Investigation on the Effect of Roasting and Leaching Parameters on Recovery of Gallium from Solid Waste Coal Fly Ash
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Alkali Roasting before Acid Leaching
2.2.2. Acid Leaching
3. Results
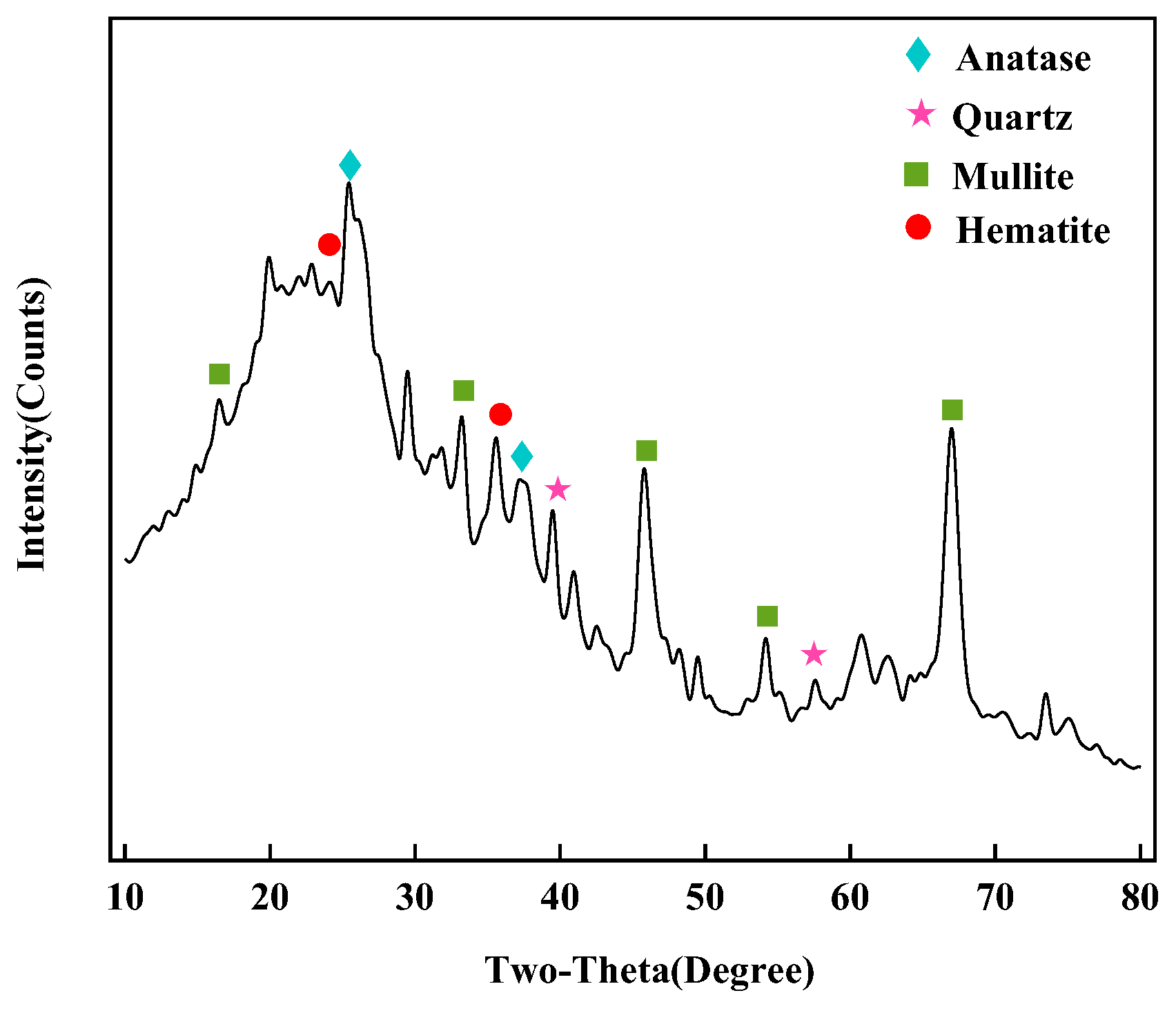
3.1. Sample Characterization
3.2. Comparison of Acid Leaching and Roasting before Acid Leaching
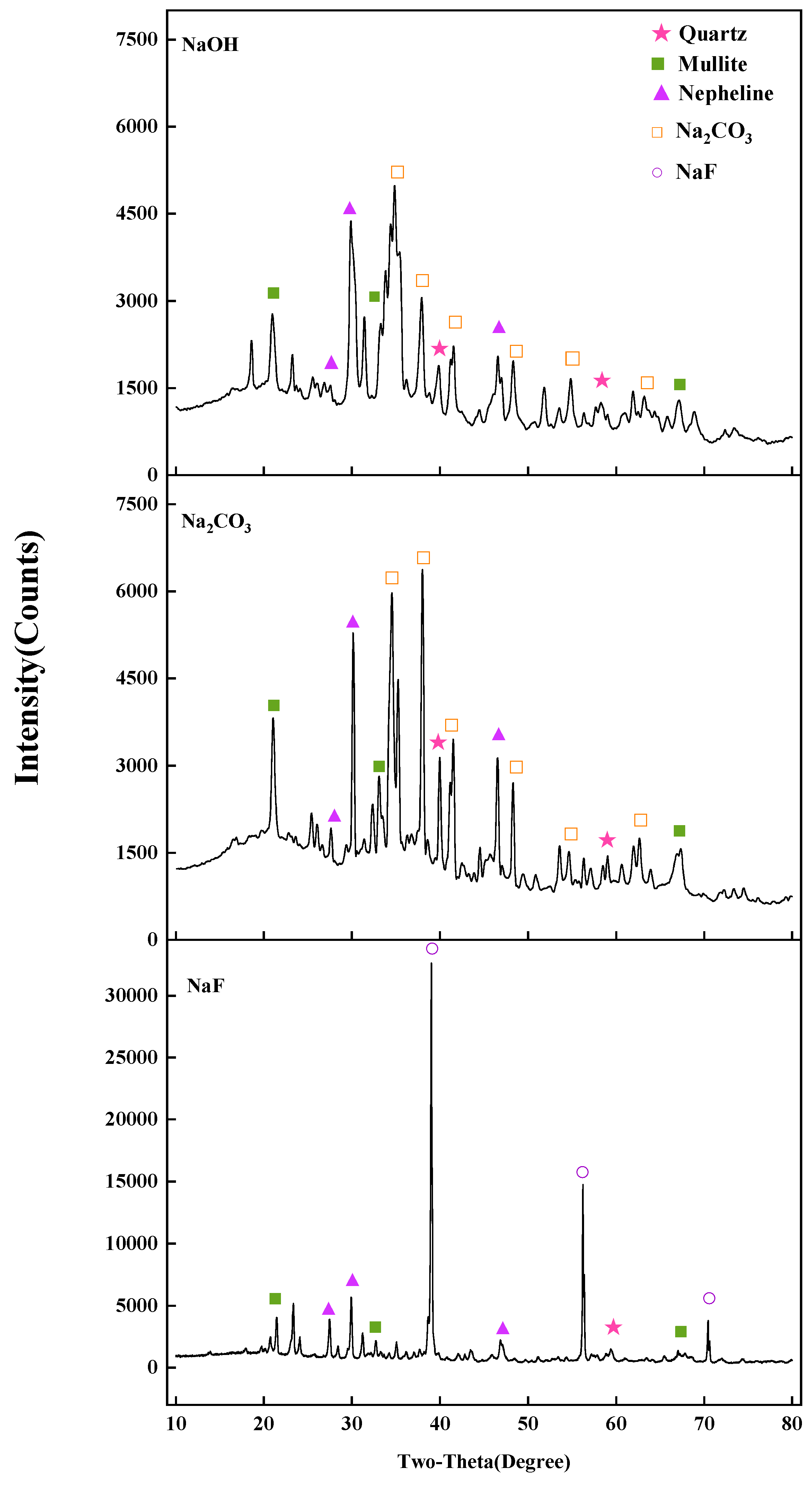
3.3. Influence of Roasting Stage
3.3.1. Effect of Roasting Temperature
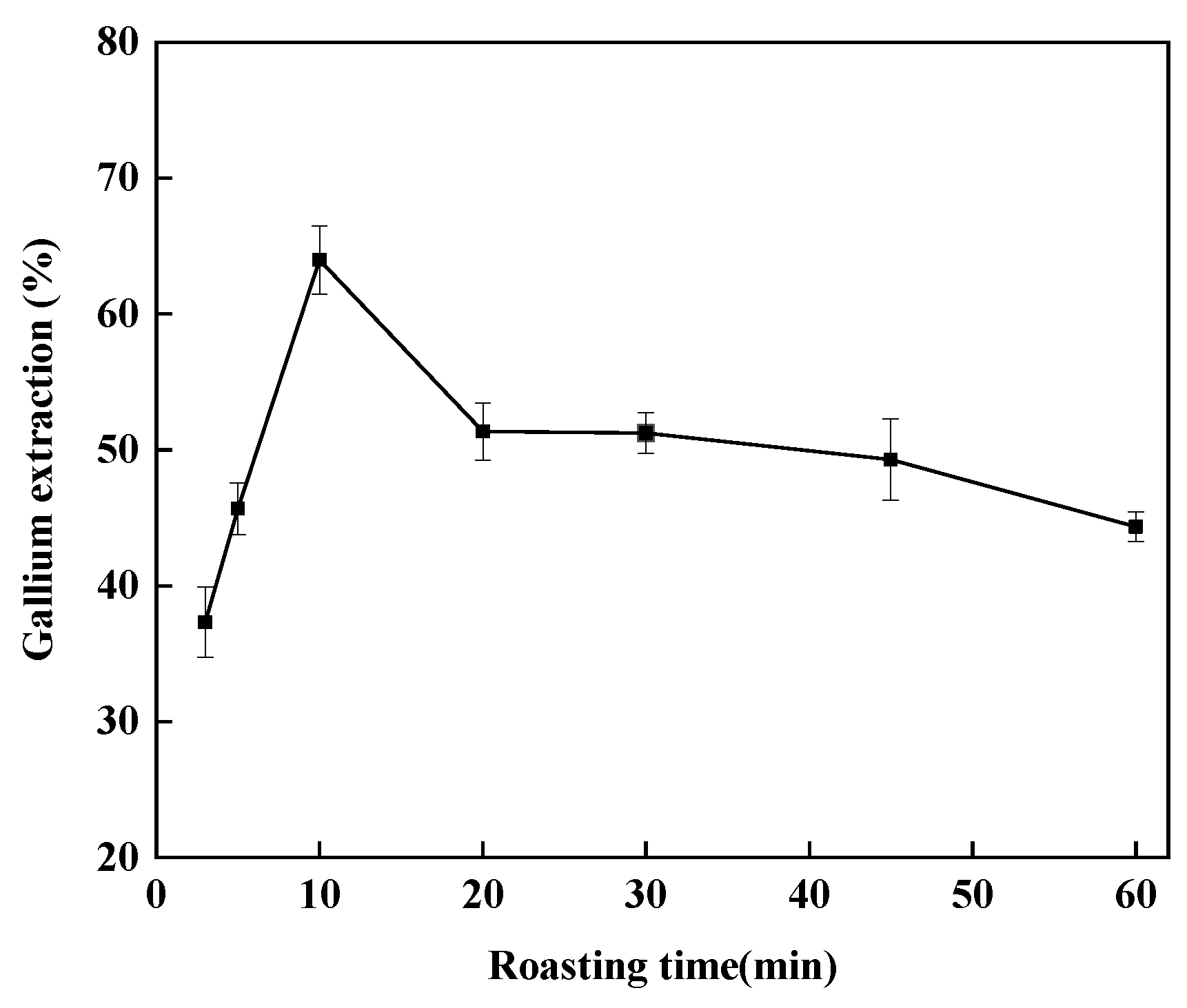
3.3.2. Effect of Roasting Time
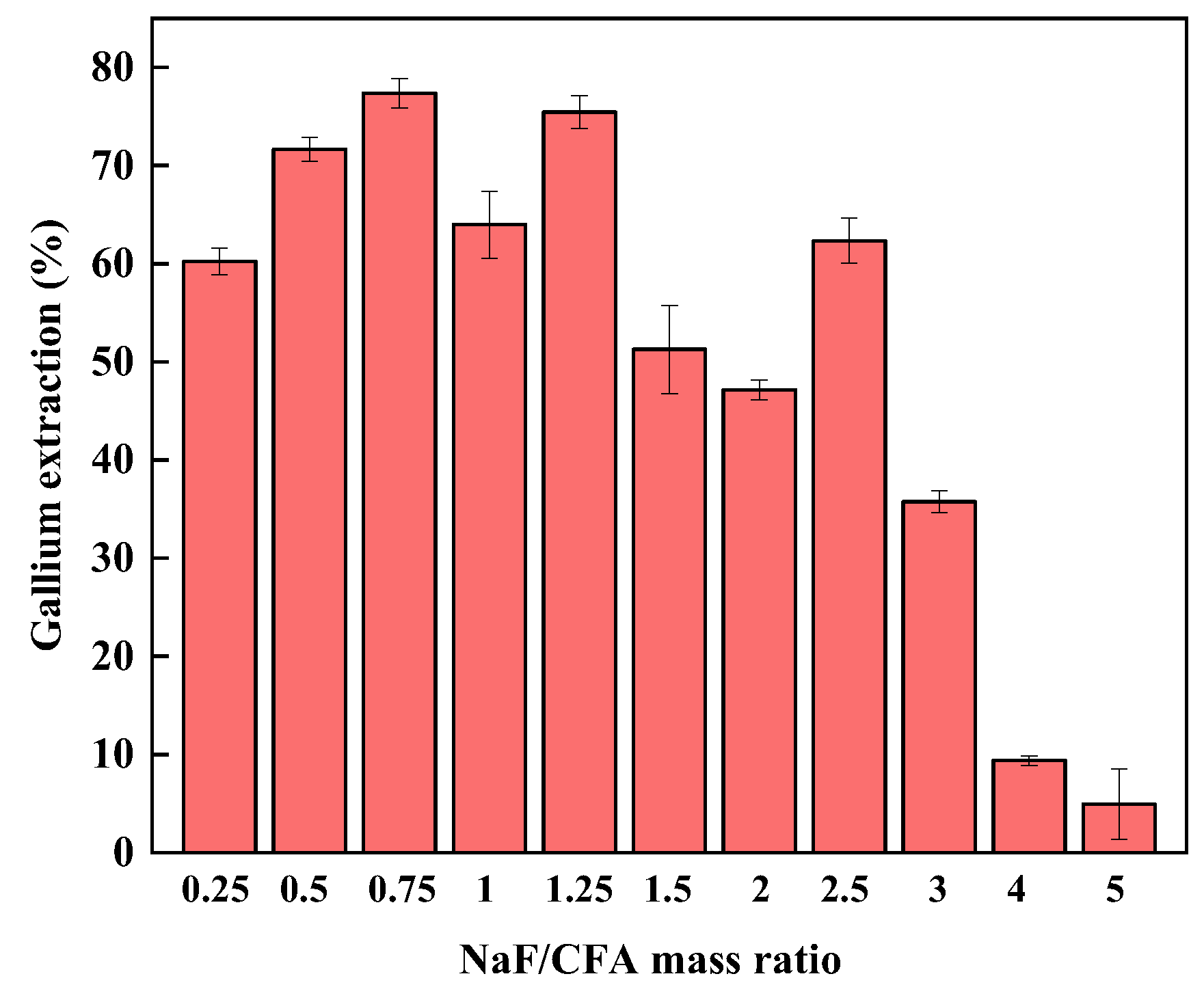
3.3.3. Effect of the Mass Ratio of NaF to CFA
3.4. Influence of Acid Leaching Stage
3.4.1. Effect of Acid Leaching Temperature
3.4.2. Effect of Acid Leaching Time and HNO3 Concentration
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Redlinger, M.; Eggert, R.; Woodhouse, M. Evaluating the availability of gallium, indium, and tellurium from recycled photovoltaic modules. Sol. Energy Mater. Sol. Cells 2015, 138, 58–71. [Google Scholar] [CrossRef]
- Yao, Z.; Ji, X.; Sarker, P.; Tang, J.; Ge, L.; Xia, M.; Xi, Y. A comprehensive review on the applications of coal fly ash. Earth Sci. Rev. 2015, 141, 105–121. [Google Scholar] [CrossRef]
- Qin, S.; Sun, Y.; Li, Y.; Wang, J.; Zhao, C.; Gao, K. Coal deposits as promising alternative sources for gallium. Earth Sci. Rev. 2015, 150, 95–101. [Google Scholar] [CrossRef]
- Dai, S.; Yan, X.; Ward, C.; Hower, J.; Zhao, L.; Wang, X.; Zhao, L.; Ren, D.; Finkelman, R. Valuable elements in Chinese coals: A review. Int. Geol. Rev. 2018, 60, 590–620. [Google Scholar] [CrossRef]
- Frenzel, M.; Ketris, M.; Seifert, T.; Gutzmer, J. On the current and future availability of gallium. Resour. Policy 2016, 47, 38–50. [Google Scholar] [CrossRef]
- Ma, Z.; Shan, X.; Cheng, F. Distribution characteristics of valuable elements, Al, Li, and Ga, and rare earth elements in feed coal, fly ash, and bottom ash from a 300 MW circulating fluidized bed boiler. ACS Omega 2019, 4, 6854–6863. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Gesser, H. Recovery of gallium from coal fly ash. Hydrometallurgy 1996, 41, 187–200. [Google Scholar] [CrossRef]
- Dai, S.; Ren, D.; Li, S. Discovery of the superlarge gallium ore deposit in Jungar, Inner Mongolia, North China. Chin. Sci. Bull. 2006, 51, 2243–2252. [Google Scholar] [CrossRef]
- Arroyo, F.; Font, O.; Maria Chimenos, J.; Fernandez-Pereira, C.; Querol, X.; Coca, P. IGCC fly ash valorisation. Optimisation of Ge and Ga recovery for an industrial application. Fuel Process. Technol. 2014, 124, 222–227. [Google Scholar] [CrossRef]
- Mketo, N.; Nomngongo, P.; Ngila, J. A single-step microwave-assisted acid extraction of total sulphur in coal samples followed by ICP-OES determination. Anal. Methods 2014, 6, 8505–8512. [Google Scholar] [CrossRef]
- Zou, J.; Tian, H.; Wang, Z. Leaching process of rare earth elements, gallium and niobium in a coal-bearing strata-hosted rare metal deposit-a case study from the late permian tuff in the Zhongliangshan mine, Chongqing. Metals 2017, 7, 174. [Google Scholar] [CrossRef]
- Taggart, R.; Hower, J.; Hsu-Kim, H. Effects of roasting additives and leaching parameters on the extraction of rare earth elements from coal fly ash. Int. J. Coal. Geol. 2018, 196, 106–114. [Google Scholar] [CrossRef]
- Tang, M.; Zhou, C.; Pan, J.; Zhang, N.; Liu, C.; Cao, S.; Hu, T.; Ji, W. Study on extraction of rare earth elements from coal fly ash through alkali fusion-Acid leaching. Miner. Eng. 2019, 136, 36–42. [Google Scholar] [CrossRef]
- Wen, K.; Jiang, F.; Zhou, X.; Sun, Z. Recovery of gallium from corundum flue dust by two-stage alkali leaching, carbonation, acid leaching and solvent extraction process. Metals 2018, 8, 545. [Google Scholar] [CrossRef]
- Wang, J.; Nakazato, T.; Sakanishi, K.; Yamada, O.; Tao, H.; Saito, I. Microwave digestion with HNO3/H2O2 mixture at high temperatures for determination of trace elements in coal by ICP-OES and ICP-MS. Anal. Chim. Acta 2004, 514, 115–124. [Google Scholar] [CrossRef]
- Shao, P.; Wang, W.; Chen, L.; Duan, P.; Qian, F.; Ma, M.; Xiong, W.; Yu, S. Distribution, occurrence, and enrichment of gallium in the Middle Jurassic coals of the Muli Coalfield, Qinghai, China. J. Geochem. Explor. 2018, 185, 116–129. [Google Scholar] [CrossRef]





| Sample | Al2O3, % | SiO2, % | CaO, % | TiO2, % | Fe2O3, % | Ga, % | Loss on Ignition, % |
|---|---|---|---|---|---|---|---|
| CFA | 41.73 | 22.51 | 2.44 | 1.79 | 1.47 | 0.00671 | 9.19 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Wang, Y.; Zhou, G.; Gu, Y. Investigation on the Effect of Roasting and Leaching Parameters on Recovery of Gallium from Solid Waste Coal Fly Ash. Metals 2019, 9, 1251. https://doi.org/10.3390/met9121251
Huang J, Wang Y, Zhou G, Gu Y. Investigation on the Effect of Roasting and Leaching Parameters on Recovery of Gallium from Solid Waste Coal Fly Ash. Metals. 2019; 9(12):1251. https://doi.org/10.3390/met9121251
Chicago/Turabian StyleHuang, Jing, Yingbin Wang, Guanxuan Zhou, and Yu Gu. 2019. "Investigation on the Effect of Roasting and Leaching Parameters on Recovery of Gallium from Solid Waste Coal Fly Ash" Metals 9, no. 12: 1251. https://doi.org/10.3390/met9121251




