Recovery of Rare Earth Oxide from Waste NiMH Batteries by Simple Wet Chemical Valorization Process
Abstract
:1. Introduction
- All battery recycling processes by various companies around the world that have been reviewed mostly follow pyrometallurgical or pyrometallurgical-dominated processes, in contrast to our developed process which is hydrometallurical.
- It is a simple acid leaching and precipitation process for the recovery of REMs.
- Recovered REM sulfate is value-added through a simple carbocation reaction.
2. Materials and Methods
2.1. Materials
2.2. Leaching and REM Separation
2.3. Characterization
3. Results and Discussion
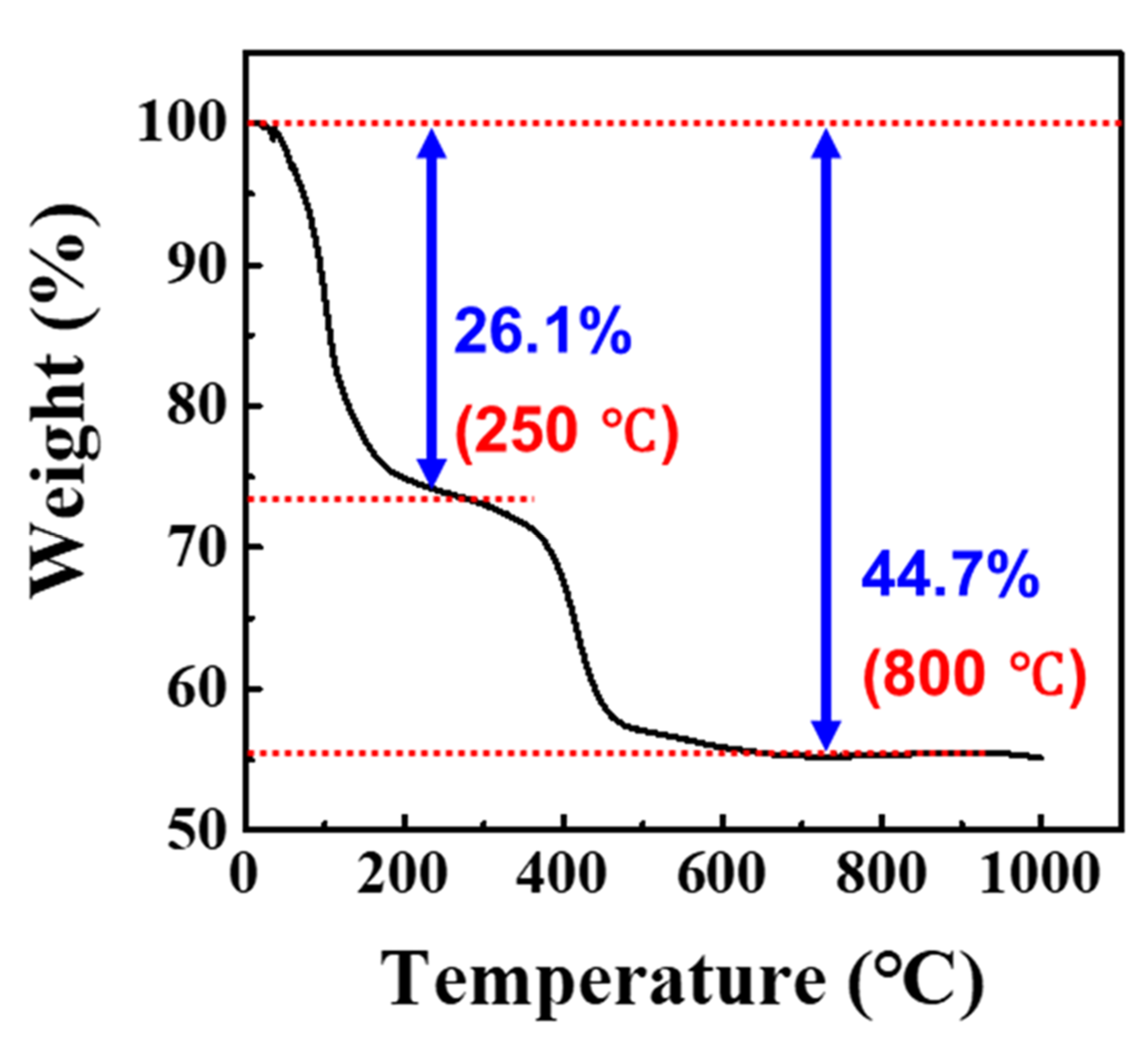
3.1. Characterization of Waste NiMH Battery Material
3.2. Leaching Optimization of Waste NiMH Battery Material
3.3. Separation and Recovery of REM by Metathesis Reaction
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Critical Raw Materials. Available online: http://ec.europa.eu/growth/sectors/raw-materials/specific-interest/critical_en (accessed on 12 September 2019).
- E.U. Commission. Study on the review of the list of Critical Raw Materials Critical Raw Materials Factsheets Publications Office of the European Union; European Commission: Brussels, Belgium, 2017. [Google Scholar]
- U.S. Department of Energy Critical Metal Strategy; U.S. Department of Energy: Washington, DC, USA, 2011.
- Jaffe, R.; Ceder, G.; Eggert, R.; Graedel, T.; Gschneidner, K.; Hitzman, M.; Houle, F.; Hurd, A.; Kelley, R.; King, A.; et al. Energy Critical Elements. Available online: https://www.aps.org/policy/reports/popa-reports/upload/elementsreport.pdf (accessed on 21 October 2019).
- Ellis, T.W.; Mirza, A.H. Battery Recycling: Defining the Market and Identifying the Technology Required to Keep High Value Materials in the Economy and Out of the Waste Dump. Available online: http://www.nist.gov/tip/wp/pswp/upload/245_battery_recycling_defining_the_market.pdf (accessed on 14 April 2016).
- European Li-Ion Battery Advanced Manufacturing For Electric Vehicles. Available online: https://elibama.files.wordpress.com/2014/10/v-d-batteries-recycling1.pdf (accessed on 14 April 2019).
- Müller, T.; Friedrich, B. Development of a recycling process for nickel-metal hydride batteries. J. Power Sources 2006, 158, 1498–1509. [Google Scholar] [CrossRef]
- Tenório, J.A.S.; Espinosa, D.C.R. Recovery of Ni-based alloys from spent NiMH batteries. J. Power Sources 2002, 108, 70–73. [Google Scholar] [CrossRef]
- Zhang, P.; Yokoyama, T.; Itabashi, O.; Wakui, Y.; Suzuki, T.M.; Inoue, K. Hydrometallurgical process for recovery of metal values from spent nickel-metal hydride secondary batteries. Hydrometallurgy 1998, 50, 61–75. [Google Scholar] [CrossRef]
- Tzanetakis, N.; Scott, K. Recycling of nickel–metal hydride batteries. I: Dissolution and solvent extraction of metals. J. Chem. Technol. Biotechnol. 2004, 79, 919–926. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, J.; Fang, X. Rare earth element recycling from waste nickel-metal hydride batteries. J Hazard Mater 2014, 279, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Larsson, K.; Binnemans, K. Selective extraction of metals using ionic liquids for nickel metal hydride battery recycling. Green Chem. 2014, 16, 4595–4603. [Google Scholar] [CrossRef] [Green Version]
- Tzanetakis, N.; Scott, K. Recycling of nickel–metal hydride batteries. II: Electrochemical deposition of cobalt and nickel. J. Chem. Technol. Biotechnol. 2004, 79, 927–934. [Google Scholar] [CrossRef]
- Valadares, A.; Valadares, C.F.; de Lemos, L.R.; Mageste, A.B.; Rodrigues, G.D. Separation of cobalt and nickel in leach solutions of spent nickel-metal hydride batteries using aqueous two-phase systems (ATPS). Hydrometallurgy 2018, 181, 180–188. [Google Scholar] [CrossRef]
- Xi, G.; Xu, H.; Yao, L. Study on preparation of NiCo ferrite using spent lithium-ion and nickel–metal hydride batteries. Sep. Purif. Technol. 2015, 145, 50–55. [Google Scholar] [CrossRef]
- Xia, Y.; Xiao, L.; Tian, J.; Li, Z.; Zeng, L. Recovery of rare earths from acid leach solutions of spent nickel-metal hydride batteries using solvent extraction. J. Rare Earths 2015, 33, 1348–1354. [Google Scholar] [CrossRef]
- Korkmaz, K.; Alemrajabi, M.; Rasmuson, Å.; Forsberg, K. Recoveries of Valuable Metals from Spent Nickel Metal Hydride Vehicle Batteries via Sulfation, Selective Roasting, and Water Leaching. J. Sustain. Metall. 2018, 4, 313–325. [Google Scholar] [CrossRef] [Green Version]
- Korkmaz, K.; Alemrajabi, M.; Rasmuson, Å.; Forsberg, K. Sustainable Hydrometallurgical Recovery of Valuable Elements from Spent Nickel–Metal Hydride HEV Batteries. Metals 2018, 8, 1062. [Google Scholar] [CrossRef]
- Innocenzi, V.; Vegliò, F. Recovery of rare earths and base metals from spent nickel-metal hydride batteries by sequential sulphuric acid leaching and selective precipitations. J. Power Sources 2012, 211, 184–191. [Google Scholar] [CrossRef]
- Pietrelli, L.; Bellomo, B.; Fontana, D.; Montereali, M.R. Rare earths recovery from NiMH spent batteries. Hydrometallurgy 2002, 66, 135–139. [Google Scholar] [CrossRef]









| Company | Battery Type | Process/Technology Used | Location |
|---|---|---|---|
| Retriev Technologies | All types | Pyrometallurgy Hydrometallurgy | Trail, BC, Canada; Baltimore, OH, USA |
| Salesco Systems | All types | Pyrometallurgy | Phoenix, AZ, USA |
| AERC | All types | Pyrometallurgy | Allentown PA, USA; Hayward CA, USA; West Melbourne FL, USA |
| Dowa | All types | Pyrometallurgy | Japan |
| Japan Recycle | All types | Pyrometallurgy | Osaka, Japan |
| Sony Corp. & Sumitomo Metals and Mining Co. | All types | Pyrometallurgy | Japan |
| XStrata | All types | Pyrometallurgy Electrowinning | Horne Que, Nikkelverk Norway; Sudbury, ON., Canada |
| Accurec | All types | Pyrometallurgy | Mulhiem, Grenada |
| DK | All types | Pyrometallurgy | Duisburg, Greece |
| AFE Group (Valdi) | All types | Pyrometallurgy | Zurich, Switzerland; Rogerville, France |
| Citron | All types | Pyrometallurgy | Zurich, Switzerland; Rogerville, France |
| Euro Dieuze/SARP | All types | Hydrometallurgy | Lorraine, France |
| SNAM | Cd, Ni, MH, Li | Pyrometallurgy | Saint Quentin Fallavier, France |
| IPGNA Ent. (Recupyl) | All types | Hydrometallurgy | Grenoble, France |
| Umicore | All types | Pyrometallurgy Hydrometallurgy Electrowinning | Hooboken, Belgium |
| Rare Earth Elements (REMs) | Total REMs | Ni | Co | Others | |||
|---|---|---|---|---|---|---|---|
| Ce | La | Nd | |||||
| Weight (%) | 10.4 | 6.7 | 3.1 | 20.2 | 45.8 | 8.5 | 25.5 |
| # | Rare Earth Elements | Ni + Co | ||
|---|---|---|---|---|
| Ce | La | Nd | ||
| Weight (%) | 17.2 | 13.1 | 5.44 | 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, N.-K.; Swain, B.; Shim, H.-W.; Kim, D.-W. Recovery of Rare Earth Oxide from Waste NiMH Batteries by Simple Wet Chemical Valorization Process. Metals 2019, 9, 1151. https://doi.org/10.3390/met9111151
Ahn N-K, Swain B, Shim H-W, Kim D-W. Recovery of Rare Earth Oxide from Waste NiMH Batteries by Simple Wet Chemical Valorization Process. Metals. 2019; 9(11):1151. https://doi.org/10.3390/met9111151
Chicago/Turabian StyleAhn, Nak-Kyoon, Basudev Swain, Hyun-Woo Shim, and Dae-Weon Kim. 2019. "Recovery of Rare Earth Oxide from Waste NiMH Batteries by Simple Wet Chemical Valorization Process" Metals 9, no. 11: 1151. https://doi.org/10.3390/met9111151





