The Impact of Particle Reinforcement with Al2O3, TiB2, and TiC and Severe Plastic Deformation Treatment on the Combination of Strength and Electrical Conductivity of Pure Aluminum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reinforcing Particles
2.2. Obtaining the Alloys
2.3. Research
3. Results and Discussion
3.1. Master Alloys Obtained in the SHS Mode
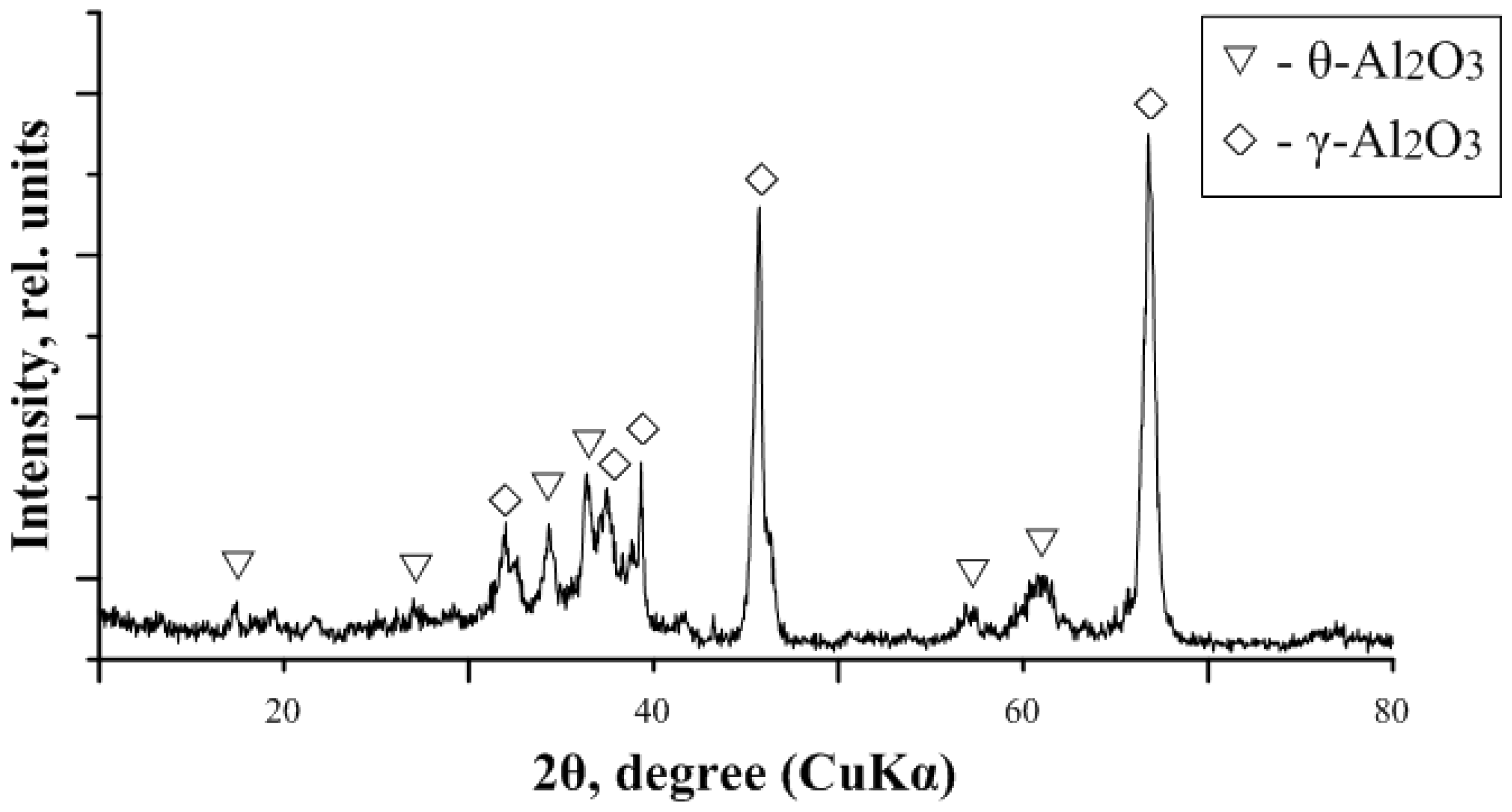
3.2. Nanosize Al2O3 Powder
3.3. Aluminium Alloys
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Flores, F.U.; Seidman, D.N.; Dunand, D.C.; Vo, N.Q. Development of High-Strength and High-Electrical-Conductivity Aluminum Alloys for Power Transmission Conductors. In TMS Annual Meeting & Exhibition; Springer: Cham, Switzerland, 2018; pp. 247–251. [Google Scholar]
- Cui, X.; Wu, Y.; Zhang, G.; Liu, Y.; Liu, X. Study on the improvement of electrical conductivity and mechanical properties of low alloying electrical aluminum alloys. Compos. Part B Eng. 2017, 110, 381–387. [Google Scholar] [CrossRef]
- Karabay, S. Modification of AA-6201 alloy for manufacturing of high conductivity and extra high conductivity wires with property of high tensile stress after artificial aging heat treatment for all-aluminium alloy conductors. Mater. Des. 2006, 27, 821–832. [Google Scholar] [CrossRef]
- Valiev, R.Z.; Murashkin, M.Y.; Sabirov, I. A nanostructural design to produce high-strength Al alloys with enhanced electrical conductivity. Scr. Mater. 2014, 76, 13–16. [Google Scholar] [CrossRef]
- Yin, Z.; Pan, Q.; Zhang, Y.; Jiang, F. Effect of minor Sc and Zr on the microstructure and mechanical properties of Al–Mg based alloys. Mater. Sci. Eng. A 2000, 280, 151–155. [Google Scholar] [CrossRef]
- Feng, X.; Liu, H.; Babu, S.S. Effect of grain size refinement and precipitation reactions on strengthening in friction stir processed Al–Cu alloys. Scr. Mater. 2011, 65, 1057–1060. [Google Scholar] [CrossRef]
- Kendig, K.L.; Miracle, D.B. Strengthening mechanisms of an Al-Mg-Sc-Zr alloy. Acta Mater. 2002, 50, 4165–4175. [Google Scholar] [CrossRef]
- Bobruk, E.V.; Murashkin, M.Y.; Kazykhanov, V.U.; Valiev, R.Z. Aging behavior and properties of ultrafine-grained aluminum alloys of Al-Mg-Si system. Rev. Adv. Mater. Sci. 2012, 31, 109–115. [Google Scholar]
- Murashkin, M.Y.; Sabirov, I.; Kazykhanov, V.U.; Bobruk, E.V.; Dubravina, A.A.; Valiev, R.Z. Enhanced mechanical properties and electrical conductivity in ultrafine-grained Al alloy processed via ECAP-PC. J. Mater. Sci. 2013, 48, 4501–4509. [Google Scholar] [CrossRef]
- Wang, X. The formation of AlB2 in an Al–B master alloy. J. Alloy. Compd. 2005, 403, 283–287. [Google Scholar] [CrossRef]
- Sabirov, I.; Murashkin, M.Y.; Valiev, R.Z. Nanostructured aluminium alloys produced by severe plastic deformation: New horizons in development. Mater. Sci. Eng. A 2013, 560, 1–24. [Google Scholar] [CrossRef]
- Vorozhtsov, S.; Zhukov, I.; Vorozhtsov, A.; Zhukov, A.; Eskin, D.; Kvetinskaya, A. Synthesis of micro-and nanoparticles of metal oxides and their application for reinforcement of Al-based alloys. Adv. Mater. Sci. Eng. 2015, 2015, 718207. [Google Scholar] [CrossRef]
- Vorozhtsov, S.; Minkov, L.; Dammer, V.; Khrustalyov, A.; Zhukov, I.; Promakhov, V.; Khmeleva, M. Ex Situ Introduction and Distribution of Nonmetallic Particles in Aluminum Melt: Modeling and Experiment. JOM 2017, 69, 2653–2657. [Google Scholar] [CrossRef]
- Naydenkin, E.; Mishin, I.; Khrustalyov, A.; Vorozhtsov, S.; Vorozhtsov, A. Influence of Combined Helical and Pass Rolling on Structure and Residual Porosity of an AA6082-0.2 wt% Al2O3 Composite Produced by Casting with Ultrasonic Processing. Metals 2017, 7, 544. [Google Scholar] [CrossRef]
- Vorozhtsov, S.; Zhukov, I.; Promakhov, V.; Naydenkin, E.; Khrustalyov, A.; Vorozhtsov, A. The Influence of ScF3 Nanoparticles on the Physical and Mechanical Properties of New Metal Matrix Composites Based on A356 Aluminum Alloy. JOM 2016, 68, 3101–3106. [Google Scholar] [CrossRef]
- Vorozhtsov, S.A.; Promakhov, V.V.; Eskin, D.G.; Vorozhtsov, A.B.; Zhukov, I.A. The use of alumina and zirconia nanopowders for optimization of the Al-based light alloys. In TMS Annual Meeting & Exhibition; Springer: Cham, Switzerland, 2015; pp. 25–32. [Google Scholar]
- Zhukov, I.A.; Ziatdinov, M.K.; Vorozhtsov, A.B.; Zhukov, A.S.; Vorozhtsov, S.A.; Promakhov, V.V. Self-Propagating High-Temperature Synthesis of Al and Ti Borides. Russ. Phys. J. 2016, 59, 1324–1326. [Google Scholar] [CrossRef]
- Zhukov, I.A.; Promakhov, V.V.; Matveev, A.E.; Platov, V.V.; Khrustalev, A.P.; Dubkova, Y.A.; Potekaev, A.I. Principles of Structure and Phase Composition Formation in Composite Master Alloys of the Al–Ti–B/B4C Systems Used for Aluminum Alloy Modification. Russ. Phys. J. 2018, 60, 2025–2031. [Google Scholar] [CrossRef]
- Vorozhtsov, S.; Vorozhtsov, A.; Kudryashova, O.; Zhukov, I.; Promakhov, V. Structural and mechanical properties of aluminium-based composites processed by explosive compaction. Powder Technol. 2017, 313, 251–259. [Google Scholar] [CrossRef]
- Zhukov, I.A.; Bondarchuk, S.S.; Basalaev, S.A.; Vorozhtsov, A.B.; Platov, V.V.; Titov, S.S. Nonlinear Dependence of Sprayed Drop Sizes on the Mass Fraction of a Salt Precursor Component. Russ. Phys. J. 2018, 60, 1845–1847. [Google Scholar] [CrossRef]
- Zhukov, I.; Bondarchuk, S.; Borisov, B.V. Double Continuum Model of Evolution of Precursor Droplets in the Flow of Heat Transfer Medium of a Plasma Chemical Reactor. MATEC Web Conf. 2016, 72, 01016. [Google Scholar] [CrossRef] [Green Version]
- Zhukov, I.; Promakhov, V.; Vorozhtsov, S.; Kozulin, A.; Khrustalyov, A.; Vorozhtsov, A. Influence of Dispersion Hardening and Severe Plastic Deformation on Structure, Strength and Ductility Behavior of an AA6082 Aluminum Alloy. JOM 2018, 70, 2731–2738. [Google Scholar] [CrossRef]
- Sauter, C.; Emin, M.A.; Schuchmann, H.P.; Tavman, S. Influence of hydrostatic pressure and sound amplitude on the ultrasound induced dispersion and de-agglomeration of nanoparticles. Ultrason. Sonochem. 2008, 15, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Mayadas, A.F. Intrinsic resistivity and electron mean free path in aluminum films. J. Appl. Phys. 1968, 39, 4241–4245. [Google Scholar] [CrossRef]
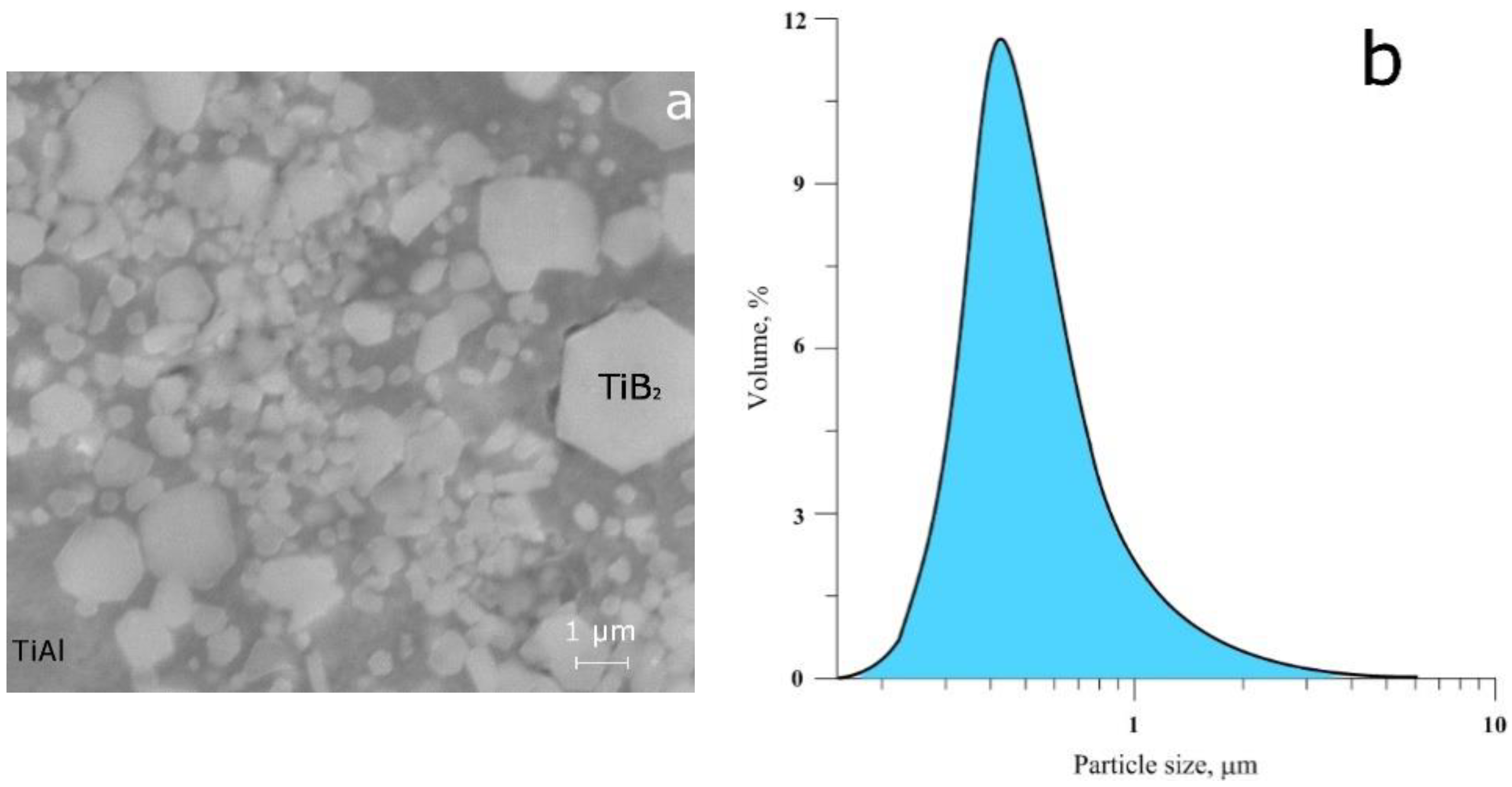
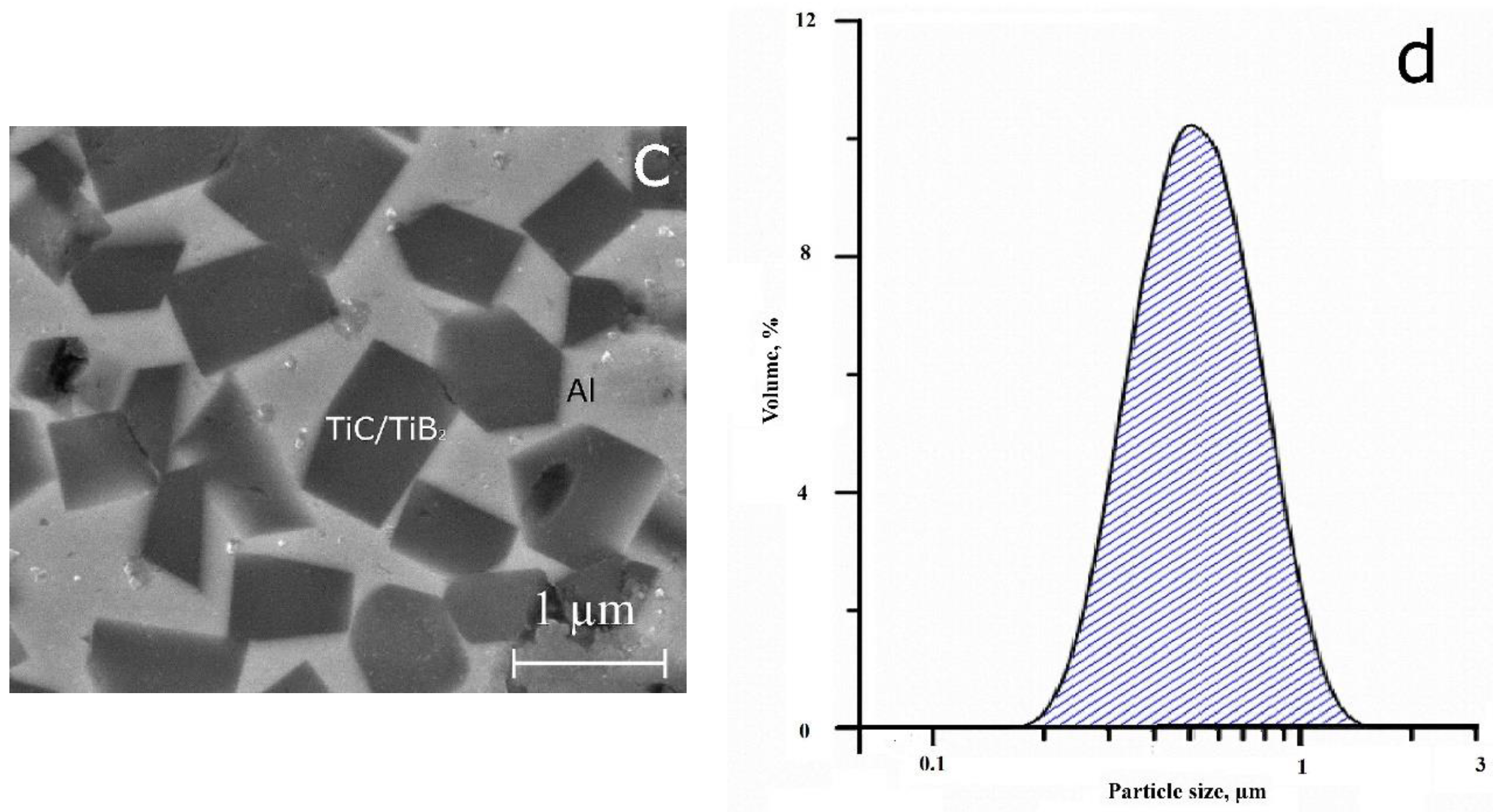



| Master-Alloy | Phases Detected in the Alloyage | Phase Content (wt.%) | Lattice Parameters (Ǻ) | CSR Size (nm) | Δd/d × 10−3 |
|---|---|---|---|---|---|
| (2) | TiB2 | 57 | a = 3.0336 c = 3.2327 | 45 | 0.1 |
| TiAl | 43 | a = 4.0620 c = 4.0456 | – | – | |
| (1) | TiB2 | 42 | a = 3.0238 c = 3.2208 | 41 | 1.7 |
| Al | 36 | a = 4.0472 | 46 | 0.5 | |
| TiC | 22 | a = 4.3184 | 64 | 0.4 |
| SPD | Alloy | ω (MS/m) | IACS (%) | HV (MPa) | YS (MPa) | UTS (MPa) |
|---|---|---|---|---|---|---|
| No cycles | Ref (without particles) | 26.65 ± 1.25 | 54.1 ± 2.1 | 308 ± 9.1 | 40 ± 0.08 | 70 ± 0.14 |
| (i) | 27.45 ± 0.65 | 52.67 ± 1.13 | 329 ± 3.3 | 38 ± 0.076 | 73 ± 0.146 | |
| (ii) | 27.3 ± 0.6 | 52.93 ± 1 | 366 ± 7.9 | 39 ± 0.078 | 68 ± 0.136 | |
| (iii) | 37.1 ± 0.8 | 36.03 ± 1.38 | 359 ± 7.1 | 42 ± 0.084 | 78 ± 0.156 | |
| Two cycles | Ref (without particles) | 24.3 ± 0.7 | 58.1 ± 1.21 | 468 ± 8.7 | 90 ± 0.18 | 119 ± 0.238 |
| (i) | 25.6 ± 1.1 | 55.9 ± 1.94 | 527 ± 11.5 | 86 ± 0.172 | 107 ± 0.214 | |
| (ii) | 29.1 ± 0.5 | 49.8 ± 1.1 | 576 ± 17.8 | 100 ± 0.2 | 129 ± 0.258 | |
| (iii) | 36.3 ± 0.4 | 37.4 ± 0.7 | 562 ± 21.8 | 102 ± 0.204 | 133 ± 0.266 | |
| Four cycles | Ref (without particles) | 21.4 ± 1.1 | 63.1 ± 1.9 | 542 ± 28.3 | 100 ± 0.2 | 149 ± 298 |
| (i) | 24.8 ± 0.7 | 57.2 ± 1.2 | 562 ± 13.5 | 93 ± 0.186 | 153 ± 0.306 | |
| (ii) | 26.7 ± 1 | 53.9 ± 1.7 | 665 ± 18.2 | 103 ± 0.206 | 165 ± 0.33 | |
| (iii) | 31.2 ± 0.8 | 46.2 ± 1.38 | 593 ± 11.7 | 97 ± 0.194 | 159 ± 0.318 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhukov, I.A.; Kozulin, A.A.; Khrustalyov, A.P.; Matveev, A.E.; Platov, V.V.; Vorozhtsov, A.B.; Zhukova, T.V.; Promakhov, V.V. The Impact of Particle Reinforcement with Al2O3, TiB2, and TiC and Severe Plastic Deformation Treatment on the Combination of Strength and Electrical Conductivity of Pure Aluminum. Metals 2019, 9, 65. https://doi.org/10.3390/met9010065
Zhukov IA, Kozulin AA, Khrustalyov AP, Matveev AE, Platov VV, Vorozhtsov AB, Zhukova TV, Promakhov VV. The Impact of Particle Reinforcement with Al2O3, TiB2, and TiC and Severe Plastic Deformation Treatment on the Combination of Strength and Electrical Conductivity of Pure Aluminum. Metals. 2019; 9(1):65. https://doi.org/10.3390/met9010065
Chicago/Turabian StyleZhukov, Ilya A., Alexander A. Kozulin, Anton P. Khrustalyov, Alexey E. Matveev, Vladimir V. Platov, Alexander B. Vorozhtsov, Tatyana V. Zhukova, and Vladimir V. Promakhov. 2019. "The Impact of Particle Reinforcement with Al2O3, TiB2, and TiC and Severe Plastic Deformation Treatment on the Combination of Strength and Electrical Conductivity of Pure Aluminum" Metals 9, no. 1: 65. https://doi.org/10.3390/met9010065





