A-242 Aluminium Alloy Foams Manufacture from the Recycling of Beverage Cans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Aluminium Alloy Foam
2.2. Morphological Characterization
2.3. Mechanical Characterization
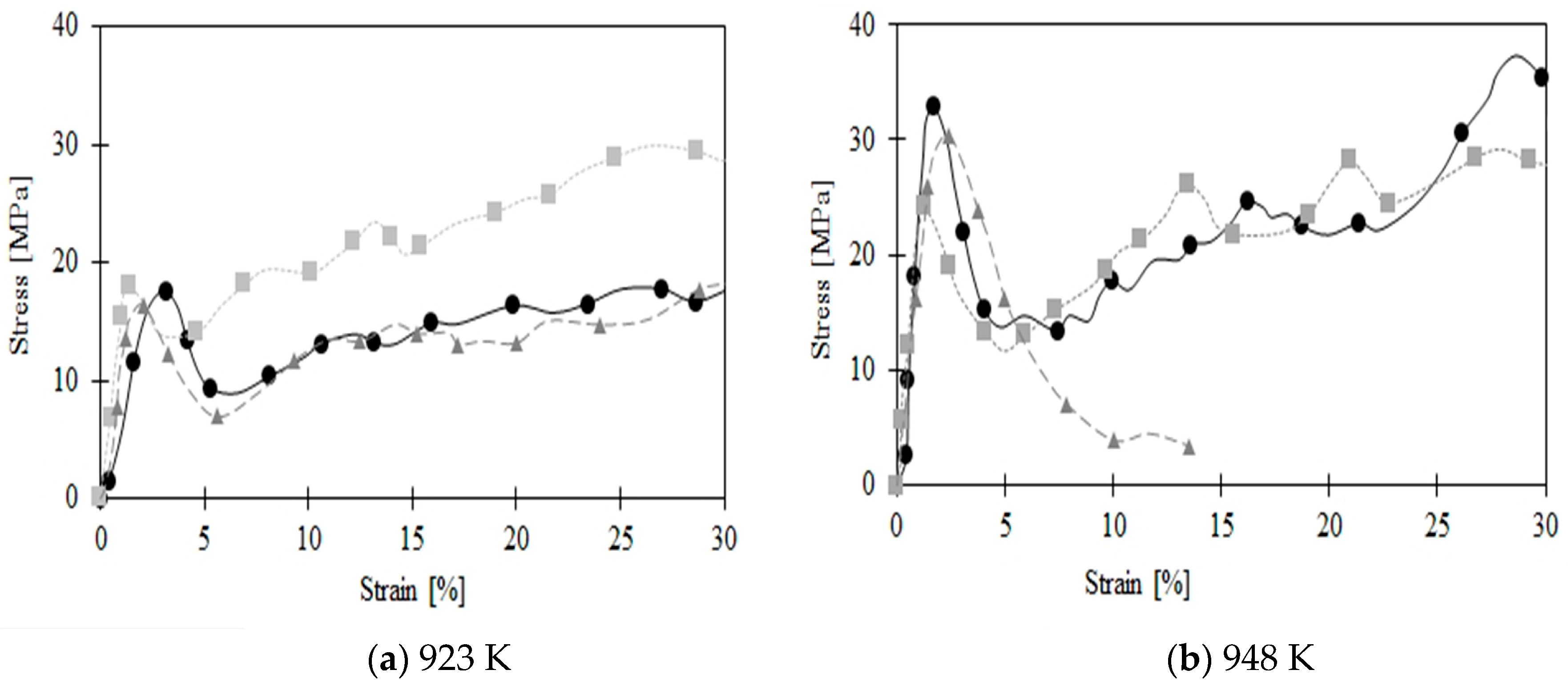
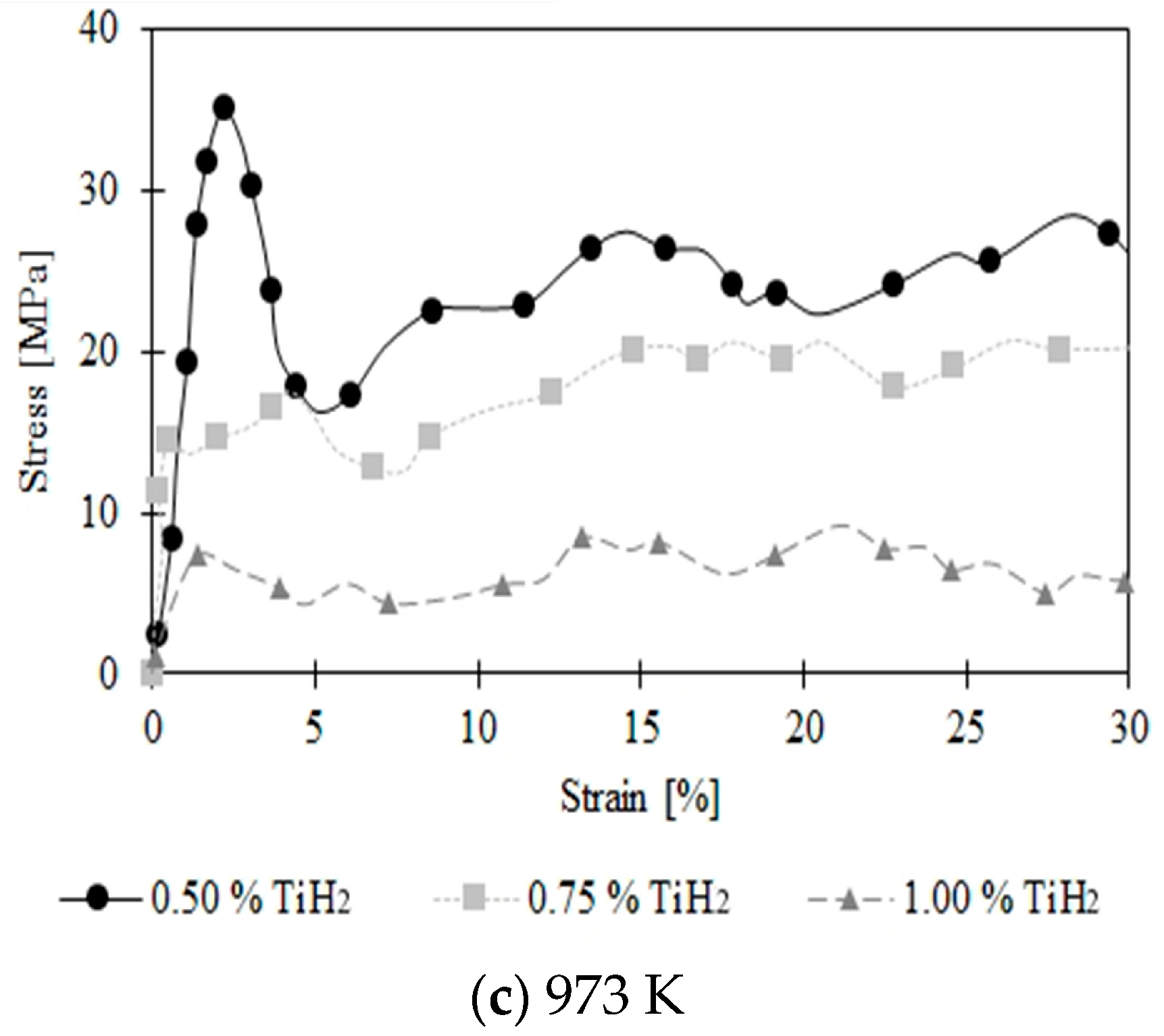
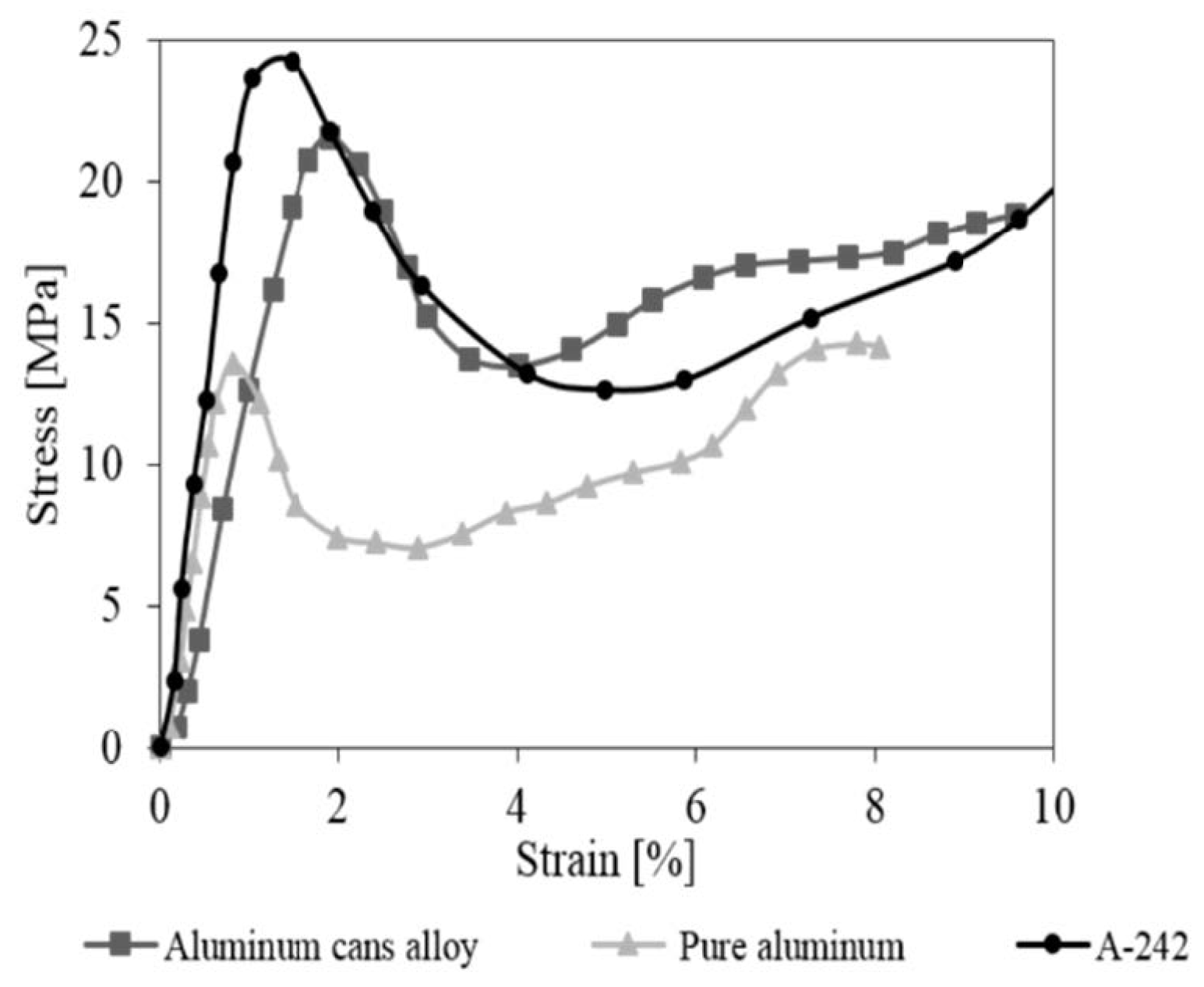
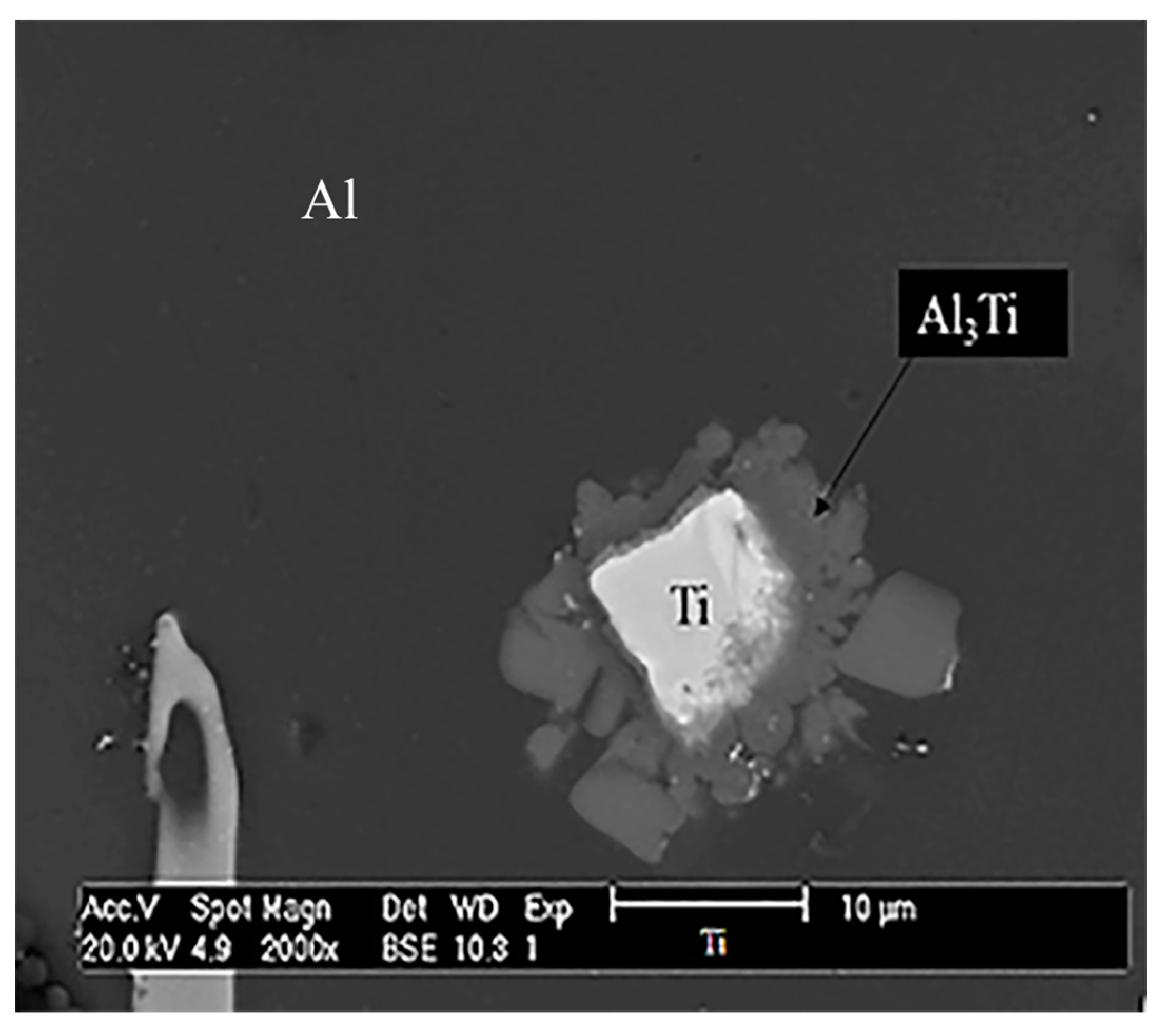
3. Results and Discussion
3.1. Foam Morphology
3.2. Compression Behavior
3.3. Microstructural Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Castillo, E.; Hector, E. Caracterización y Recuperación (Reciclado) de Chatarra de Aluminio (Latas). Bachelor’s Thesis, Universidad Nacional Autónoma de México, Mexico City, Mexico, 1995. [Google Scholar]
- Stotz, P.; Niero, M.; Bey, N.; Paraskevas, D. Environmental screening of novel technologies to increase material circularity: A case study on aluminium cans. Resour. Conserv. Recycl. 2017, 127, 96–106. [Google Scholar] [CrossRef]
- Bauxite Index. Available online: https://thebauxiteindex.com/en/cbix/industry-101/alumina-101/production-trade/outlook#alumina_101 (accessed on 10 July 2018).
- European Aluminium. Available online: https://www.european-aluminium.eu/about-aluminium/aluminium-in-use/ (accessed on 16 July 2018).
- Paraskevas, D.; Kellens, K.; Dewulf, W.; Duflou, J.R. Environmetal modelling of aluminium recycling: A Life Cycle Assessment tool for sustainable metal management. J. Clean. Prod. 2015, 105, 357–370. [Google Scholar] [CrossRef]
- United Nations Environment Programme. Available online: https://wedocs.unep.org/bitstream/handle/20.500.11822/8702/Recycling%20rates%20of%20metals:%20A20status%20report2011Recycling_Rates.pdf?amp%3BisAllowed=&sequence=3 (accessed on 24 April 2018).
- United Nations Environment Programme. Available online: https://www.unenvironment.org/resources/report/metal-recycling-opportunities-limits-infrastructure (accessed on 24 April 2018).
- Modaresi, R.; Müller, D.B. The role of automobiles for the future of aluminum recycling. Environ. Sci. Technol. 2012, 46, 8587–8594. [Google Scholar] [CrossRef]
- Paraskevas, D.; Kellens, K.; Van de Voorde, A.; Dewulf, W.; Duflou, J.R. Environmetal impact analysis of primary aluminium production at country level. Procedia CIRP 2016, 40, 209–213. [Google Scholar] [CrossRef]
- Comercio Exterior Bancomext. Available online: www.revistacomercioexterior.com/articulo.php?id=120&t=la-industria-del-aluminio-en-mexico (accessed on 16 July 2018).
- American Society for Metals. ASM Metals Handbook, Properties and Selection: Nonferrous Alloys and Special-Purpose Materials; ASM International: Geauga County, OH, USA, 1990; Volume 2, pp. 583–590. [Google Scholar]
- American Society for Metals. ASM Metals Handbook, Casting; ASM International: Geauga County, OH, USA, 2008; pp. 44–49. [Google Scholar]
- Fuki, T.; Nonaka, Y.; Suzuki, S. Fabrication of Al-Cu-Mg Alloy Foams Using Mg as Thickener Through Melt Route and Reinforcement of Cell Walls by Heat Treatment. Procedia Mater. Sci. 2014, 4, 33–37. [Google Scholar] [CrossRef]
- Gibson, L.J. Mechanical Behavior of Metallic Foams. Ann. Rev. Mater. Sci. 2002, 30, 191–227. [Google Scholar] [CrossRef]
- Banhart, J.; Baumeister, J. Deformation characteristics of metal foams. Mater. Sci. 1998, 33, 1431–1440. [Google Scholar] [CrossRef]
- García-Moreno, F. Commercial Application of Metal Foams: Their Properties and Production. J. Mater. Sci. 2016, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Banhart, J. Manufacture, characterization and application of cellular metals and metal foam. Prog. Mater. Sci. 2001, 46, 559–632. [Google Scholar] [CrossRef]
- Davies, G.J.; Zhen, S. Metallic foams: Their production, properties and applications. J. Mater. Sci. 1983, 18, 1899–1911. [Google Scholar] [CrossRef]
- Nakajima, H. Fabrication, properties and application of porous metals with directional pores. Prog. Mater. Sci. 2007, 52, 1091–1173. [Google Scholar] [CrossRef]
- Kranzlin, N.; Niederberger, M. Controlled fabrication of porous metals from the nanometer to the macroscopic scale. Mater. Horiz. 2015, 2, 359–377. [Google Scholar] [CrossRef]
- Qin, J.; Chen, Q.; Yang, C.; Huang, Y. Research process on property andapplication of metal porous materials. J. Alloys Compd. 2016, 654, 39–44. [Google Scholar] [CrossRef]
- Lefebvre, L.P.; Banhart, J.; Dunand, D.C. Porous metals and metallic foams: Current status and recent developments. Adv. Eng. Mater. 2008, 10, 775–787. [Google Scholar] [CrossRef]
- Ashby, M.F.; Evans, A.G.; Fleck, N.A.; Gibson, L.J.; Hutchinson, J.W.; Wadley, H.N.G. Metal Foams: A Design Guide; Butterworth-Heinemann: Boston, MA, USA, 2000. [Google Scholar]
- Baumeister, J.; Weise, J. Metallic foams. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000. [Google Scholar]
- Weaire, D.; Cox, S.J.; Banhart, J. Methods and models of metallic foam fabrication. In Proceedings of the 8th Annual International Conference on Composites Engineering, Canary Islands, Spain, 5–11 August 2001; pp. 977–978. [Google Scholar]
- Gibson, L.; Ashby, M. Cellular Solids: Structure and Properties; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Akiyama, S.; Ueno, H.; Imagawa, K.; Kitahara, A.; Nagata, S.; Morimoto, K.; Nishikawa, T.; Itoh, M. Foamed Metal and Method of Producing Same. U.S. Patent 4,713,277, 15 December 1987. [Google Scholar]
- Jin, I.; Kenny, L.D.; Sang, H. Method of Producing Lightweight Foamed Metal. U.S. Patent 4,973,358, 27 November 1990. [Google Scholar]
- Allen, B.C.; Mote, M.W.; Sabroff, A.M. Method of Making Foamed Metal. U.S. Patent 3,087,807, 30 April 1963. [Google Scholar]
- Asavavisithchai, S.; Kennedy, A.R. Effect of powder oxide content on the expansion and stability of PM-route Al foams. J. Colloid Interface Sci. 2006, 297, 715–723. [Google Scholar] [CrossRef]
- Weaire, D.; Hutzler, S. The Physics of Foams; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Hall, I.W.; Guden, M.; Yu, C.-J. Crushing of aluminum closed cell foams: Density and strain rate effects. Scr. Mater. 2000, 43, 515–521. [Google Scholar] [CrossRef]
- Olurin, O.B.; Fleck, N.A.; Ashby, M.F. Deformation and fracture of aluminium foams. Mater. Sci. Eng. A 2000, 291, 136–146. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, A.R. Aspects of the reproducibility of mechanical properties in Al based foams. J. Mater. Sci. 2004, 39, 3085–3088. [Google Scholar] [CrossRef]
- Ramamurty, U.; Paul, A. Variability in mechanical properties of a metal foam. Acta Mater. 2004, 52, 869–876. [Google Scholar] [CrossRef]
- Beals, J.T.; Thomson, M.S. Density gradient effects on aluminium foam compression behaviour. J. Mater. Sci. 1997, 32, 3595–3600. [Google Scholar] [CrossRef]
- Antônio, A.; Oliveira, J.R.; Marques, R. Thixoforging of Al-3.8% Si alloy recycled from aluminum cans. Mater. Sci. Eng. A 2014, A607, 219–225. [Google Scholar] [CrossRef]
- Soltani, R.; Sarajan, Z.; Soltani, M. Foaming of pure aluminium by TiH2. Mater. Res. Innov. 2014, 18, 401–406. [Google Scholar] [CrossRef]
- Geramipour, T.; Oveisi, H. Effects of foaming parameters on microstructure and compressive properties of aluminum foams produced by powder metallurgy method. Trans. Nonferrous Met. Soc. China 2014, 27, 1569–1579. [Google Scholar] [CrossRef]
- Duarte, I.; Banhart, J. A study of aluminium foam formation kinetics and microstructure. Acta Mater. 2000, 48, 2349–2362. [Google Scholar] [CrossRef]
- Baumeister, J.; Banhart, J.; Weber, M. Aluminium Foam for Transport Industry. Mater. Des. 1997, 18, 217–220. [Google Scholar] [CrossRef]
- Prieto, V.; Torres, J.; Flores, A. Recycling of aluminum beverage cans for metallic foams manufacturing. J. Porous Mat. 2017, 24, 707–712. [Google Scholar] [CrossRef]
- Li, P.; Kandalova, E.G.; Nikitin, V.I. Grain refining performance of Al-Ti master alloys with different microstructures. Mater. Lett. 2005, 59, 723–727. [Google Scholar] [CrossRef]
- Huang, L.; Wang, H.; Yang, D.H.; Ye, F.; Wang, S.Q.; Lu, Z.P. Effects of calcium on mechanical properties of celular Al-Cu foams. Mater. Sci. Eng. A 2014, A618, 471–478. [Google Scholar] [CrossRef]
- Mishin, Y.; Herzing, C. Diffusion in the Ti-Al system. Acta Mater. 2000, 48, 589–623. [Google Scholar] [CrossRef]







| Al | Si | Fe | Cu | Mn | Mg | Cr | Ni | Zn | Ti | Others |
|---|---|---|---|---|---|---|---|---|---|---|
| Balance | 0.70 | 1.00 | 3.5–4.5 | 0.35 | 1.2–1.8 | 0.25 | 1.7–2.3 | 0.35 | 0.25 | 0.15 |
| Alloy | Al | Si | Fe | Cu | Mn | Mg | Ni | Ti | Cr | Zn |
|---|---|---|---|---|---|---|---|---|---|---|
| Body A-3004 | Balance | 0.30 | 0.70 | 0.25 | 1.00–1.50 | 0.80–1.30 | - | - | - | 0.25 |
| Lid A-5182 | Balance | 0.20 | 0.35 | 0.15 | 0.20–0.50 | 4.00–5.00 | - | 0.10 | 0.10 | 0.25 |
| Seal A-5082 | Balance | 0.20 | 0.35 | 0.15 | 0.15 | 4.00–5.00 | - | 0.10 | 0.15 | 0.25 |
| Base alloy | Balance | 0.27 | 0.72 | 0.16 | 0.89 | 0.86 | 0.01 | 0.01 | 0.02 | 0.09 |
| Alloy | Al | Si | Fe | Cu | Mn | Mg | Ni | Ti | Cr | Zn |
|---|---|---|---|---|---|---|---|---|---|---|
| A-242 | Balance | 0.25 | 0.66 | 3.68 | 0.81 | 0.77 | 1.94 | - | 0.02 | 0.09 |
| Sample | % TiH2 | Foaming Temperature (K) |
|---|---|---|
| A1 | 0.50 | 923 |
| A2 | 0.75 | 923 |
| A3 | 1.00 | 923 |
| A4 | 0.50 | 948 |
| A5 | 0.75 | 948 |
| A6 | 1.00 | 948 |
| A7 | 0.50 | 973 |
| A8 | 0.75 | 973 |
| A9 | 1.00 | 973 |
| Sample | Linear Expansion (%) | Relative Density | Porosity (%) | Pore Diameter (µm) | σ Pore Diameter (µm) | Wall Thickness (µm) | σ Wall Thickness (µm) |
|---|---|---|---|---|---|---|---|
| A1 | 79.50 | 0.1293 | 87.07 | 633.87 | 279.74 | 148.11 | 66.84 |
| A2 | 75.01 | 0.1512 | 84.88 | 864.40 | 362.54 | 90.07 | 56.54 |
| A3 | 79.21 | 0.1451 | 85.49 | 1220.96 | 742.55 | 124.74 | 65.61 |
| A4 | 77.20 | 0.1006 | 89.94 | 1753.93 | 848.31 | 87.56 | 29.32 |
| A5 | 71.56 | 0.1327 | 86.73 | 1071.93 | 486.78 | 89.44 | 45.04 |
| A6 | 77.63 | 0.0883 | 91.17 | 2149.79 | 979.64 | 181.69 | 75.13 |
| A7 | 77.35 | 0.1410 | 85.90 | 823.51 | 268.20 | 97.48 | 38.31 |
| A8 | 80.21 | 0.1118 | 88.82 | 1388.27 | 867.70 | 92.55 | 55.82 |
| A9 | 76.98 | 0.0604 | 93.96 | 1710.11 | 699.65 | 120.43 | 54.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trejo Rivera, N.M.; Torres Torres, J.; Flores Valdés, A. A-242 Aluminium Alloy Foams Manufacture from the Recycling of Beverage Cans. Metals 2019, 9, 92. https://doi.org/10.3390/met9010092
Trejo Rivera NM, Torres Torres J, Flores Valdés A. A-242 Aluminium Alloy Foams Manufacture from the Recycling of Beverage Cans. Metals. 2019; 9(1):92. https://doi.org/10.3390/met9010092
Chicago/Turabian StyleTrejo Rivera, Nallely Montserrat, Jesús Torres Torres, and Alfredo Flores Valdés. 2019. "A-242 Aluminium Alloy Foams Manufacture from the Recycling of Beverage Cans" Metals 9, no. 1: 92. https://doi.org/10.3390/met9010092





