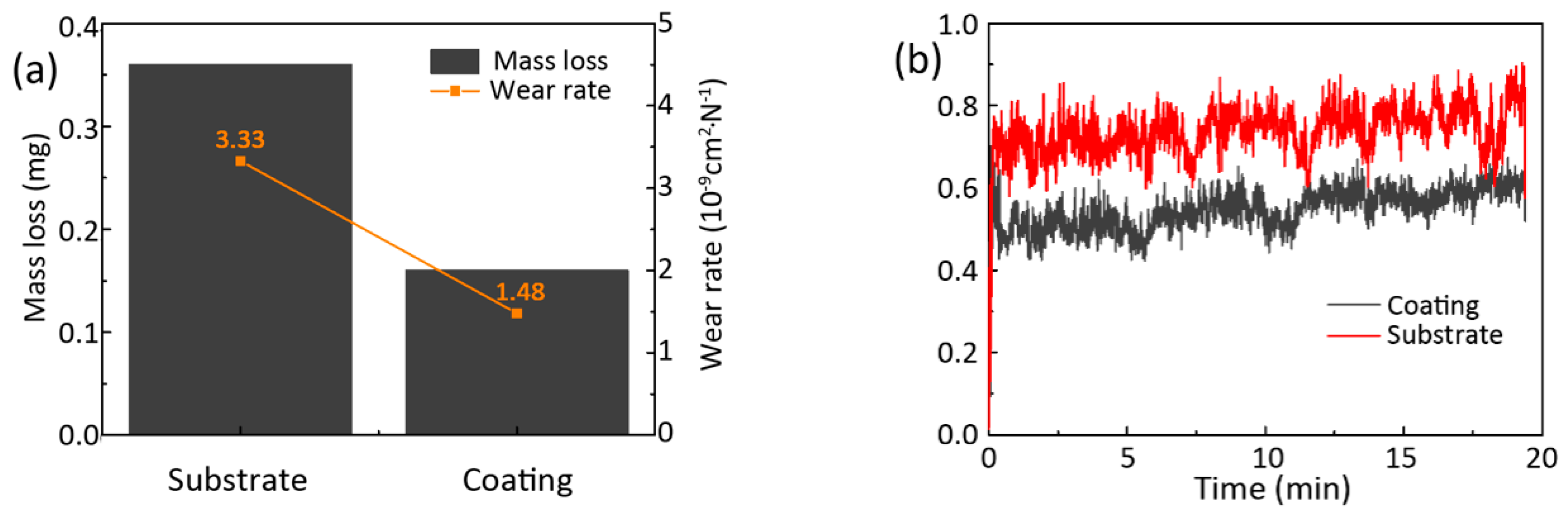
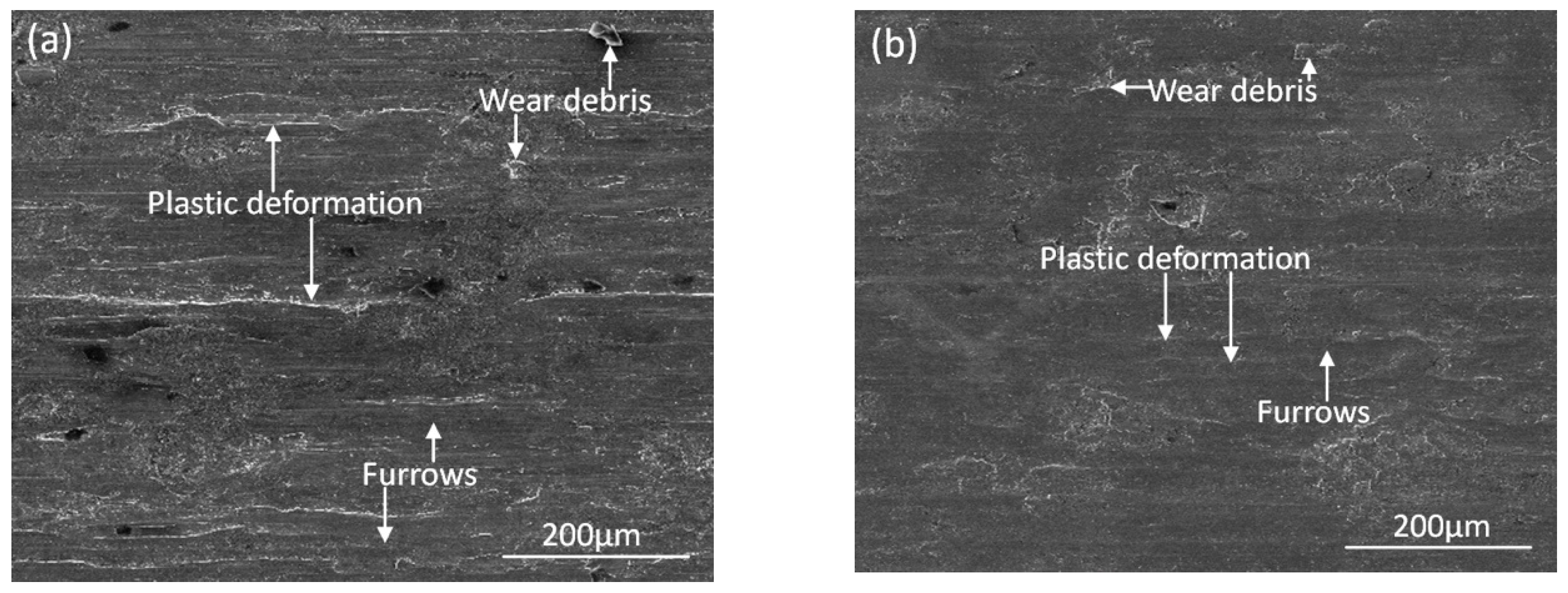
3.4. Wear Resistance
4. Conclusions
- (1)
- The Ti6Al4V coating with a thickness of more than 550 µm was successfully prepared by electro-spark deposition. The interface between the deposited coating and the underlying substrate is even and consecutive, which implies that a good metallurgical bond was obtained.
- (2)
- The deposited coating is mainly composed of α’ martensite. The average hardness of the ESD coating is 540 HV which is about 1.6 times that of the substrate.
- (3)
- The deposited coating shows better wear resistance, thus the cumulative mass loss was less than that of the substrate and the friction coefficient decreased by 0.19. The enhancement on hardness of the deposited coatings is the main reason for this improvement of wear resistance.
Author Contributions
Funding
Conflicts of Interest
References
- Makama, Z.; Doble, I.; Nicolson, D.; Webb, M.E.; Beech, I.B.; Campbell, S.A.; Smith, J.R. Surface preparation of stainless steel 316L, bronze CW451K and titanium Ti6Al4V for bonding to polyurethane in marine cable connector assemblies. Trans. Inst. Met. Finish. 2011, 89, 237–243. [Google Scholar] [CrossRef]
- Balasubramanian, M.; Jayabalan, V.; Balasubramanian, V. A mathematical model to predict impact toughness of pulsed-current gas tungsten arc-welded titanium alloy. Int. J. Adv. Manuf. Technol. 2008, 35, 852–858. [Google Scholar] [CrossRef]
- Cvijović-Alagić, I.; Gubeljak, N.; Rakin, M.; Cvijović, Z.; Gerić, K. Microstructural morphology effects on fracture resistance and crack tip strain distribution in Ti–6Al–4V alloy for orthopedic implants. Mater. Des. 2014, 53, 870–880. [Google Scholar] [CrossRef]
- Ceschini, L.; Lanzoni, E.; Martini, C.; Prandstraller, D.; Sambogna, G. Comparison of dry sliding friction and wear of Ti6Al4V alloy treated by plasma electrolytic oxidation and PVD coating. Wear 2008, 264, 86–95. [Google Scholar] [CrossRef]
- Lütjering, G. Influence of processing on microstructure and mechanical properties of (α+β) titanium alloys. Mater. Sci. Eng. A 1998, 243, 32–45. [Google Scholar] [CrossRef]
- Yetim, A.F.; Celik, A.; Alsaran, A. Improving tribological properties of Ti6Al4V alloy with duplex surface treatment. Surf. Coat. Technol. 2010, 205, 320–324. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.P.; Deng, C.Y.; Huo, L.X.; Wang, L.J.; Cao, S. Feasibility study on preparation of coatings on Ti–6Al–4V by combined ultrasonic impact treatment and electrospark deposition. Mater. Des. 2014, 63, 488–492. [Google Scholar] [CrossRef]
- Obadele, B.A.; Andrews, A.; Olubambi, P.A.; Mathew, M.T.; Pityana, S. Effect ofZrO2 addition onthedryslidingwearbehavioroflaserclad Ti6Al4V alloy. Wear 2015, 328–329, 295–300. [Google Scholar] [CrossRef]
- Korkmaz, K. Investigation and characterization of electrospark deposited chromium carbide-based coating on the steel. Surf. Coat. Technol. 2015, 272, 1–7. [Google Scholar] [CrossRef]
- Çakir, A.; Yilmaz, M.S.; Ribalko, A.; Korkmaz, K. A Study on modification of micro-alloy steel surfaces with different hard materials via electro-spark deposition method. Acta Phys. Polonica A 2015, 127, 1410–1413. [Google Scholar] [CrossRef]
- Reynolds, J.L.; Holdren, R.L.; Brown, L.E. Electro-spark deposition. Adv. Mater. Process. 2003, 161, 35–37. [Google Scholar]
- Li, Z.W.; Gao, W.; Yoshihara, M.; He, Y.D. Improving oxidation resistance of Ti3Al and TiAl intermetallic compounds with electro-spark deposit coatings. Mater. Sci. Eng. A 2003, 347, 243–252. [Google Scholar] [CrossRef]
- Tang, J.M. Mechanical and tribological properties of the TiC–TiB2compositecoating deposited on 40Cr-steel by electro spark deposition. Appl. Surf. Sci. 2016, 365, 202–208. [Google Scholar] [CrossRef]
- Wei, X.; Chen, Z.G.; Zhong, J.; Wang, L.; Hou, Z.W.; Zhang, Y.; Tan, F.L. Facile preparation of nanocrystalline Fe2B coating by direct electrospark deposition of coarse-grained Fe2B electrode material. J. Alloys Compd. 2017, 717, 31–40. [Google Scholar] [CrossRef]
- Liu, D.Y.; Gao, W.; Li, Z.W.; Zhang, H.F.; Hu, Z.Q. Electro-spark deposition of Fe-based amorphous alloy coatings. Mater. Lett. 2007, 61, 165–167. [Google Scholar] [CrossRef]
- Patel, M.R.; Barrufet, M.A.; Eubank, P.T.; DiBitonto, D.D. Theoretical models of the electrical discharge machining process. II. The anode erosion model. J. Appl. Phys. 1989, 66, 4104–4111. [Google Scholar] [CrossRef]
- Liu, J.F.; Guo, Y.B. Thermal modeling of EDM with progression of massive random electrical discharges. Procedia Manuf. 2016, 5, 495–507. [Google Scholar] [CrossRef]
- Lee, H.T.; Tai, T.Y. Relationship between EDM parameters and surface crack formation. J. Mater. Process. Technol. 2003, 142, 676–683. [Google Scholar] [CrossRef]
- Ahmed, T.; Rack, H.J. Phase transformations during cooling in α+β titanium alloys. Mater. Sci. Eng. A 1998, 243, 206–211. [Google Scholar] [CrossRef]
- Xie, Y.J.; Wang, M.C. Epitaxial MCrAlY coating on a Ni-base superalloy produced by electrospark deposition. Surf. Coat. Technol. 2006, 201, 3564–3570. [Google Scholar] [CrossRef]
- Jain, P.; Mandal, T.; Prakash, P.; Garg, A.; Balani, K. Electrophoretic deposition of nanocrystalline hydroxyapatite on Ti6Al4V/TiO2 substrate. J. Coat. Technol. Res. 2013, 10, 263–275. [Google Scholar] [CrossRef]
- Sundaresan, S.; Ram, G.D.J. Use of magnetic arc oscillation for grain refinement of gas tungsten arc welds in α–β titanium alloys. Sci. Technol. Weld. Join. 2013, 4, 151–160. [Google Scholar] [CrossRef]
- Huang, Z.Y.; Guo, Z.J.; Wen, G.P.; Li, Q. Mechanical properties of TC4 alloy annealed with different processes. Heat Treat. Met. 2015, 40, 175–179. (In Chinese) [Google Scholar] [CrossRef]
- Tang, R.Z.; Tian, R.Z. Binary Alloy Phase Diagrams and Crystal Structure of IntermediadePhase, 1st ed.; Central South University Press: Changsha, China, 2009; p. 909. (In Chinese) [Google Scholar]
- Na, T.W.; Kim, W.R.; Yang, S.M.; Kwon, O.; Park, J.M.; Kim, G.H.; Jung, K.H.; Lee, C.W.; Park, H.K.; Kim, H.G. Effect of laser power on oxygen and nitrogen concentration of commercially pure titanium manufactured by selective laser melting. Mater. Charact. 2018, 143, 110–117. [Google Scholar] [CrossRef]
- Archard, J.F. Contact and rubbing of flat surfaces. J. Appl. Phys. 1953, 24, 981–988. [Google Scholar] [CrossRef]
- Archard, J.F.; Hirst, W. The wear of metals under unlubricated conditions. Proc. R. Soc. Lond. A 1956, 236, 397–410. [Google Scholar] [CrossRef]
| Temperature (°C) | Density (Kg/m3) | Coefficient of Thermal Expansion (per °C) | Thermal Conductivity (W/m °C) | Specific Heat (J/kg °C) |
|---|---|---|---|---|
| 20 | 4500 | 8.6 × 10−6 | 7.95 | 611 |
| 500 | 4410 | 9.7 × 10−6 | 11.8 | 703 |
| 1000 | 4350 | 10.2 × 10−6 | 15.5 | 1030 |
| 1500 | 4330 | 10.6 × 10−6 | 21.1 | 1850 |
| 2000 | 4300 | 11.1 × 10−6 | 22.7 | 1852 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Han, C. Microstructure and Wear Resistance of Ti6Al4V Coating Fabricated by Electro-Spark Deposition. Metals 2019, 9, 23. https://doi.org/10.3390/met9010023
Wang W, Han C. Microstructure and Wear Resistance of Ti6Al4V Coating Fabricated by Electro-Spark Deposition. Metals. 2019; 9(1):23. https://doi.org/10.3390/met9010023
Chicago/Turabian StyleWang, Weifu, and Chao Han. 2019. "Microstructure and Wear Resistance of Ti6Al4V Coating Fabricated by Electro-Spark Deposition" Metals 9, no. 1: 23. https://doi.org/10.3390/met9010023




