Microstructure and Properties of High-Entropy AlxCoCrFe2.7MoNi Alloy Coatings Prepared by Laser Cladding
Abstract
:1. Introduction
2. Materials and Methods
3. Experimental Results
3.1. Crystal Structure
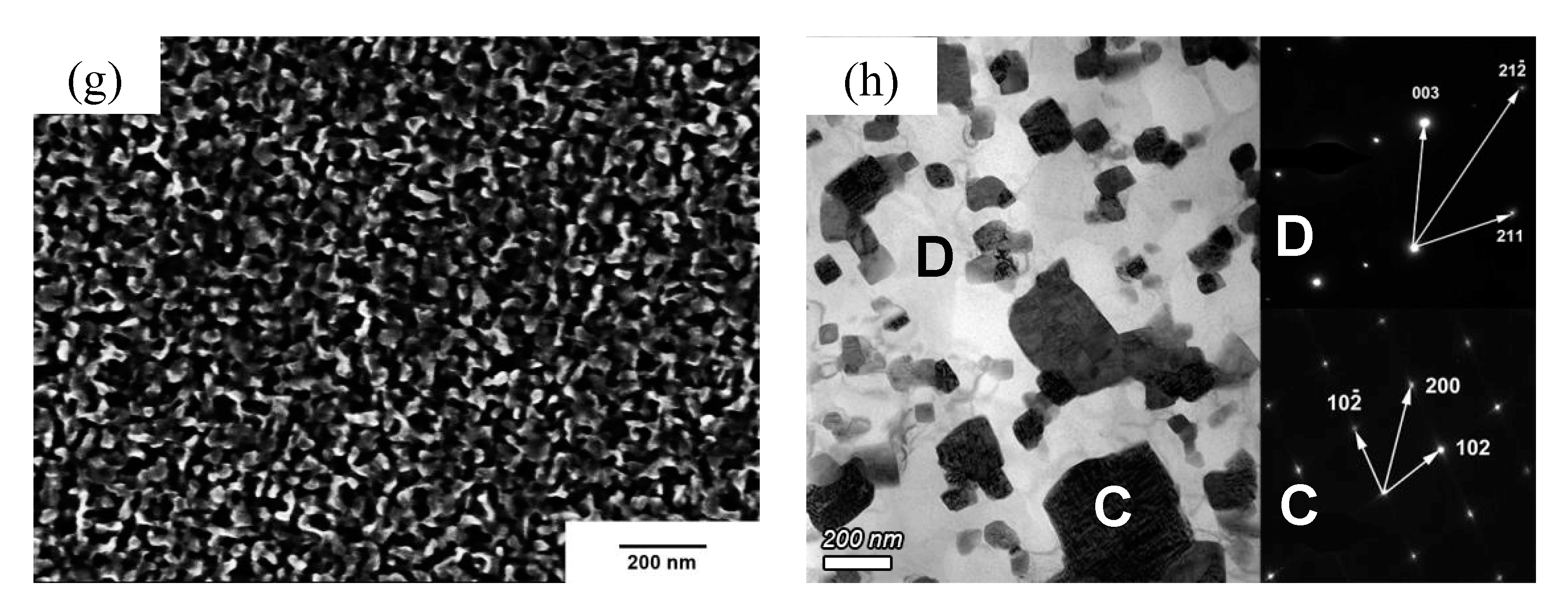
3.2. Microstructure
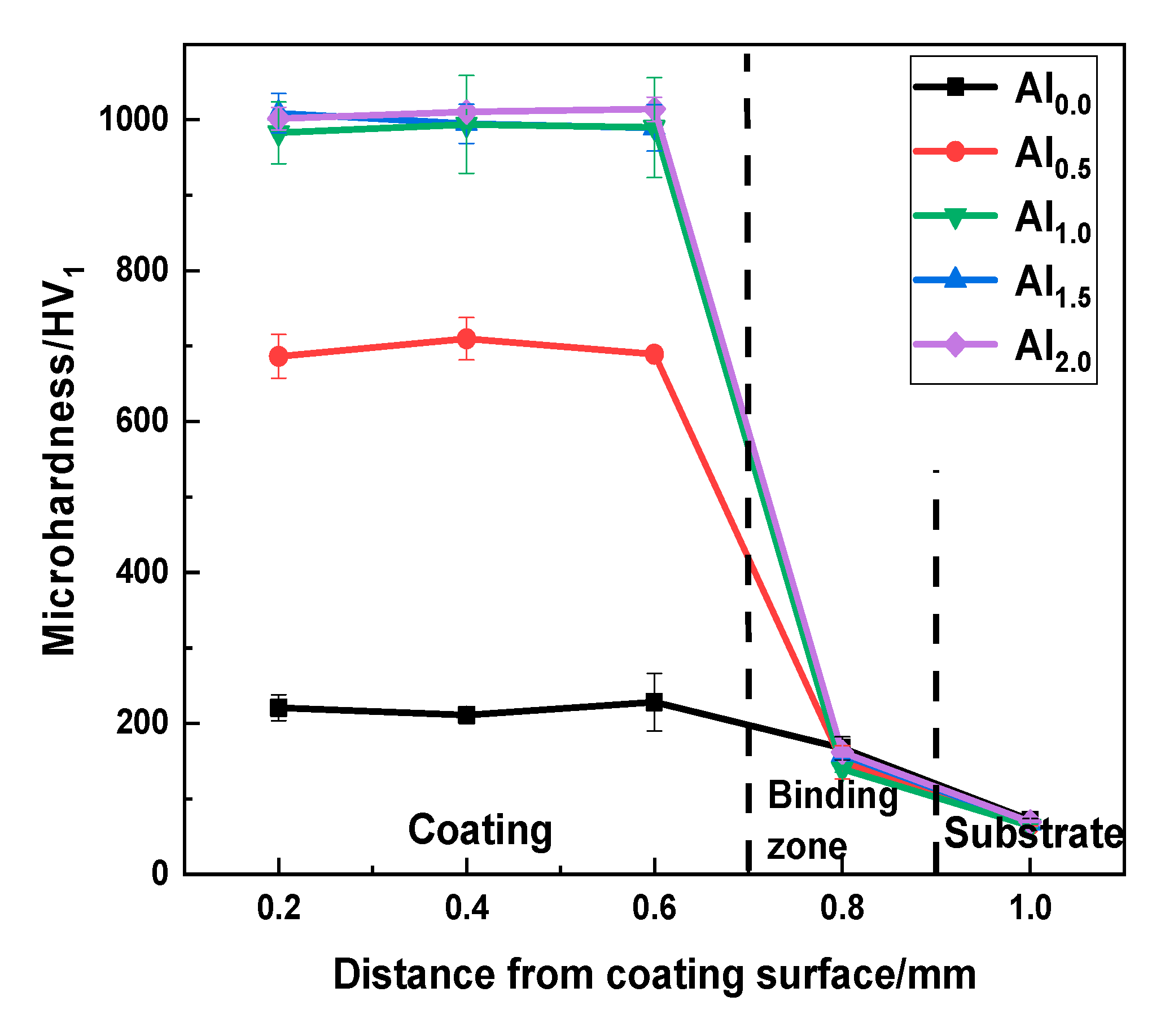
3.3. Microhardness
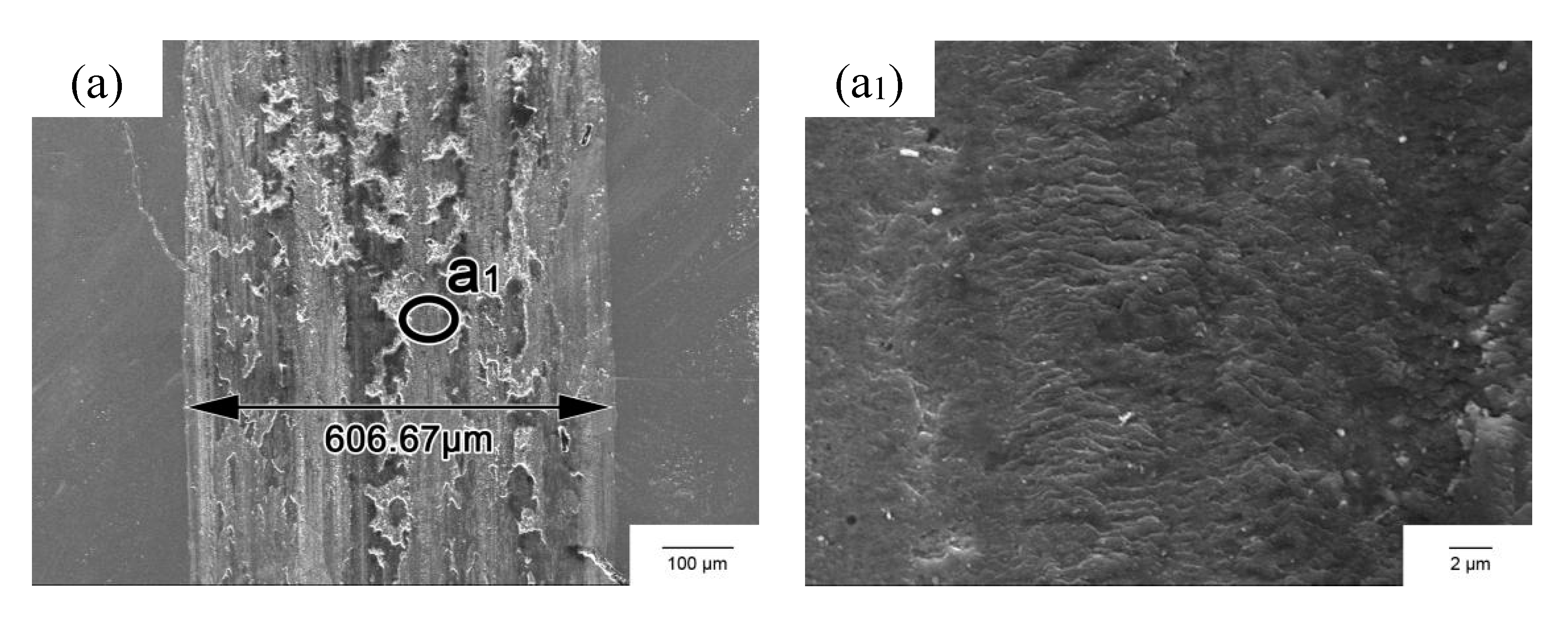
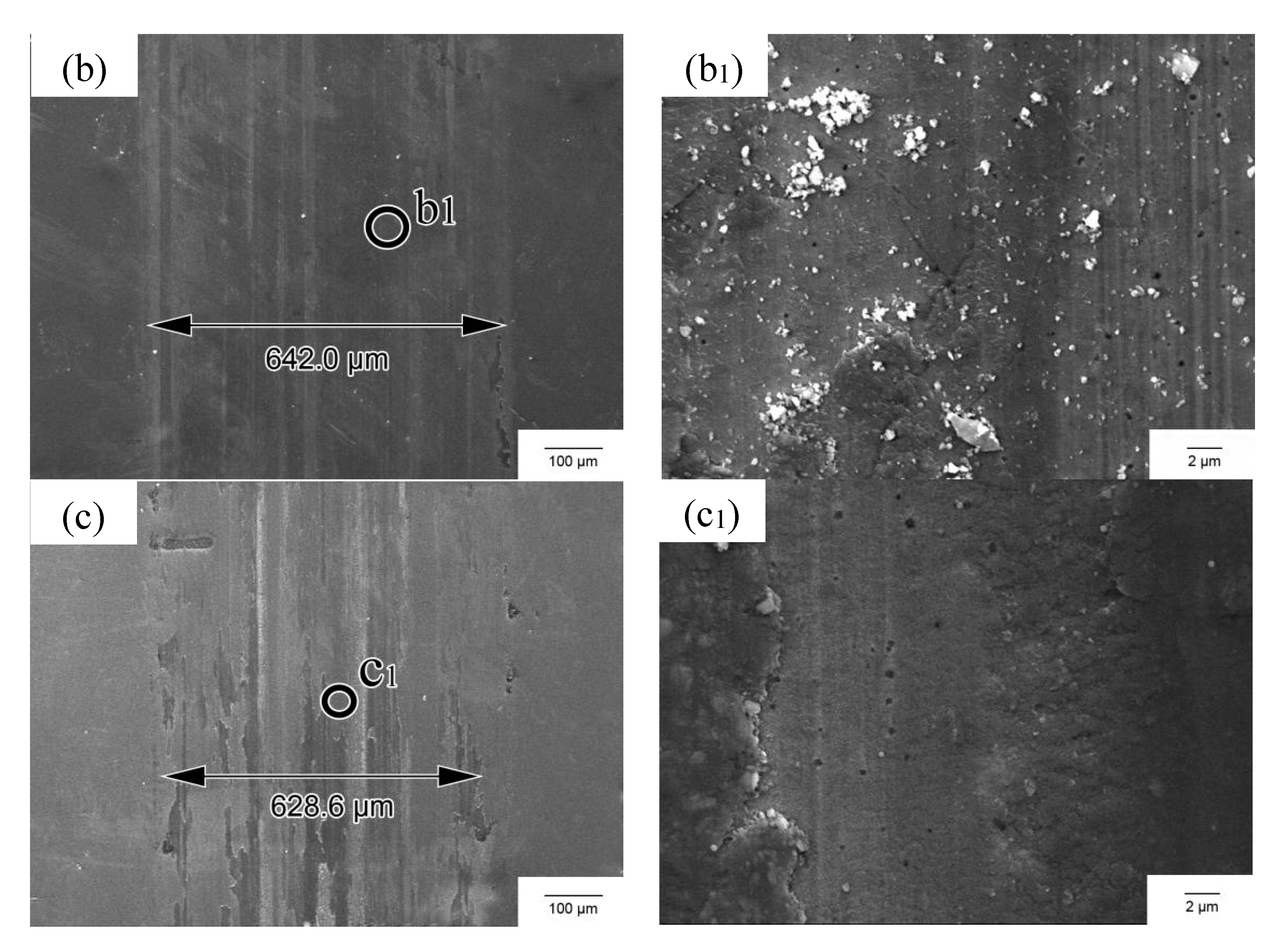
3.4. Wear Resistance
3.5. Corrosion Resistance
4. Discussion
4.1. Microstructure and Phase
4.2. Hardness and Wear Resistance
4.3. Corrosion Resistance
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Senkov, O.N.; Wilks, G.B.; Miracle, D.B.; Chuang, C.P.; Liaw, P.K. Refractory high-entropy alloys. Intermetallics 2010, 18, 1758–1765. [Google Scholar] [CrossRef]
- Miracle, D.B.; Miller, J.D.; Senkov, O.N.; Woodward, C.; Uchic, M.D.; Tiley, J. Exploration and development of high entropy alloys for structural applications. Entropy 2014, 16, 494–525. [Google Scholar] [CrossRef]
- Yeh, J.W. Alloy design strategies and future trends in high-entropy alloys. JOM 2013, 65, 1759–1771. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Gao, M.C.; Liaw, P.K.; Yeh, J.W.; Zhang, Y. High-Entropy Alloys: Fundamentals and Applications; Springer: Berlin, Germany, 2016. [Google Scholar]
- Lu, Y.; Dong, Y.; Guo, S.; Jiang, L.; Kang, H.; Wang, T.; Wen, B.; Wang, Z.; Jie, J.; Cao, Z. A promising new class of high-temperature alloys: Eutectic high-entropy alloys. Sci. Rep. 2014, 4, 6200. [Google Scholar] [CrossRef]
- Lei, Z.; Liu, X.; Wu, Y.; Wang, H.; Jiang, S.; Wang, S.; Hui, X.; Wu, Y.; Gault, B.; Kontis, P.; et al. Enhanced strength and ductility in a high-entropy alloy via ordered oxygen complexes. Nature 2018, 563, 546–550. [Google Scholar] [CrossRef]
- Huang, H.; Wu, Y.; He, J.; Wang, H.; Liu, X.; An, K.; Wu, W.; Lu, Z. Phase-transformation ductilization of brittle high-entropy alloys via metastability engineering. Adv. Mater. 2017, 29, 1701678. [Google Scholar] [CrossRef]
- He, J.Y.; Wang, H.; Huang, H.L.; Xu, X.D.; Chen, M.W.; Wu, Y.; Liu, X.J.; Nieh, T.G.; An, K.; Lu, Z.P. A precipitation-hardened high-entropy alloy with outstanding tensile properties. Acta. Mater. 2016, 102, 187–196. [Google Scholar] [CrossRef]
- Lu, Y.; Gao, X.; Jiang, L.; Chen, Z.; Wang, T.; Jie, J.; Kang, H.; Zhang, Y.; Guo, S.; Ruan, H.; et al. Directly cast bulk eutectic and near-eutectic high entropy alloys with balanced strength and ductility in a wide temperature range. Acta Mater. 2017, 124, 143–150. [Google Scholar] [CrossRef]
- Lu, Y.; Jiang, H.; Guo, S.; Wang, T.; Cao, Z.; Li, T. A new strategy to design eutectic high-entropy alloys using mixing enthalpy. Intermetallics 2017, 91, 124–128. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, C.L.; Zhang, C.H.; Guan, M.; Tan, J.Z. Laser surface alloying of FeCoCrAlNi high-entropy alloy on 304 stainless steel to enhance corrosion and cavitation erosion resistance. Opt. Laser. Technol. 2016, 84, 23–31. [Google Scholar] [CrossRef]
- Niu, X.; Julius, J.; Dan, Z.; Yang, G.; You, Y. Microstructure and corrosion properties of AlxFeCoCrNiCu (x=0.25, 0.5, 1.0) thin coatings on steel substrates deposited by electron beam evaporation. Rare Metal Mater. Eng. 2017, 46, 3621–3625. [Google Scholar] [CrossRef]
- Kao, Y.F.; Lee, T.D.; Chen, S.K.; Chang, Y.S. Electrochemical passive properties of AlxCoCrFeNi (x = 0, 0.25, 0.50, 1.00) alloys in sulfuric acids. Corros. Sci. 2010, 52, 1026–1034. [Google Scholar] [CrossRef]
- Li, Q.H.; Yue, T.M.; Guo, Z.N.; Lin, X. Microstructure and corrosion properties of AlCoCrFeNi high entropy alloy coatings deposited on AISI 1045 steel by the electrospark process. Metall. Mater. Trans. A 2013, 44, 1767–1778. [Google Scholar] [CrossRef]
- Qiao, J.W.; Ma, S.G.; Huang, E.W.; Chuang, C.P.; Liaw, P.K.; Zhang, Y. Microstructural characteristics and mechanical behaviors of AlCoCrFeNi high-entropy alloys at ambient and cryogenic temperatures. Mater. Sci. Forum. 2011, 688, 419–425. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, W.; Fu, H. Effect of solid aluminization on microstructure of AlCoCrFeNi high-entropy alloy. Heat. Treat. Met. 2015, 29, 250–252. [Google Scholar] [CrossRef]
- Manzoni, A.; Daoud, H.; Völkl, R.; Glatzel, U.; Wanderka, N. Phase separation in equiatomic AlCoCrFeNi high-entropy alloy. Ultramicroscopy 2013, 132, 212–215. [Google Scholar] [CrossRef]
- Ma, S.G.; Zhang, Y. Effect of Nb addition on the microstructure and properties of AlCoCrFeNi high-entropy alloy. Mat. Sci. Eng. A 2012, 532, 480–486. [Google Scholar] [CrossRef]
- Na, Y.S.; Lim, K.R.; Chang, H.J.; Kim, J. Effect of trace additions of Ti on the microstructure of AlCoCrFeNi-based high entropy alloy. Sci. Adv. Mater. 2016, 8, 1984–1988. [Google Scholar] [CrossRef]
- Yong, D.; Zhou, K.; Lu, Y.; Gao, X.; Wang, T.; Li, T. Effect of vanadium addition on the microstructure and properties of AlCoCrFeNi high entropy alloy. Mater. Des. 2014, 57, 67–72. [Google Scholar] [CrossRef]
- Cunat, P. Alloying Elements in Stainless Steel and Other Chromium-Containing Alloys; ICDA: Paris, France, 2004. [Google Scholar]
- Li, X.C.; Dou, D.; Zheng, Z.Y.; Li, J.C. Microstructure and properties of FeAlCrNiMox high-entropy alloys. J. Mater. Eng. Perform. 2016, 25, 2164–2169. [Google Scholar] [CrossRef]
- Jiang, L.; Cao, Z.Q.; Jie, J.C.; Zhang, J.J.; Lu, Y.P.; Wang, T.M.; Li, T.J. Effect of Mo and Ni elements on microstructure evolution and mechanical properties of the CoFeNixVMoy high entropy alloys. J. Alloy. Compd. 2015, 689, 585–590. [Google Scholar] [CrossRef]
- Yong, D.; Lu, Y.; Kong, J.; Zhang, J.; Li, T. Microstructure and mechanical properties of multi-component AlCrFeNiMox high-entropy alloys. J. Alloy. Compd. 2013, 573, 96–101. [Google Scholar] [CrossRef]
- Liu, W.H.; Lu, Z.P.; He, J.Y.; Luan, J.H.; Wang, Z.J.; Liu, B.; Yong, L.; Chen, M.W.; Liu, C.T. Ductile CoCrFeNiMox high entropy alloys strengthened by hard intermetallic phases. Acta Mater. 2016, 116, 332–342. [Google Scholar] [CrossRef]
- Tsai, M.H.; Yuan, H.; Cheng, G.M.; Xu, W.Z.; Jiang, W.W. Significant hardening due to the formation of a sigma phase matrix in a high entropy alloy. Intermetallics 2013, 33, 81–86. [Google Scholar] [CrossRef]
- Wei, W.; Li, J.; Hui, J.; Pan, X.; Cao, Z.; Deng, D.; Wang, T.; Li, T. Phase evolution and properties of Al2CrFeNiMox high-entropy alloys coatings by laser cladding. J. Therm. Spray. Technol. 2015, 24, 1333–1340. [Google Scholar] [CrossRef]
- Chou, H.P.; Chang, Y.S.; Chen, S.K.; Yeh, J.W. Microstructure, thermophysical and electrical properties in AlxCoCrFeNi (0 ≤ x ≤ 2) high-entropy alloys. Mat. Sci. Eng. B 2009, 163, 184–189. [Google Scholar] [CrossRef]
- Tong, C.J.; Chen, M.R.; Yeh, J.W.; Lin, S.J.; Chen, S.K.; Shun, T.T.; Chang, S.Y. Mechanical performance of the AlxCoCrCuFeNi high-entropy alloy system with multiprincipal elements. Metall. Mater. Trans. A 2005, 36, 1263–1271. [Google Scholar] [CrossRef]
- Kao, Y.F.; Chen, T.J.; Chen, S.K.; Yeh, J.W. Microstructure and mechanical property of as-cast, -homogenized, and -deformed AlxCoCrFeNi (0 ≤ x ≤ 2) high-entropy alloys. J. Alloy. Compd. 2009, 488, 57–64. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Collins, L.; Rui, F.; Zhang, C.; Balke, N.; Liaw, P.K.; Yang, B. Homogenization of AlxCoCrFeNi high-entropy alloys with improved corrosion resistance. Corros. Sci. 2018, 133, 120–131. [Google Scholar] [CrossRef]



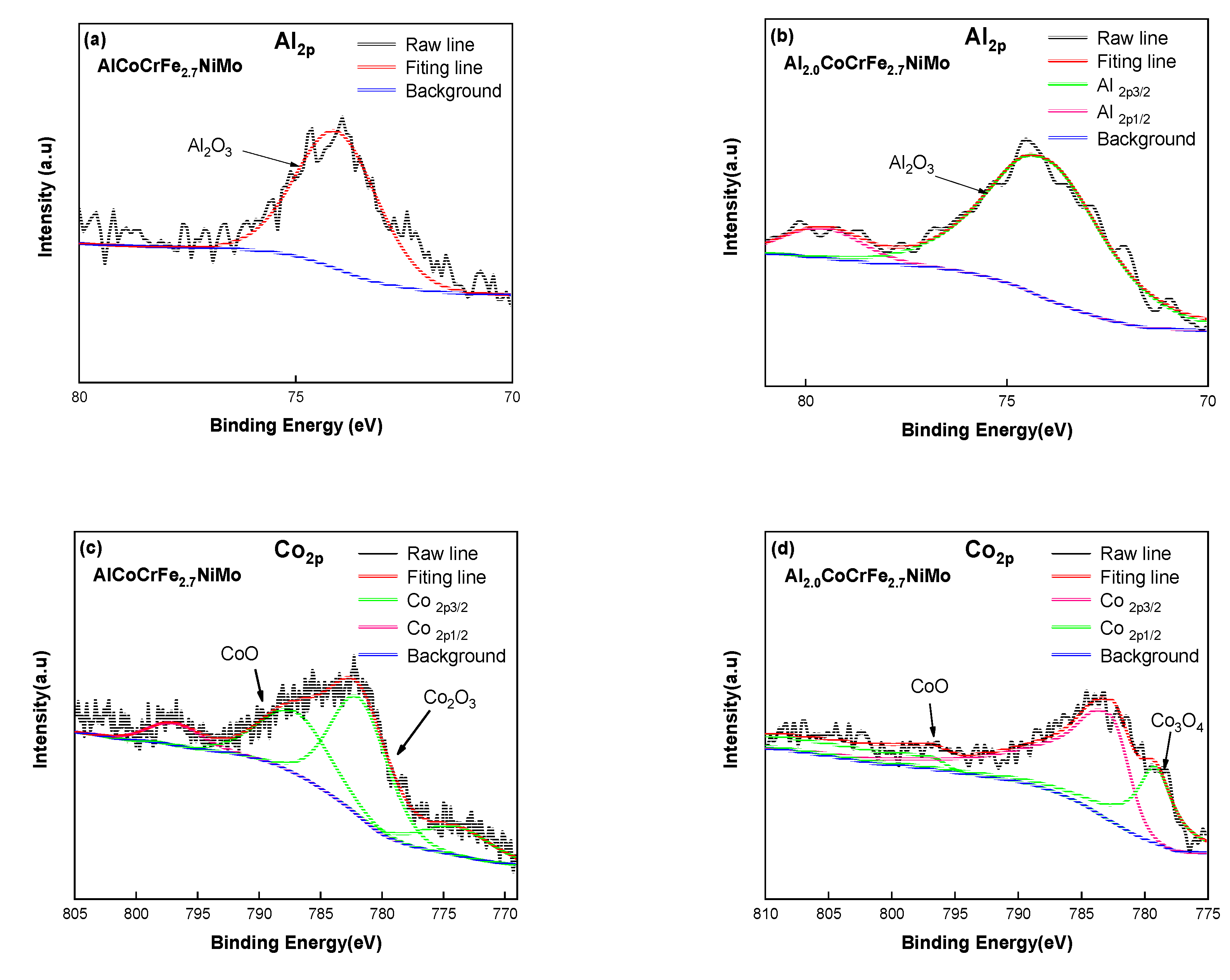

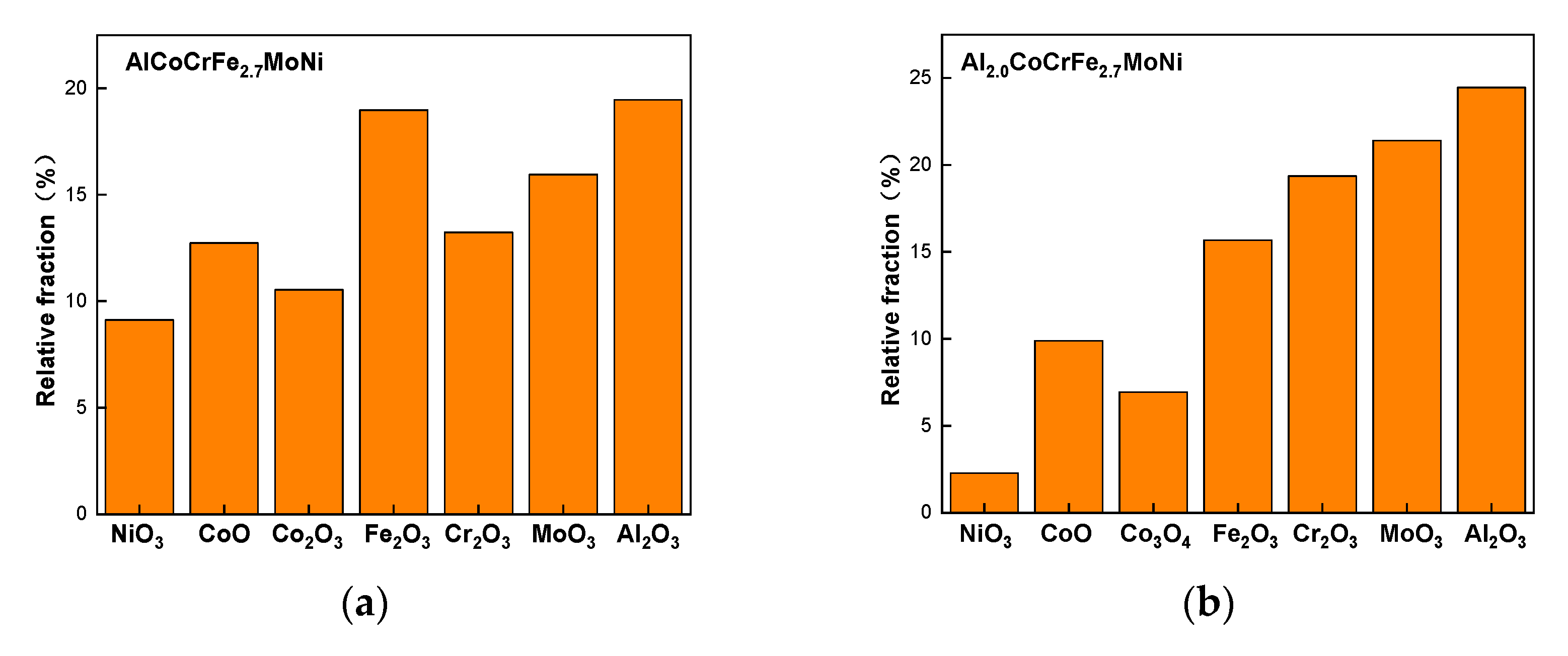
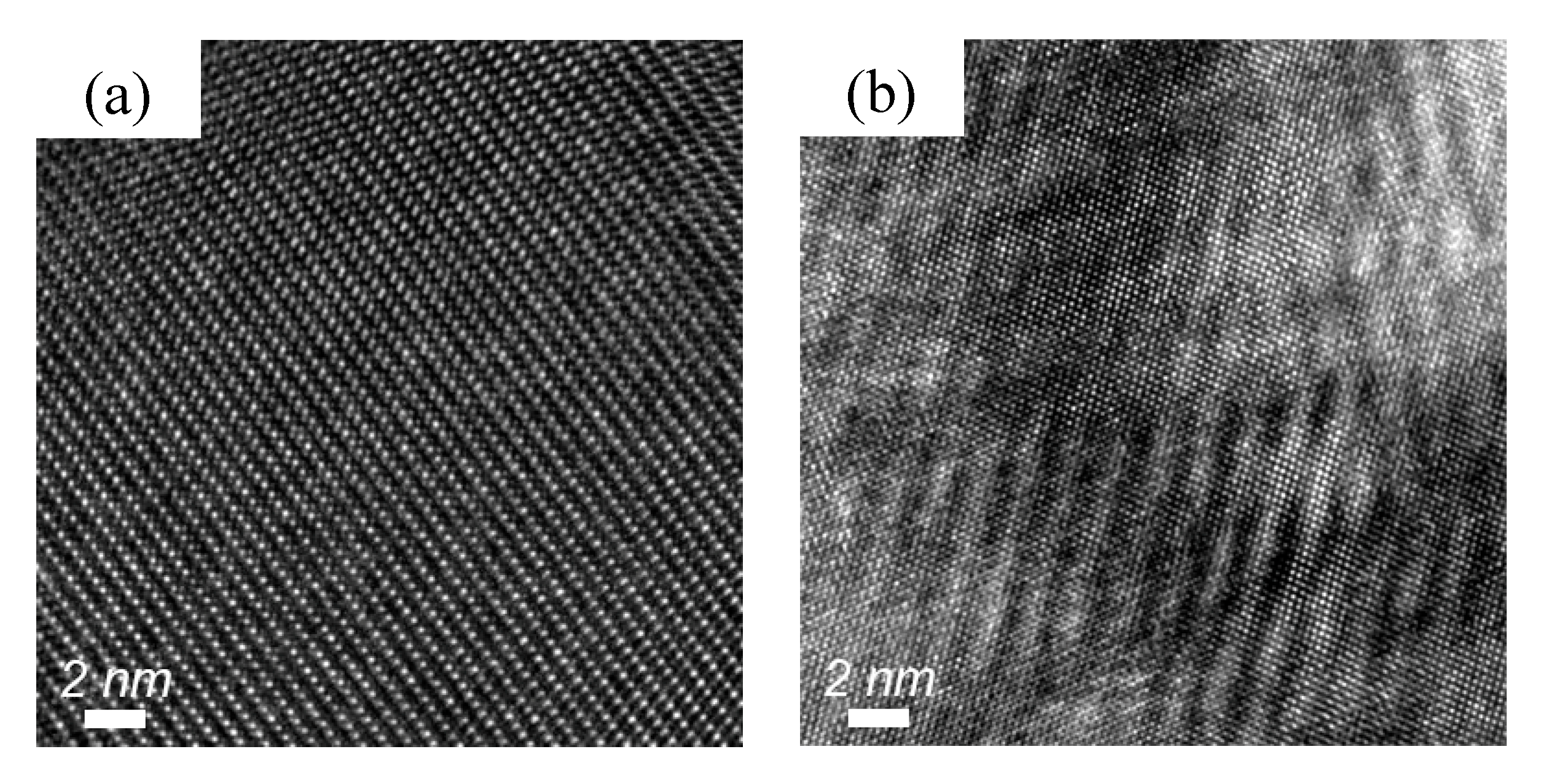

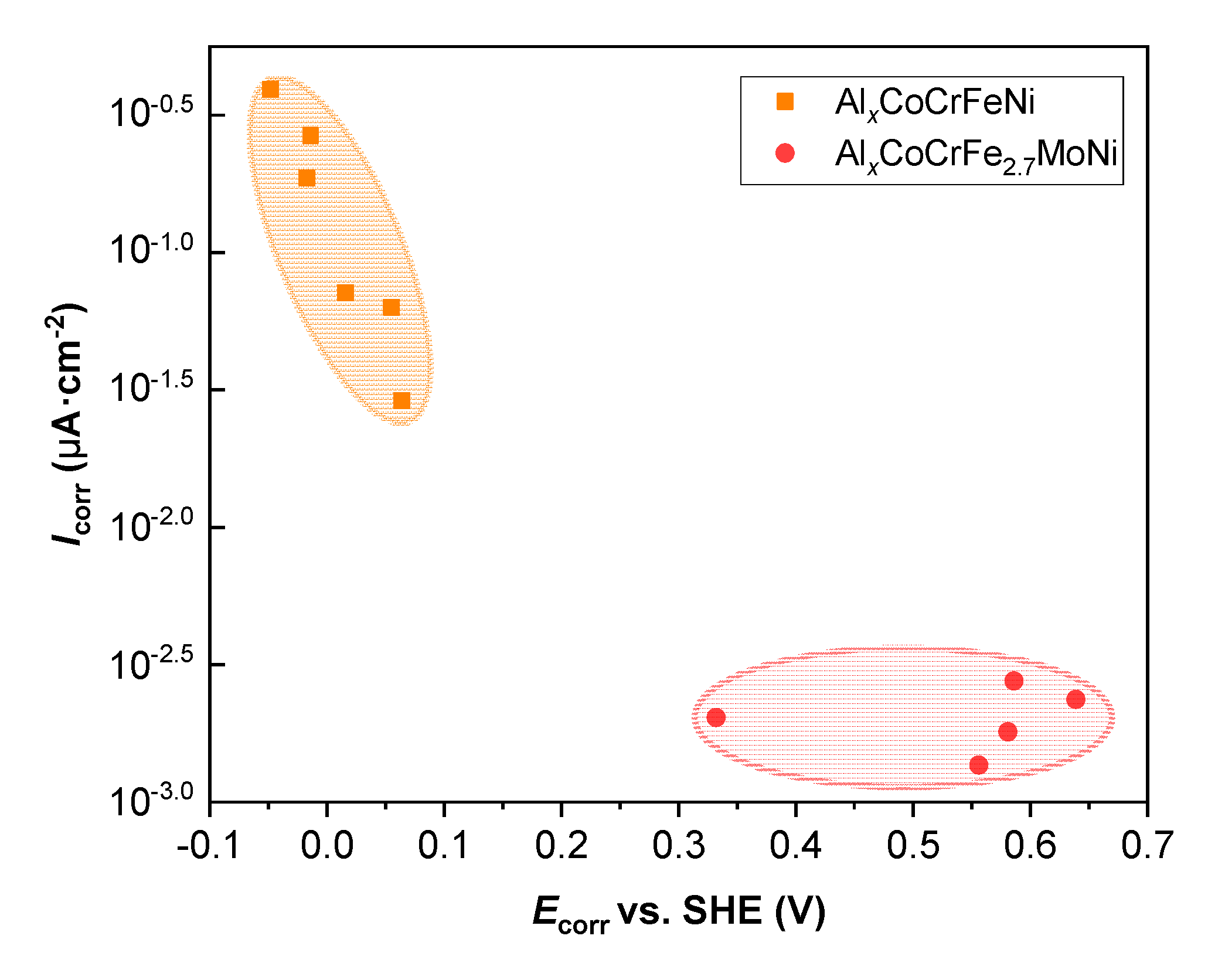
| Element | Fe | Al | S | P | Mn | Si | C |
|---|---|---|---|---|---|---|---|
| Content | 99.457 | 0.22 | 0.014 | 0.011 | 0.120 | 0.150 | 0.028 |
| x | Al | Co | Cr | Fe | Mo | Ni |
|---|---|---|---|---|---|---|
| 0 | 0 | 18.34 | 16.18 | 17.38 | 29.85 | 18.26 |
| 0.5 | 4.03 | 17.60 | 15.52 | 16.68 | 28.65 | 17.53 |
| 1.0 | 7.74 | 16.92 | 14.92 | 16.03 | 27.54 | 16.85 |
| 1.5 | 11.18 | 16.28 | 14.37 | 15.43 | 26.51 | 16.22 |
| 2.0 | 14.38 | 15.70 | 13.85 | 14.88 | 25.56 | 15.64 |
| x | Type | Al | Cr | Fe | Co | Ni | Mo |
|---|---|---|---|---|---|---|---|
| 0 | Actual | 0 | 14.52 | 40.39 | 15.04 | 16.56 | 13.49 |
| Target | 0 | 14.93 | 40.30 | 14.93 | 14.93 | 14.93 | |
| 0.5 | Actual | 5.92 | 13.73 | 37.87 | 14.88 | 12.82 | 14.79 |
| Target | 6.94 | 13.89 | 37.50 | 13.89 | 13.89 | 13.89 | |
| 1.0 | Actual | 12.71 | 13.25 | 36.33 | 12.96 | 12.76 | 11.98 |
| Target | 12.99 | 12.99 | 35.06 | 12.99 | 12.99 | 12.99 | |
| 1.5 | Actual | 16.36 | 13.93 | 32.77 | 12.19 | 12.81 | 11.94 |
| Target | 18.29 | 12.20 | 32.93 | 12.20 | 12.20 | 12.20 | |
| 2.0 | Actual | 19.25 | 11.34 | 33.95 | 11.45 | 11.69 | 12.32 |
| Target | 22.99 | 11.49 | 31.03 | 11.49 | 11.49 | 11.49 |
| Alloy | Region | Al | Co | Cr | Fe | Ni | Mo |
|---|---|---|---|---|---|---|---|
| Al0.0 | A region | 0 | 0 | 79.98 | 20.02 | 0 | 0 |
| B region | 0 | 17.71 | 13.85 | 53.28 | 13.61 | 7.55 | |
| Al0.5 | D region | 5.25 | 11.91 | 8.59 | 53.41 | 9.24 | 11.60 |
| Al1.0 | C region | 1.76 | 10 | 18.2 | 49.44 | 2.46 | 17.75 |
| D region | 28.91 | 17.04 | 1.96 | 22.17 | 29.57 | 0.32 | |
| Al1.5 | C region | 1.01 | 8.98 | 13.72 | 58.11 | 2.01 | 34.89 |
| D region | 25.60 | 11.63 | 3.84 | 38.55 | 19.20 | 1.18 | |
| E region | 6.34 | 7.93 | 15.87 | 43.97 | 3.17 | 22.68 | |
| Al2.0 | C region | 3.53 | 7.87 | 15.21 | 53.58 | 2.16 | 17.34 |
| D region | 26.68 | 12.58 | 3.84 | 36.52 | 19.76 | 0.58 |
| Alloy | Before Abrasion/g | After Abrasion/g | Abrasion Weight Loss/mg |
|---|---|---|---|
| Al1.0 | 10.0048 | 10.0044 | 0.4 |
| Al1.5 | 8.4143 | 8.4141 | 0.2 |
| Al2.0 | 10.1304 | 10.1303 | 0.1 |
| Alloys | Icorr /μA·cm−2 | Ecorr vs. SHE/V |
|---|---|---|
| Al0.0 | 2.033 × 10−3 | 0.332 |
| Al0.5 | 1.803 × 10−3 | 0.581 |
| Al1.0 | 1.355 × 10−3 | 0.556 |
| Al1.5 | 2.364 × 10−3 | 0.639 |
| Al2.0 | 2.762 × 10−3 | 0.586 |
| Element | ||||||
|---|---|---|---|---|---|---|
| Al | Co | Cr | Fe | Ni | Mo | |
| Al | - | |||||
| Co | −19 | - | ||||
| Cr | −10 | −4 | - | |||
| Fe | −11 | −1 | −1 | - | ||
| Ni | −22 | 0 | −7 | −2 | - | |
| Mo | −5 | −5 | 0 | −2 | −7 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sha, M.; Jia, C.; Qiao, J.; Feng, W.; Ai, X.; Jing, Y.-A.; Shen, M.; Li, S. Microstructure and Properties of High-Entropy AlxCoCrFe2.7MoNi Alloy Coatings Prepared by Laser Cladding. Metals 2019, 9, 1243. https://doi.org/10.3390/met9121243
Sha M, Jia C, Qiao J, Feng W, Ai X, Jing Y-A, Shen M, Li S. Microstructure and Properties of High-Entropy AlxCoCrFe2.7MoNi Alloy Coatings Prepared by Laser Cladding. Metals. 2019; 9(12):1243. https://doi.org/10.3390/met9121243
Chicago/Turabian StyleSha, Minghong, Chuntang Jia, Jun Qiao, Wenqiang Feng, Xingang Ai, Yu-An Jing, Minggang Shen, and Shengli Li. 2019. "Microstructure and Properties of High-Entropy AlxCoCrFe2.7MoNi Alloy Coatings Prepared by Laser Cladding" Metals 9, no. 12: 1243. https://doi.org/10.3390/met9121243





