Effects of Al2O3 Addition on the Microstructure and Properties of CoCr Alloys
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Sintered Powder
2.2. Sample Preparation
2.3. Microstructural Analysis
2.4. Mechanical Properties
3. Results and Discussion
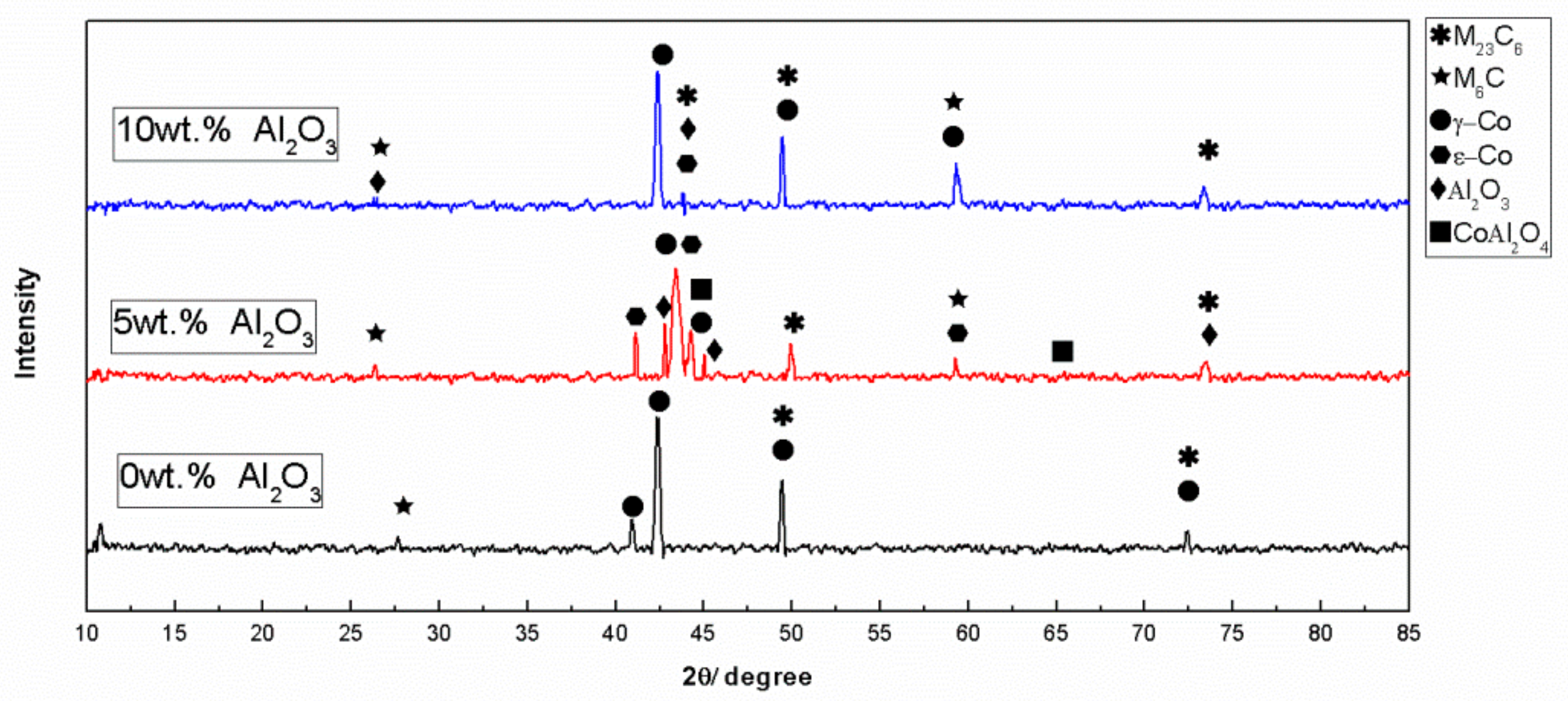
3.1. Alloy Powder Microstructure and Phase Analysis
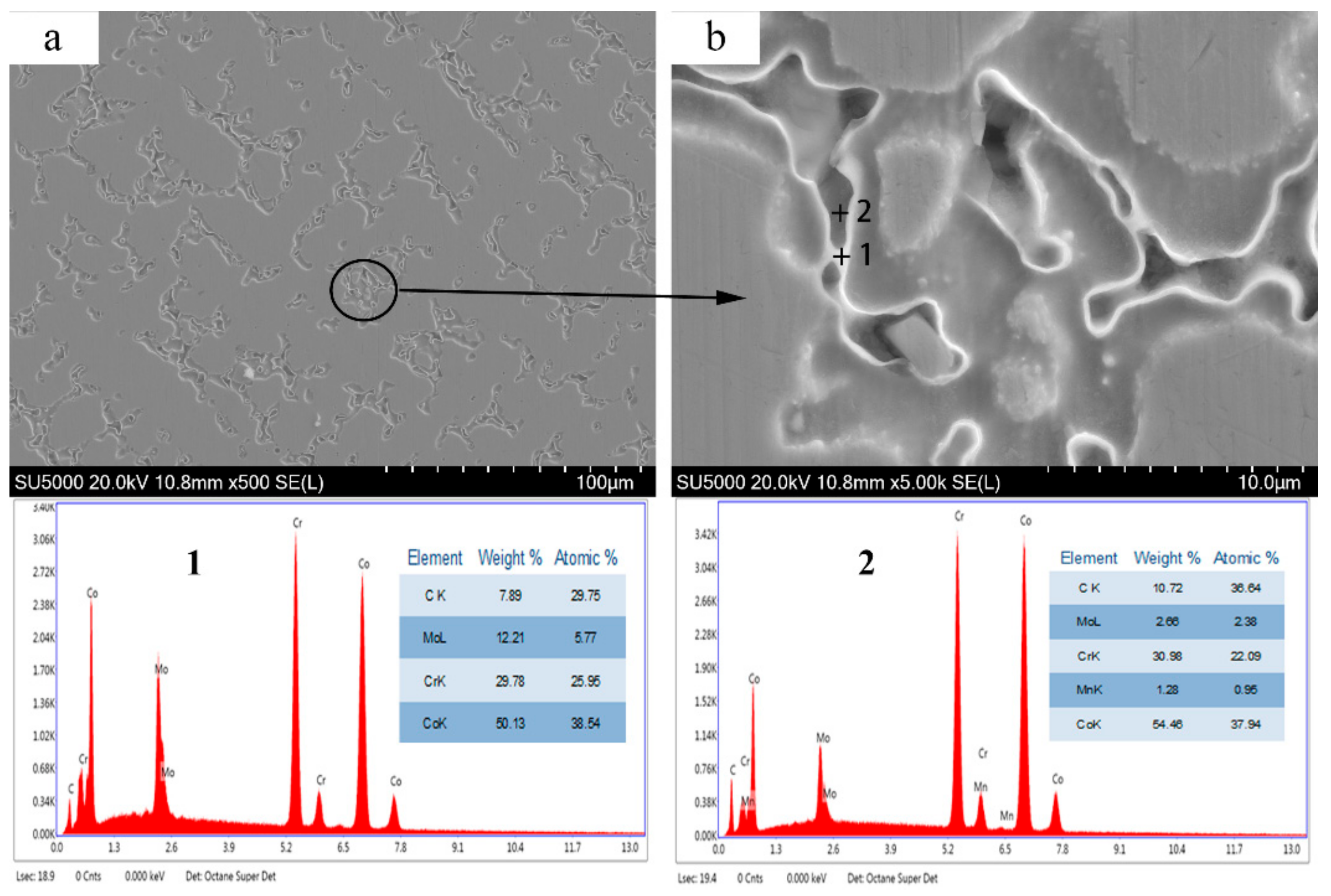
3.2. Microstructural and Phase Analysis of Pure CoCr Alloy Powder for Forming Sample Parts
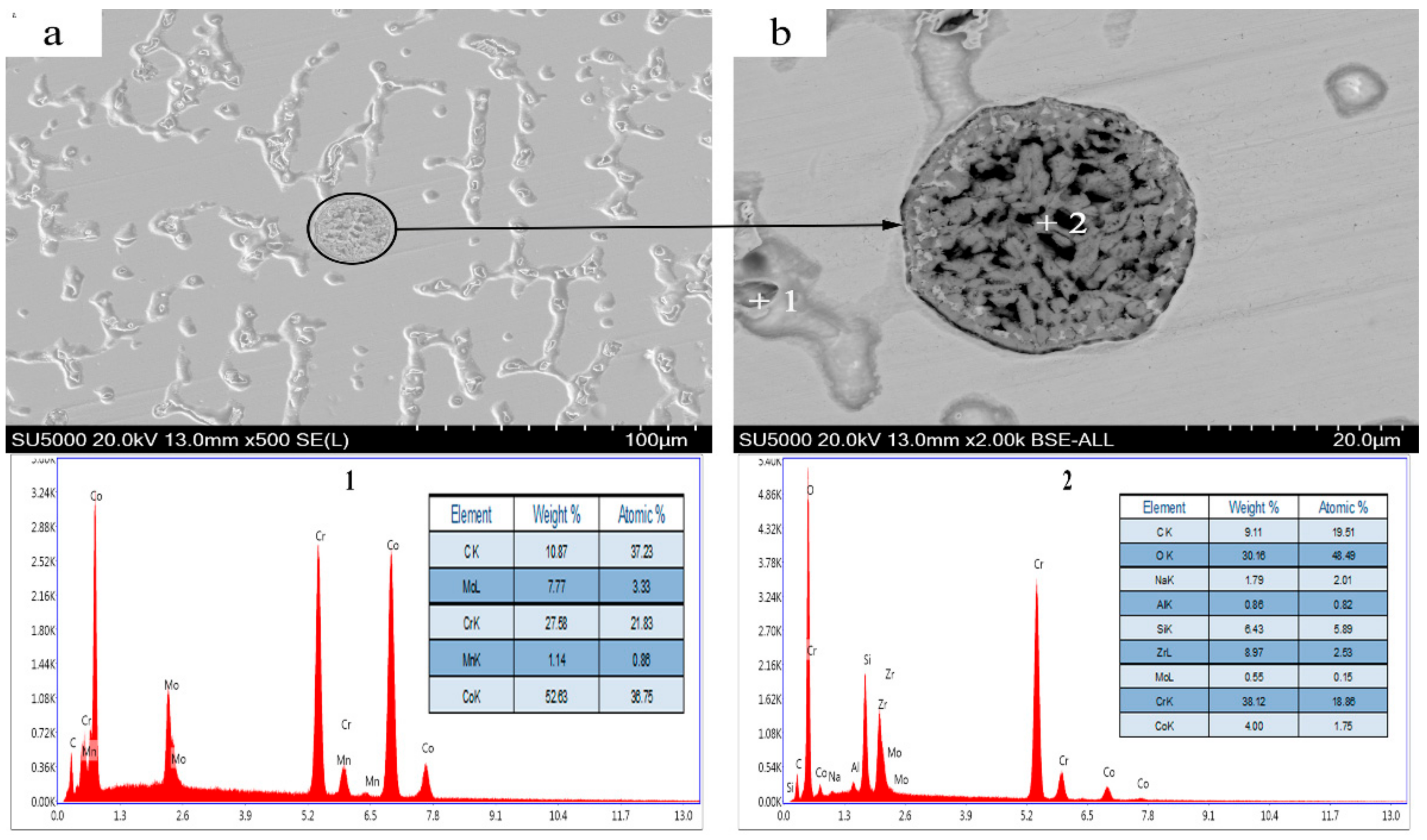
3.3. Microstructural and Phase Analysis of Alloy Powder Molded Parts After Al2O3 Addition
3.4. Mechanical Properties
3.5. Formation Mechanism of the CoAl2O4 Phase
4. Conclusions
- (1)
- Among all the ratios, the microstructure and mechanical properties of the alloy were the best when the content of Al2O3 was 5%. At that time, the deep region of the Al2O3 particles appeared in the microstructure of the alloy, which hindered the growth of dendrites. These results showed that the columnar grain size of the alloy with Al2O3 was obviously smaller than that of the alloy without adding Al2O3.
- (2)
- With the addition of Al2O3, the accumulation area of carbides in the microstructure of the alloy almost disappeared, and the M6C phase increased, which indicated a change in the microstructure and morphology of the CoCr alloy.
- (3)
- The average Vickers hardness of the alloy mixed with 5% Al2O3 was 338.775 HV, and the tensile strength was 856 MPa. From the fracture morphology analysis of the alloy, it was concluded that the local plastic zone appears during the fracture of the alloy. This suggests that the mechanical properties of the alloy were improved.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Davis, J.R. Handbook of Materials for Medical Devices. Available online: https://www.researchgate.net/publication/313151336_Handbook_of_Materials_for_Medical_Devices (accessed on 1 January 2018).
- Li, J.; Chen, C.; Liao, J.; Liu, L.; Ye, X.; Lin, S.; Ye, J. Bond strengths of porcelain to cobalt-chromium alloys made by casting, milling, and selective laser melting. J. Prosthet. Dent. 2017, 118, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Svanborg, P. A 5-Year Retrospective Study of Cobalt-Chromium–Based Fixed Dental Prostheses. Int. J. Prosthodont. 2013, 26, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Al Jabbari, Y.S.; Koutsoukis, T.; Barmpagadaki, X.; Zinelis, S. Metallurgical and interfacial characterization of PFM Co-Cr dental alloys fabricated via casting, milling or selective laser melting. Dent. Mater. 2014, 30, e79–e88. [Google Scholar] [CrossRef] [PubMed]
- Malayoglu, U.; Neville, A. Mo and W as alloying elements in Co-based alloys—Their effects on erosion–Corrosion resistance. Wear 2005, 259, 219–229. [Google Scholar] [CrossRef]
- Qin, L.; Sun, H.; Hafezi, M.; Zhang, Y. Polydopamine-Assisted Immobilization of Chitosan Brushes on a Textured CoCrMo Alloy to Improve its Tribology and Biocompatibility. Materials 2019, 12, 3014. [Google Scholar] [CrossRef] [PubMed]
- Quante, K.; Ludwig, K.; Kern, M. Marginal and internal fit of metal-ceramic crowns fabricated with a new laser melting technology. Dent. Mater. 2008, 24, 1311–1315. [Google Scholar] [CrossRef] [PubMed]
- Traini, T.; Mangano, C.; Sammons, R.L.; Mangano, F.; Macchi, A.; Piattelli, A. Direct laser metal sintering as a new approach to fabrication of an isoelastic functionally graded material for manufacture of porous titanium dental implants. Dent. Mater. 2008, 24, 1525–1533. [Google Scholar] [CrossRef]
- Alt, V.; Hannig, M.; Wostmann, B.; Balkenhol, M. Fracture strength of temporary fixed partial dentures: CAD/CAM versus directly fabricated restorations. Dent. Mater. 2011, 27, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Hedberg, Y.S.; Qian, B.; Shen, Z.; Virtanen, S.; Wallinder, I.O. In vitro biocompatibility of CoCrMo dental alloys fabricated by selective laser melting. Dent. Mater. 2014, 30, 525–534. [Google Scholar] [CrossRef]
- Qian, B.; Saeidi, K.; Kvetkova, L.; Lofaj, F.; Xiao, C.; Shen, Z. Defects-tolerant Co-Cr-Mo dental alloys prepared by selective laser melting. Dent. Mater. 2015, 31, 1435–1444. [Google Scholar] [CrossRef]
- Cassar, J.; Mallia, B.; Mazzonello, A.; Karl, A.; Buhagiar, J. Improved Tribocorrosion Resistance of a CoCrMo Implant Material by Carburising. Lubricants 2018, 6, 76. [Google Scholar] [CrossRef]
- Poolphol, N.; Sakkaew, T.; Kachin, K.; Jantaratana, P.; Vittayakorn, W. Physical, mechanical and magnetic properties of cobalt-chromium alloys prepared by conventional processing. Mater. Today Proc. 2017, 4, 6358–6364. [Google Scholar] [CrossRef]
- Li, K.C.; Prior, D.J.; Waddell, J.N.; Swain, M.V. Comparison of the microstructure and phase stability of as-cast, CAD/CAM and powder metallurgy manufactured Co-Cr dental alloys. Dent. Mater. 2015, 31, e306–e315. [Google Scholar] [CrossRef] [PubMed]
- Yager, S.; Ma, J.; Ozcan, H.; Kilinc, H.I.; Elwany, A.H.; Karaman, I. Mechanical properties and microstructure of removable partial denture clasps manufactured using selective laser melting. Addit. Manuf. 2015, 8, 117–123. [Google Scholar] [CrossRef]
- Bezzon, O.L.; De Mattos, M.D.G.; Ribeiro, R.F.; De Almeida Rollo, J.M. Effect of beryllium on the castability and resistance of ceramometal bonds in nickel-chromium alloys. J. Prosthet. Dent. 1998, 80, 570–574. [Google Scholar] [CrossRef]
- Jang, S.-H.; Min, B.; Hong, M.-H.; Kwon, T.-Y. Effect of Different Post-Sintering Temperatures on the Microstructures and Mechanical Properties of a Pre-Sintered Co–Cr Alloy. Metals 2018, 8, 1036. [Google Scholar] [CrossRef]
- Wang, J.H.; Ren, J.; Liu, W.; Wu, X.Y.; Gao, M.X.; Bai, P.K. Effect of Selective Laser Melting Process Parameters on Microstructure and Properties of Co-Cr Alloy. Materials 2018, 11, 1546. [Google Scholar] [CrossRef]
- Ayu, H.M.; Izman, S.; Daud, R.; Krishnamurithy, G.; Shah, A.; Tomadi, S.H.; Salwani, M.S. Surface Modification on CoCrMo Alloy to Improve the Adhesion Strength of Hydroxyapatite Coating. Procedia Eng. 2017, 184, 399–408. [Google Scholar] [CrossRef]
- Asri, R.I.M.; Harun, W.S.W.; Samykano, M.; Lah, N.A.C.; Ghani, S.A.C.; Tarlochan, F.; Raza, M.R. Corrosion and surface modification on biocompatible metals: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 1261–1274. [Google Scholar] [CrossRef]
- Mori, M.; Sato, N.; Yamanaka, K.; Yoshida, K.; Kuramoto, K.; Chiba, A. Development of microstructure and mechanical properties during annealing of a cold-swaged Co-Cr-Mo alloy rod. J. Mech. Behav. Biomed. Mater. 2016, 64, 187–198. [Google Scholar] [CrossRef]
- Mengucci, P.; Barucca, G.; Gatto, A.; Bassoli, E.; Denti, L.; Fiori, F.; Girardin, E.; Bastianoni, P.; Rutkowski, B.; Czyrska-Filemonowicz, A. Effects of thermal treatments on microstructure and mechanical properties of a Co-Cr-Mo-W biomedical alloy produced by laser sintering. J. Mech. Behav. Biomed. Mater. 2016, 60, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Rodriguez, M.A.L.; Mercado-Solis, R.D.; Presbitero, G.; Lozano, D.E.; Martinez-Cazares, G.M.; Bedolla-Gil, Y. Influence of Boron Additions and Heat Treatments on the Fatigue Resistance of CoCrMo Alloys. Materials 2019, 12, 1076. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, K.; Mori, M.; Chiba, A. Nanoarchitectured Co-Cr-Mo orthopedic implant alloys: Nitrogen-enhanced nanostructural evolution and its effect on phase stability. Acta Biomater. 2013, 9, 6259–6267. [Google Scholar] [CrossRef] [PubMed]
- Kotoban, D.; Nazarov, A.; Shishkovsky, I. Comparative Study of Selective Laser Melting and Direct Laser Metal Deposition of Ni 3 Al Intermetallic Alloy. Procedia IUTAM 2017, 23, 138–146. [Google Scholar] [CrossRef]
- Cai, J.; Nordin, G.P.; Kim, S.; Jiang, J. Surface properties and corrosion behavior of Co-Cr alloy fabricated with selective laser melting technique. Cell Biochem. Biophys. 2013, 67, 983–990. [Google Scholar]
- Takaichi, A.; Nakamoto, T.; Joko, N.; Nomura, N.; Tsutsumi, Y.; Migita, S.; Doi, H.; Kurosu, S.; Chiba, A.; Wakabayashi, N.; et al. Microstructures and mechanical properties of Co-29Cr-6Mo alloy fabricated by selective laser melting process for dental applications. J. Mech. Behav. Biomed. Mater. 2013, 21, 67–76. [Google Scholar] [CrossRef]
- Hagihara, K.; Nakano, T.; Sasaki, K. Anomalous strengthening behavior of Co–Cr–Mo alloy single crystals for biomedical applications. Scr. Mater. 2016, 123, 149–153. [Google Scholar] [CrossRef]
- Bettini, E.; Eriksson, T.; Boström, M.; Leygraf, C.; Pan, J. Influence of metal carbides on dissolution behavior of biomedical CoCrMo alloy: SEM, TEM and AFM studies. Electrochim. Acta 2011, 56, 9413–9419. [Google Scholar] [CrossRef]
- Huang, K.; Marthinsen, K.; Zhao, Q.; Logé, R.E. The double-edge effect of second-phase particles on the recrystallization behaviour and associated mechanical properties of metallic materials. Prog. Mater. Sci. 2018, 92, 284–359. [Google Scholar] [CrossRef]
- Liao, Y.; Pourzal, R.; Stemmer, P.; Wimmer, M.A.; Jacobs, J.J.; Fischer, A.; Marks, L.D. New insights into hard phases of CoCrMo metal-on-metal hip replacements. J. Mech. Behav. Biomed. Mater. 2012, 12, 39–49. [Google Scholar] [CrossRef]
- Chiba, A.; Kumagai, K.; Nomura, N.; Miyakawa, S. Pin-on-disk wear behavior in a like-on-like configuration in a biological environment of high carbon cast and low carbon forged Co–29Cr–6Mo alloys. Acta Mater. 2007, 55, 1309–1318. [Google Scholar] [CrossRef]
- Song, C.; Zhang, M.; Yang, Y.; Wang, D.; Jia-kuo, Y. Morphology and properties of CoCrMo parts fabricated by selective laser melting. Mater. Sci. Eng. A 2018, 713, 206–213. [Google Scholar] [CrossRef]
- Leinfelder, K.F. Porcelain esthetics for the 21st century. J. Am. Dent. Assoc. 2000, 131, 47S–51S. [Google Scholar] [CrossRef]
- Rao, C. Effect of heat treatment on corrosion behavior of weld deposited Co-Cr-Mo alloy. ARPN J. Eng. Appl. Sci. 2016, 11, 12188–12191. [Google Scholar]
- Zhou, Y.; Li, N.; Yan, J.; Zeng, Q. Comparative analysis of the microstructures and mechanical properties of Co-Cr dental alloys fabricated by different methods. J. Prosthet. Dent. 2018, 120, 617–623. [Google Scholar] [CrossRef]
- Cantor, B. Fundamentals of Rapid Solidification. In Science and Technology of the Undercooled Melt; Springer: Dordrecht, The Netherlands, 1986. [Google Scholar]
- Xian, X.; Zhong, Z.; Zhang, B.; Song, K.; Chen, C.; Wang, S.; Cheng, J.; Wu, Y. A high-entropy V 35 Ti 35 Fe 15 Cr 10 Zr 5 alloy with excellent high-temperature strength. Mater. Des. 2017, 121, 229–236. [Google Scholar] [CrossRef]
- Barin, I.; Platzki, G. Thermochemical Data of Pure Substances; VCH: Weinheim, NY, USA, 1995. [Google Scholar]
- Tavasoli, A.; Karimi, S.; Taghavi, S.; Zolfaghari, Z.; Amirfirouzkouhi, H. Comparing the deactivation behaviour of Co/CNT and Co/γ-Al2O3 nano catalysts in Fischer-Tropsch synthesis. J. Nat. Gas. Chem. 2012, 21, 605–613. [Google Scholar] [CrossRef]














| Group | Yield Strength (MPa) | Ultimate Tensile Strength (MPa) | Elongation (%) | Microhardness (HV) |
|---|---|---|---|---|
| Al2O3 (0.0%) | 686.8 | 788.7 | 11.0 | 270.1 |
| Al2O3 (2.5%) | 789.1 | 820.9 | 6.5 | 230.0 |
| Al2O3 (5.0%) | 822.3 | 865.1 | 13.6 | 338.7 |
| Al2O3 (7.5%) | 565.9 | 583.9 | 8.6 | 281.0 |
| Al2O3 (10%) | 521.6 | 535.9 | 8.0 | 225.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, Q.; Bai, P.; Wang, J. Effects of Al2O3 Addition on the Microstructure and Properties of CoCr Alloys. Metals 2019, 9, 1074. https://doi.org/10.3390/met9101074
Hong Q, Bai P, Wang J. Effects of Al2O3 Addition on the Microstructure and Properties of CoCr Alloys. Metals. 2019; 9(10):1074. https://doi.org/10.3390/met9101074
Chicago/Turabian StyleHong, Qin, Peikang Bai, and Jianhong Wang. 2019. "Effects of Al2O3 Addition on the Microstructure and Properties of CoCr Alloys" Metals 9, no. 10: 1074. https://doi.org/10.3390/met9101074
APA StyleHong, Q., Bai, P., & Wang, J. (2019). Effects of Al2O3 Addition on the Microstructure and Properties of CoCr Alloys. Metals, 9(10), 1074. https://doi.org/10.3390/met9101074




