Removal of Phosphorus from High-Phosphorus Manganese Ores by Ammonia-Ammonium Carbonate Leaching Method
Abstract
:1. Introduction
2. Material and Experimental Procedure
2.1. Materials
2.2. Experimental
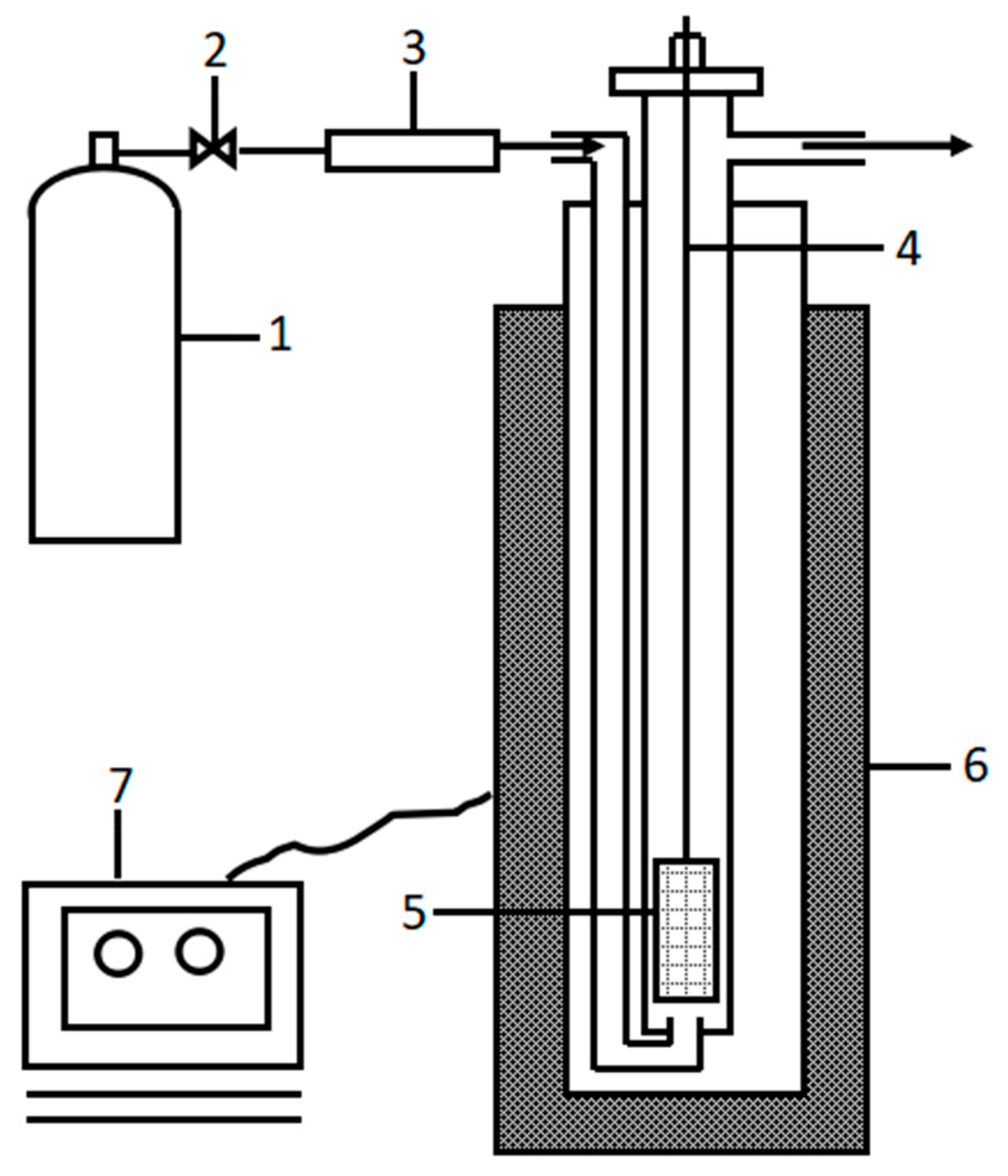
2.2.1. Roasting Procedure
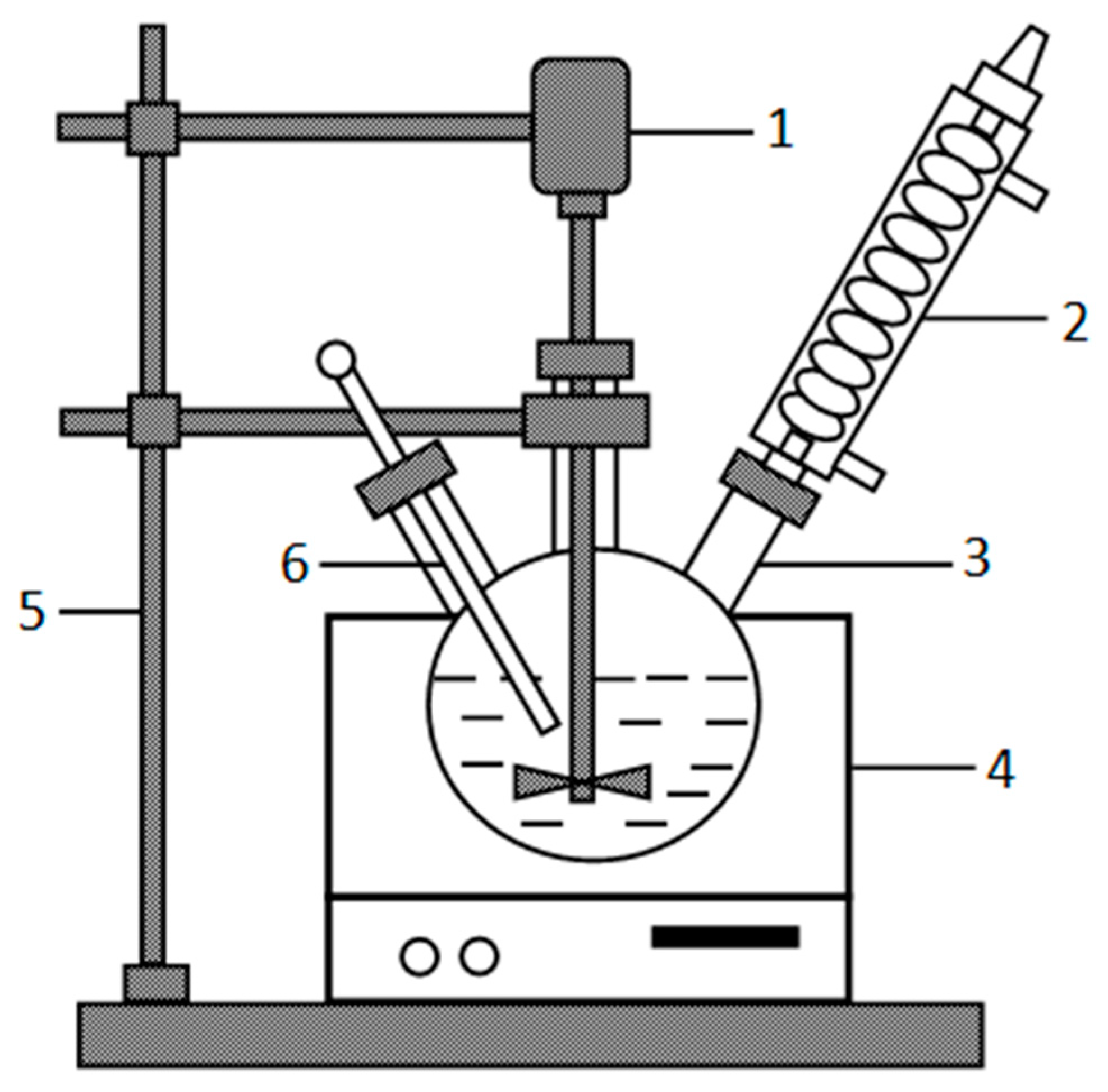
2.2.2. Leaching Procedure
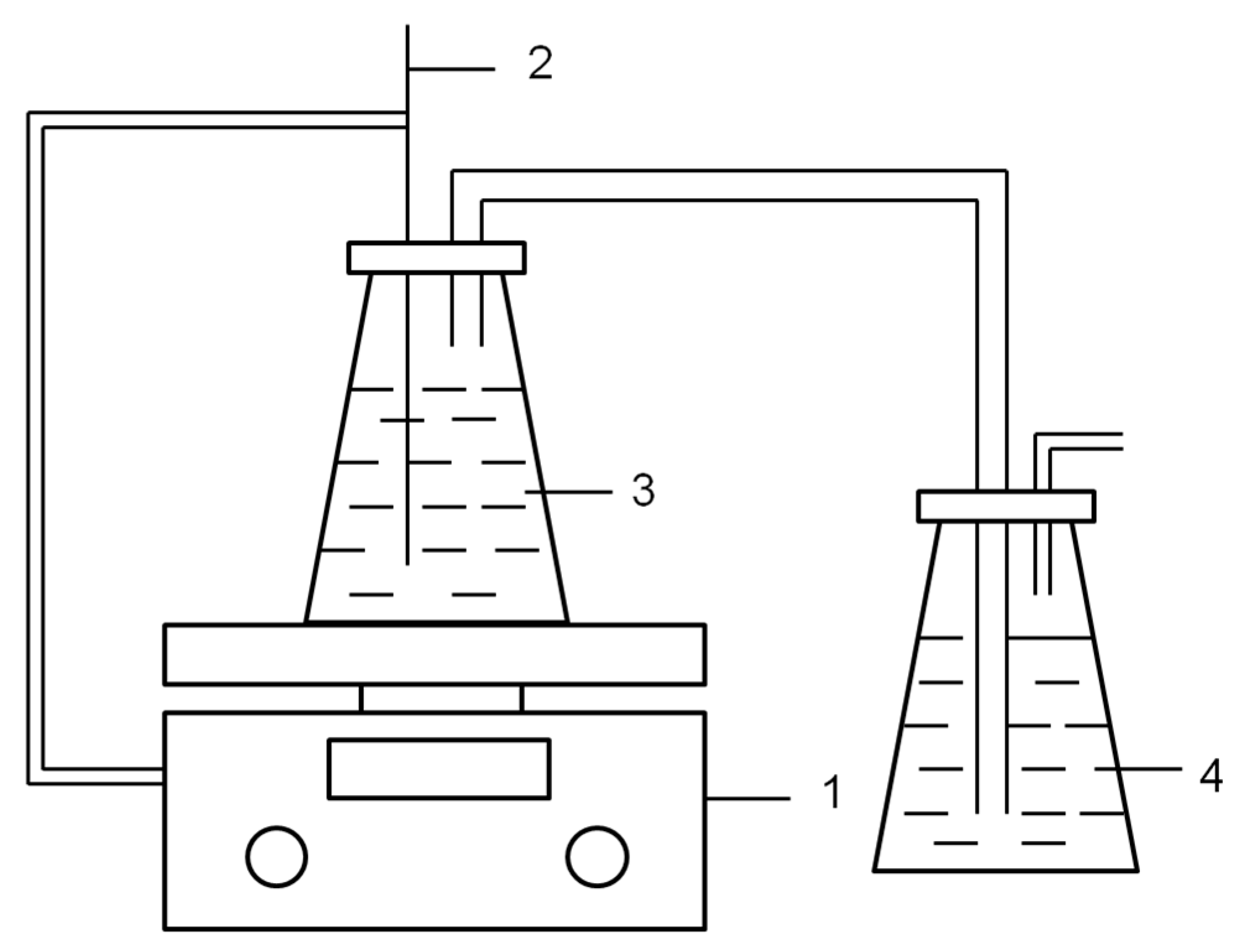
2.2.3. Evaporation Procedure
3. Results and Discussion
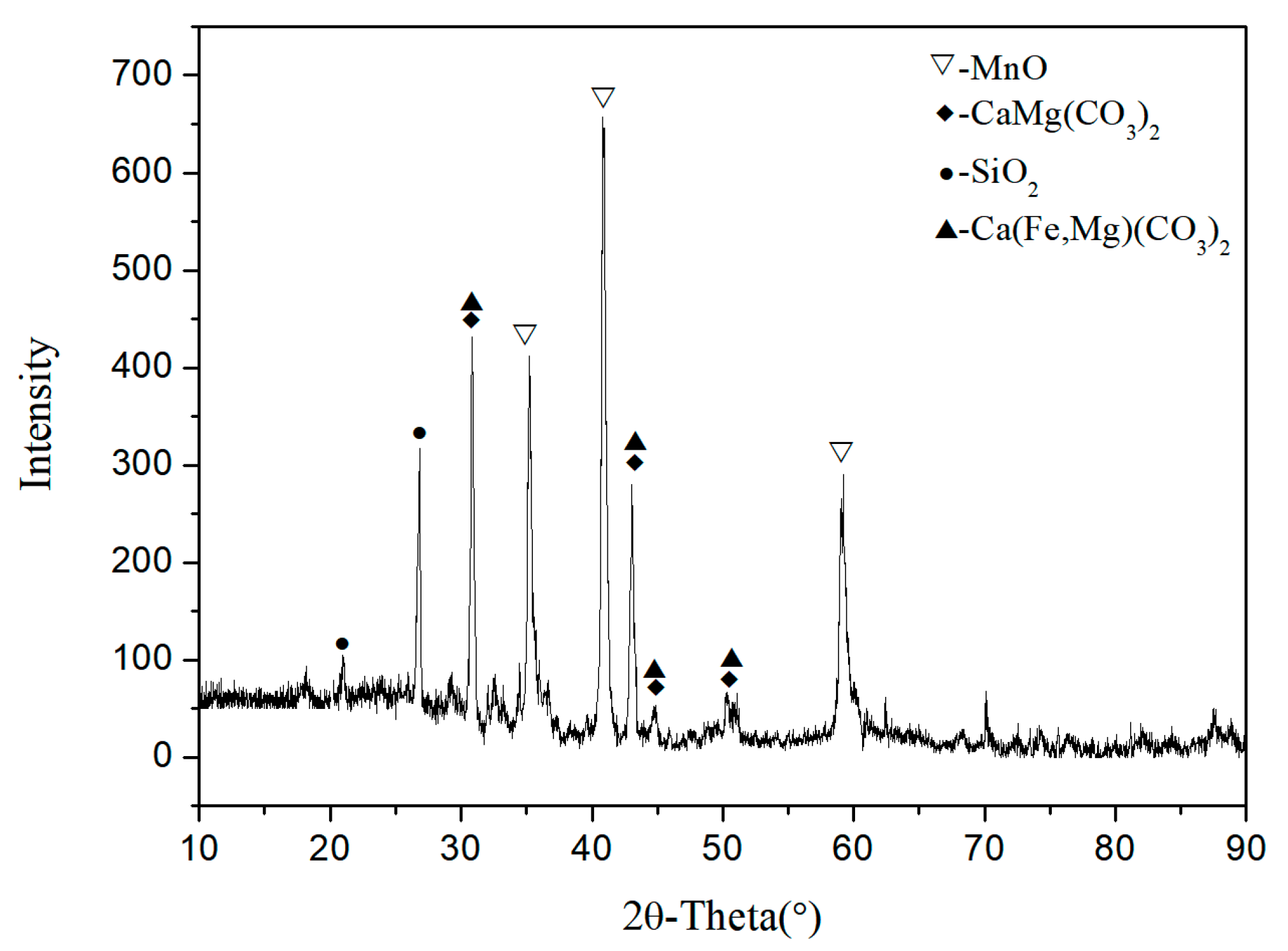
3.1. The Phases of Roasted Sample
3.2. Effect of the Ammonia to Ammonium Carbonate Concentration Ratio on Mn and P Leaching Efficiency
3.3. Effect of Leaching Temperature on Mn and P Leaching Efficiency
3.4. Effect of Liquid to Solid Ratio on Mn and P Leaching Efficiency
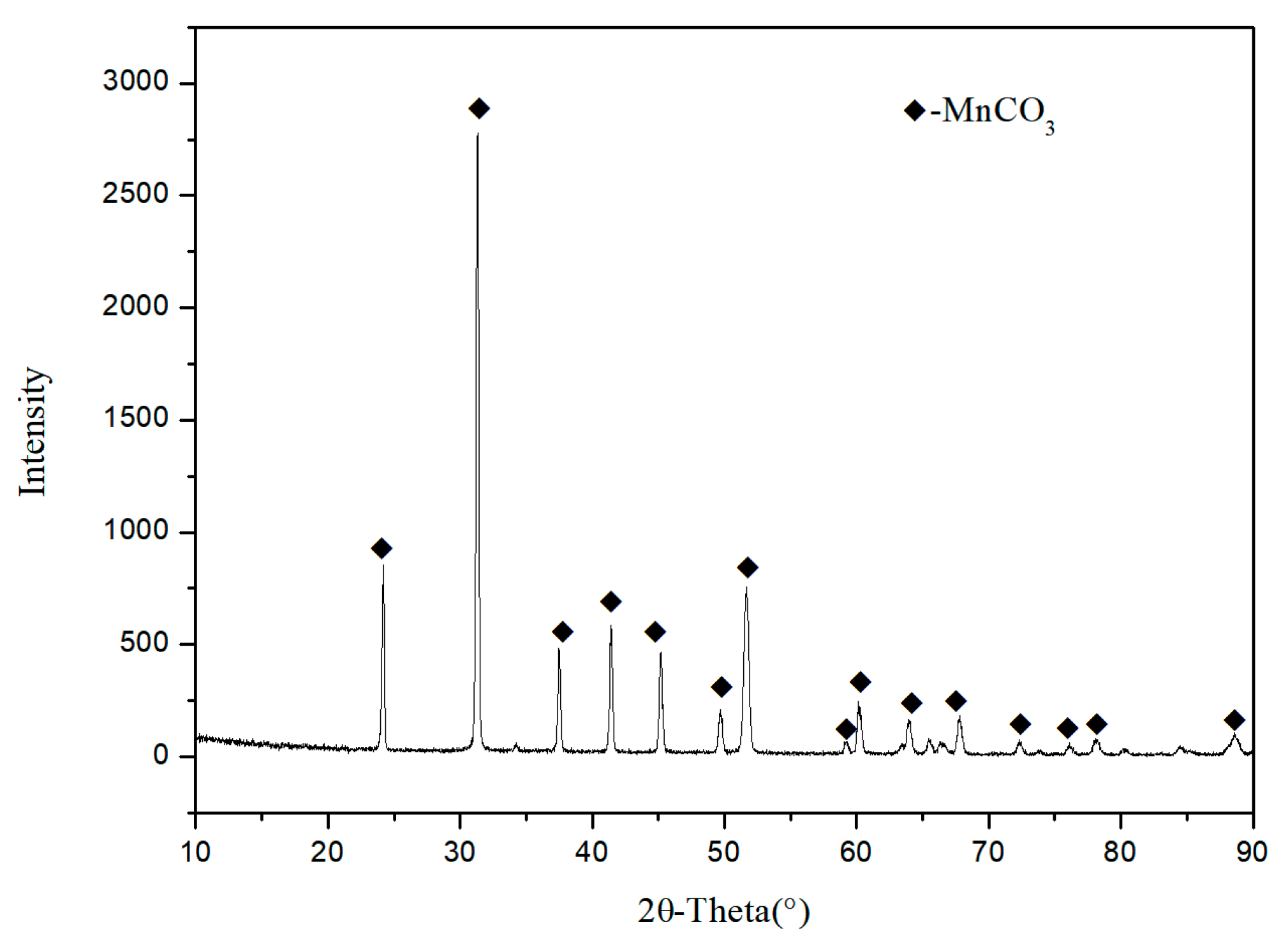
3.5. The Composition of Precipitated Manganiferous Sample
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kanungo, S.; Mishra, S. Dephosphorization of high-phosphorous manganese ores from Andhra Pradesh and southern Orissa, India, by roasting. Min. Metall. Explor. 2000, 17, 37–40. [Google Scholar] [CrossRef]
- Wu, W.; Dai, S.F.; Liu, Y. Dephosphorization stability of hot metal by double slag operation in basic oxygen furnace. J. Iron Steel Res. Int. 2017, 24, 908–915. [Google Scholar] [CrossRef]
- Zhu, D.Q.; Wang, H.; Pan, J.; Yang, C.C. Influence of Mechanical Activation on Acid Leaching Dephosphorization of High-phosphorus Iron Ore Concentrates. J. Iron Steel Res. Int. 2016, 23, 661–668. [Google Scholar] [CrossRef]
- Mani, K.; Subrahmanyam, D. Utilisation of low grade/off grade ores and mine waste. Proc. Indian Natn. Sci. Acad. A 1984, 50, 509–522. [Google Scholar]
- Naik, P.; Reddy, P.; Misra, V. Flock flotation studies of ultra fine siliceous manganese ore by linear orthogonal saturated design. J. Min. Metall. A Min. 2005, 41, 11–25. [Google Scholar]
- Wu, Y.; Shi, B.; Ge, W.; Yan, C.J.; Yang, X. Magnetic Separation and Magnetic Properties of Low-Grade Manganese Carbonate Ore. JOM 2015, 67, 361–368. [Google Scholar] [CrossRef]
- Hu, Y.P.; Xu, X.M. Study on high gradient magnetic separation of phosphorus removal from microparticle rhodophanite. Youse Jinshu Gongcheng 1989, 4, 49–54. [Google Scholar]
- Su, E.Q. Study on manganese enrichment and phosphorus reduction by mechanical beneficiation of manganese ore with high phosphorus content. China’s Manganese Ind. 1990, 6, 8–12. [Google Scholar]
- Kanungo, S.; Sant, B. Dephosphorization of phosphorus-rich manganese ores by selective leaching with dilute hydrochloric acid. Int. J. Miner. Process. 1981, 8, 359–375. [Google Scholar] [CrossRef]
- Kanungo, S.; Mishra, S. Reduction of phosphorus content of certain high phosphorus manganese ores of india by roasting with sodium chloride followed by leaching in acid medium. I: Statistical design of roasting experiments. Trans. Indian Inst. Met. 2002, 55, 81–89. [Google Scholar]
- Zhang, Y. Study on phosphorus enrichment and phosphorus reduction in huayuan type high phosphorus manganese ore. China’s Manganese Ind. 1992, 6, 12–16. [Google Scholar]
- Fei, T.; Luo, S.; Mu, W.; Lei, X.; Xin, H.; Zhai, Y.; Dai, Y. Manganese Extraction from Low-Grade Pyrolusite by Roasting with H2SO4. JOM 2018, 70, 2008–2014. [Google Scholar]
- Radmehr, V.; Koleini, S.M.J.; Khalesi, M.R.; Mohammadi, M.R.T. Ammonia Leaching: A new approach of copper industry in hydrometallurgical processes. J. Inst. Eng. (India) Ser. D 2013, 94, 95–104. [Google Scholar] [CrossRef]
- Heindl, R.; Ruppert, J.; Skow, M.; Conley, J. Manganese from Steel-Plant Slags by a Lime-Clinkering and Carbonate-Leaching Process: Part I, Lab. Development (in Two Parts). BuMines Rep. Inv. 1955, 5124, 23–61. [Google Scholar]
- Mcintosh, S.N.; Baglin, E.G. Recovery of Manganese from Steel Plant Slag by Carbamate Leaching; US Department of the Interior, Bureau of Mines: Washington, DC, USA, 1992.
- Chen, J.B. Reduction roasting and ammonia leaching of high-iron manganese ore from Zunyi. China’s Manganese Ind. 1995, 13, 38–43. [Google Scholar]
- Chen, Y.M.; Yi, B.B. The theory and practice of ammonia leaching in the treatment of low-grade manganese. J. Wuhan Univ. Technol. 2004, 13, 100–105. [Google Scholar]
- Liu, Y.; Lin, Q.; Li, L.; Fu, J.; Zhu, Z.; Wang, C.; Qian, D. Study on hydrometallurgical process and kinetics of manganese extraction from low-grade manganese carbonate ores. Int. J. Min. Sci. Technol. 2014, 24, 567–571. [Google Scholar] [CrossRef]
- Baba, A.A.; Ghosh, M.K.; Pradhan, S.R.; Rao, D.S.; Baral, A.; Adekola, F.A. Characterization and kinetic study on ammonia leaching of complex copper ore. Trans. Nonferrous Met. Soc. China 2014, 24, 1587–1595. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Z.; Li, Q.; Cao, Z.; Yang, T. Dissolution behavior of willemite in the (NH4)2SO4–NH3–H2O system. Hydrometallurgy 2012, 125–126, 50–54. [Google Scholar] [CrossRef]
- Bingöl, D.; Canbazoğlu, M.; Aydoğan, S. Dissolution kinetics of malachite in ammonia/ammonium carbonate leaching. Hydrometallurgy 2005, 76, 55–62. [Google Scholar] [CrossRef]
- Langeloth, M.; Chiku, M.; Einaga, Y. Anodic stripping voltammetry of zinc at boron-doped diamond electrodes in ammonia buffer solution. Electrochim. Acta 2010, 55, 2824–2828. [Google Scholar] [CrossRef]
- Ding, Z.; Yin, Z.; Hu, H.; Chen, Q. Dissolution kinetics of zinc silicate (hemimorphite) in ammoniacal solution. Hydrometallurgy 2010, 104, 201–206. [Google Scholar] [CrossRef]
- Dian, Y. Application of EH-pH of Cu-NH3-H2O, Fe-NH3-H2O and Mn-NH3-H2O in hydrometallurgical process. Chongqing Shifan Daxue Xuebao Ziran Kexueban 1991, 2, 004. [Google Scholar]
- Li, H.Y.; Wang, K.; Hua, W.-H.; Yang, Z.; Zhou, W.; Xie, B. Selective leaching of vanadium in calcification-roasted vanadium slag by ammonium carbonate. Hydrometallurgy 2016, 160, 18–25. [Google Scholar] [CrossRef]
- Mei, X.C.; Tang, X.M. Summary of production process of chemical manganese dioxide abroad. China’s Manganese Ind. 1984, 2, 39–43. [Google Scholar]
- Devuyst, E. Reductive Dissolution of Goethite and Pyrolusite in Alkaline Solution. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 1970. [Google Scholar]
- Li, C.; Li, H.-Y.; Tu, C.-B.; Zhang, T.; Fang, H.-X.; Xie, B. Selective extraction of vanadium from the APV-precipitated waste water. In REWAS 2013; Springer: Berlin/Heidelberg, Germany, 2013; pp. 302–308. [Google Scholar]






| Researchers | Year | Object | Results | Reference |
|---|---|---|---|---|
| Heindl; Ruppert; Skow; Conley | 1955 | Open-hearth slag | Ferromanganese product suitable for use as blast furnace feed was produced. | [14] |
| Mcintosh; Baglin | 1992 | BOF slag | About 80% of the manganese could be extracted. | [15] |
| Chen | 1994 | High-iron manganese ore | Manganese extraction reaches 59.9% to 64.8%. | [16] |
| Chen; Yi | 2004 | Low-grade manganese ore | Manganese extraction reaches 81.5% to 91.2%. | [17] |
| Element | Mn | Ca | Si | Mg | Fe | S | Al | P |
|---|---|---|---|---|---|---|---|---|
| Content | 17.83 | 12.60 | 3.05 | 4.25 | 1.24 | 0.51 | 1.30 | 0.40 |
| Number | Ammonia to Ammonium Concentration Ratio | Leaching Temperature (°C) | Solid to Liquid Ratio |
|---|---|---|---|
| 1 | 14:0 | 25 | 5:1 |
| 2 | 14:0.5 | 25 | 5:1 |
| 3 | 14:1 | 25 | 5:1 |
| 4 | 14:1.5 | 25 | 5:1 |
| 5 | 14:2 | 25 | 5:1 |
| 6 | 14:2.5 | 25 | 5:1 |
| 7 | 14:3 | 25 | 5:1 |
| 8 | 14:2 | 15 | 5:1 |
| 9 | 14:2 | 25 | 5:1 |
| 10 | 14:2 | 35 | 5:1 |
| 11 | 14:2 | 45 | 5:1 |
| 12 | 14:2 | 55 | 5:1 |
| 13 | 14:2 | 25 | 3:1 |
| 14 | 14:2 | 25 | 4:1 |
| 15 | 14:2 | 25 | 5:1 |
| 16 | 14:2 | 25 | 6:1 |
| 17 | 14:2 | 25 | 7:1 |
| 18 | 14:2 | 25 | 8:1 |
| Component | Mn | MnCO3 | Fe | P | CaCO3 | MgCO3 | Si |
|---|---|---|---|---|---|---|---|
| Content | 44.12 | 92.25 | - | 0.02 | 3.42 | 1.85 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, Z.; Liang, X.; Wang, Y.; Wu, C. Removal of Phosphorus from High-Phosphorus Manganese Ores by Ammonia-Ammonium Carbonate Leaching Method. Metals 2019, 9, 1354. https://doi.org/10.3390/met9121354
Tu Z, Liang X, Wang Y, Wu C. Removal of Phosphorus from High-Phosphorus Manganese Ores by Ammonia-Ammonium Carbonate Leaching Method. Metals. 2019; 9(12):1354. https://doi.org/10.3390/met9121354
Chicago/Turabian StyleTu, Zhongbing, Xiaoping Liang, Yu Wang, and Chengbo Wu. 2019. "Removal of Phosphorus from High-Phosphorus Manganese Ores by Ammonia-Ammonium Carbonate Leaching Method" Metals 9, no. 12: 1354. https://doi.org/10.3390/met9121354




