Investigation of NbC/TiC Heterogeneous Nucleation Interface by First-Principles and Experimental Methods
Abstract
:1. Introduction
2. Calculation Method and Experimental Details
2.1. Calculation Methods and Details
2.2. Experimental Methods
3. Results and Discussion
3.1. Surface Properties of NbC and TiC
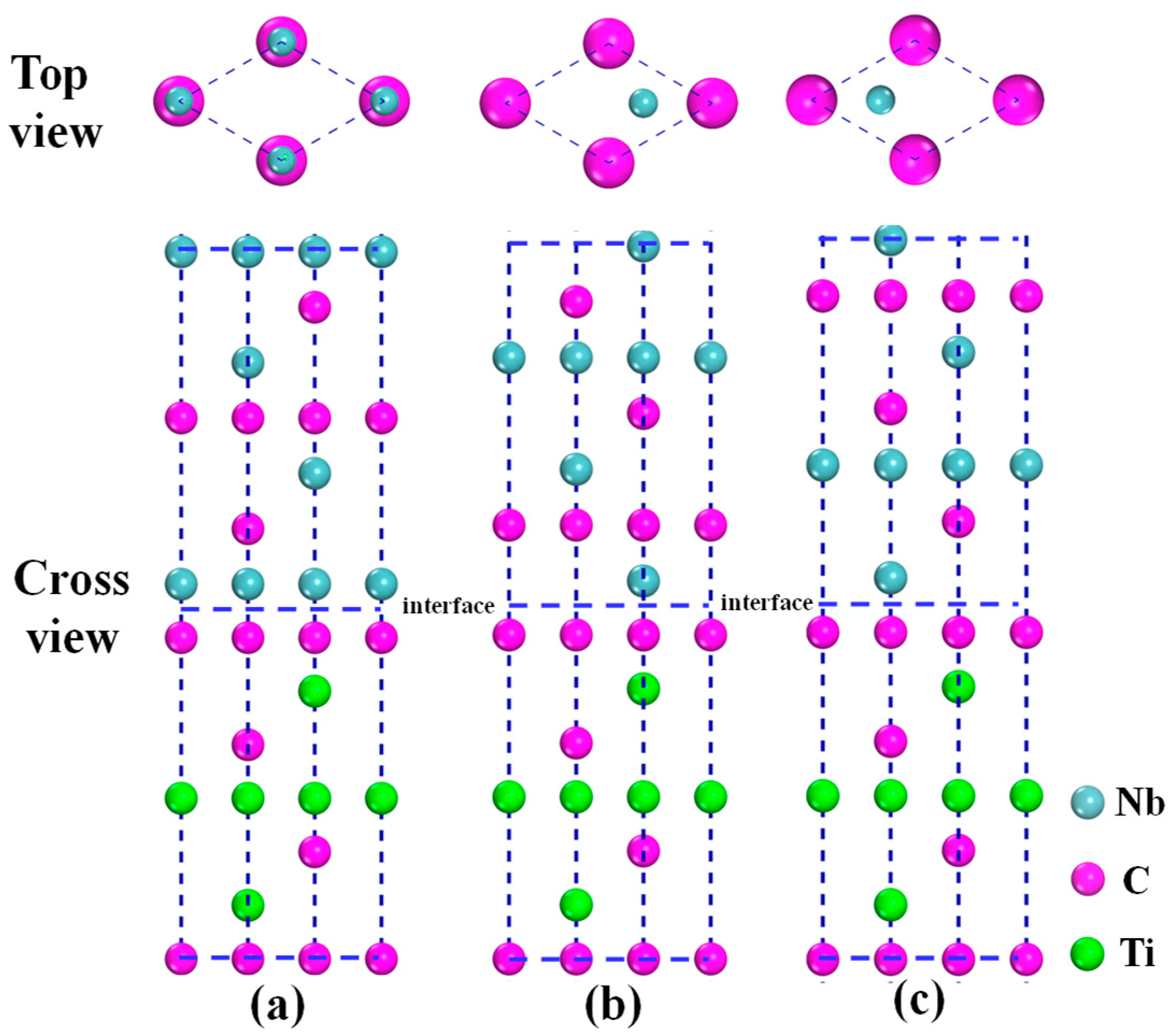
3.2. Models of NbC/TiC Interfaces
3.3. Interface Properties of NbC and TiC
3.3.1. Work of Adhesion
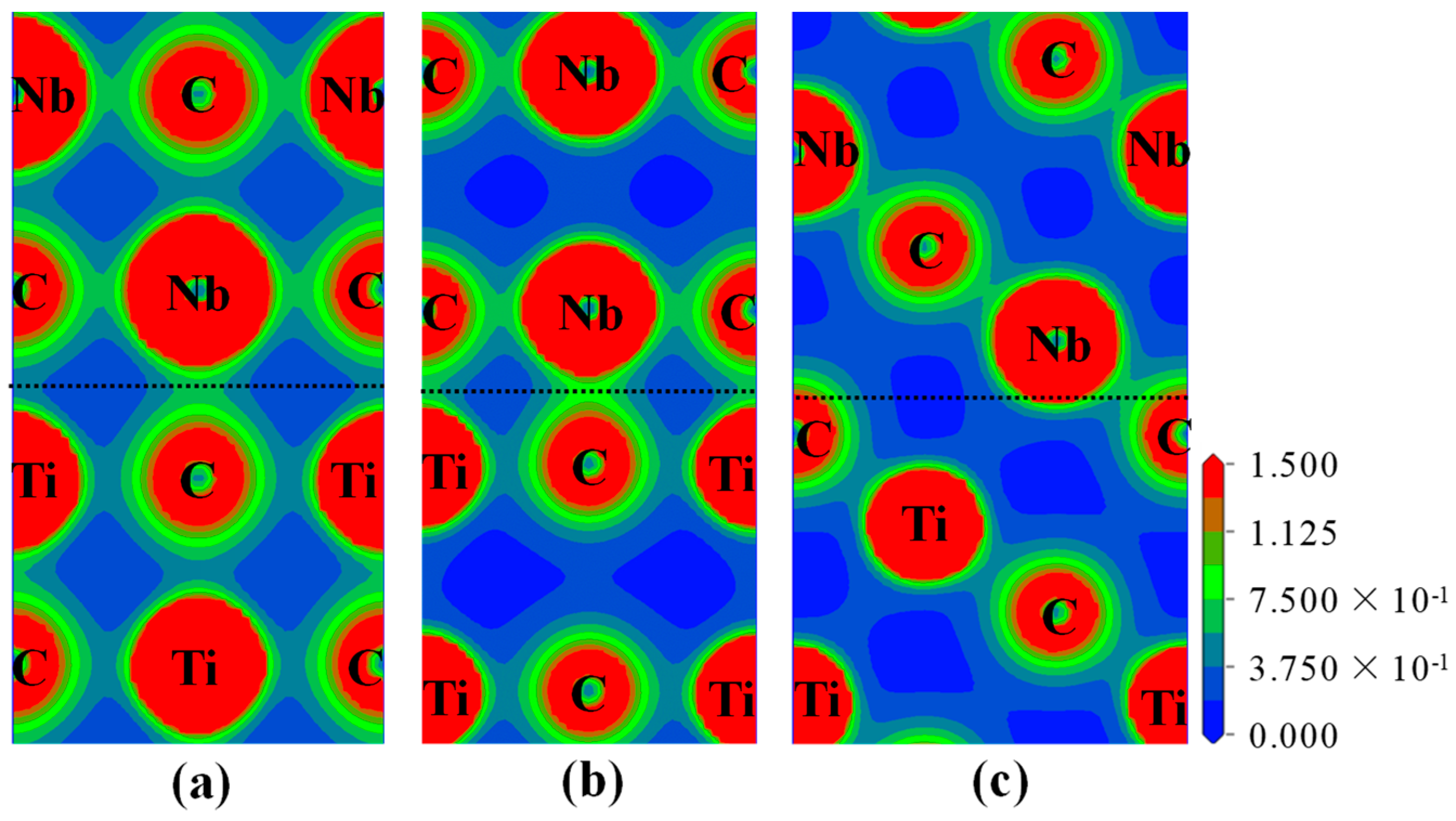
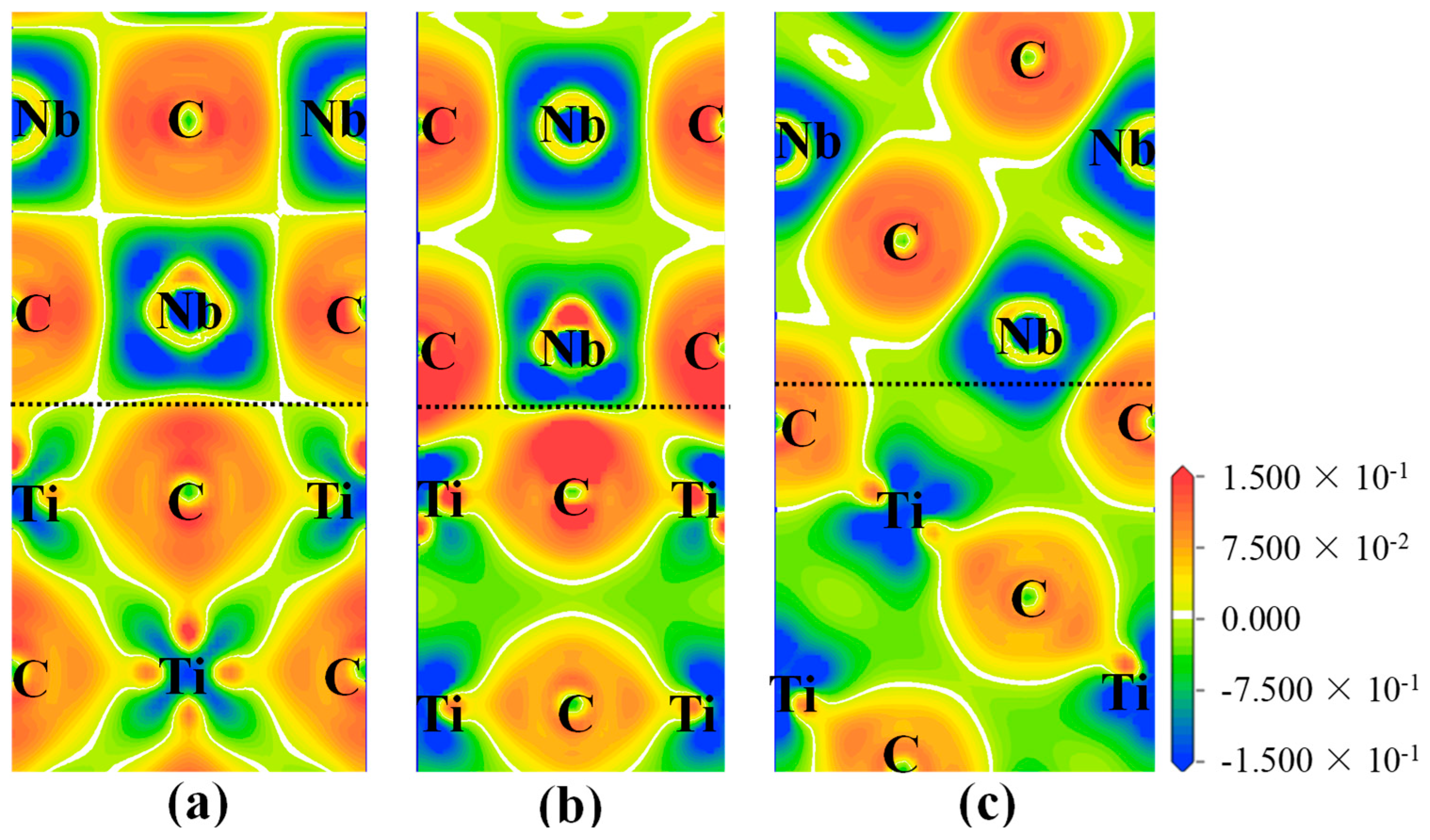
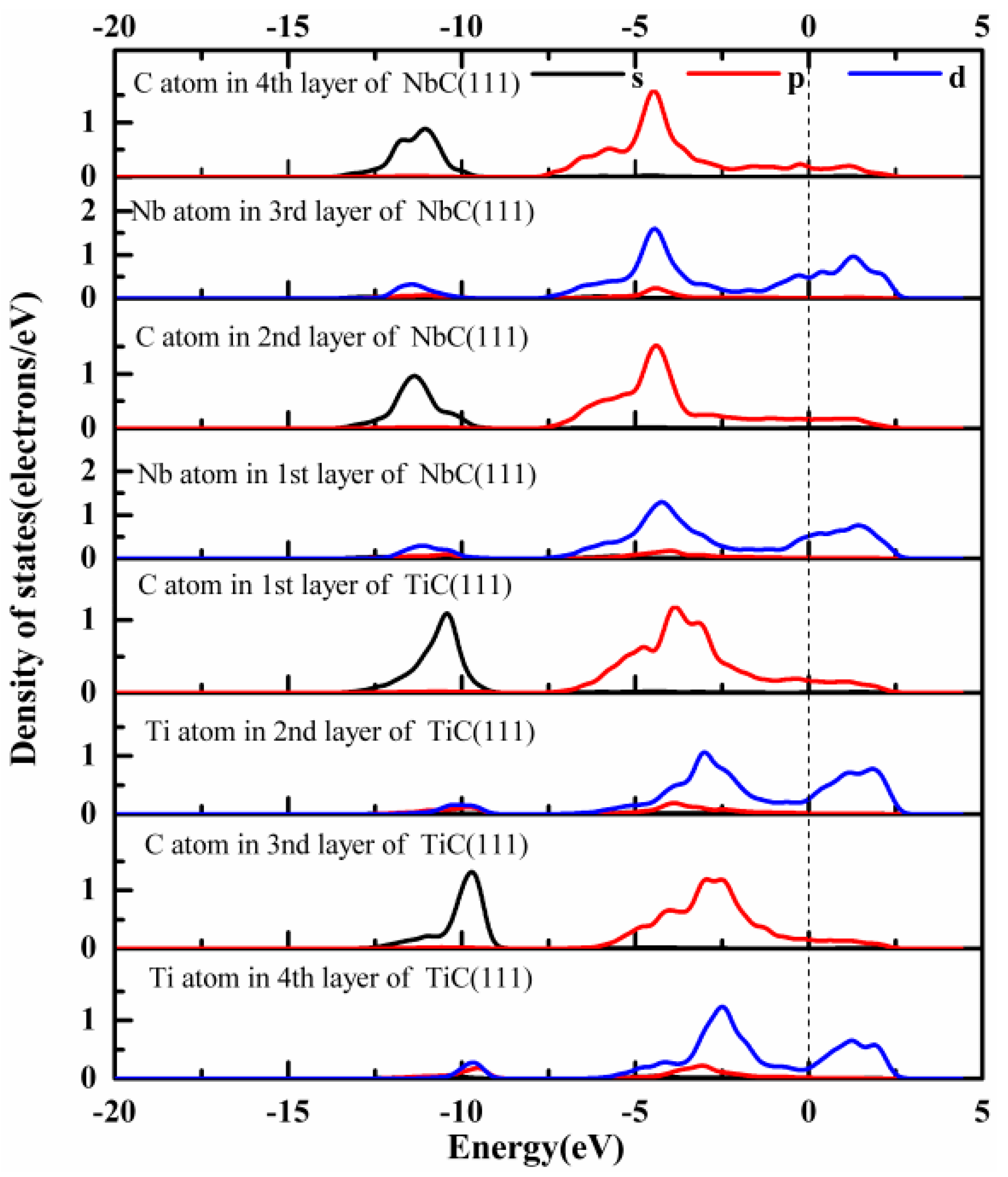
3.3.2. Electronic Structure and Bond Characteristics
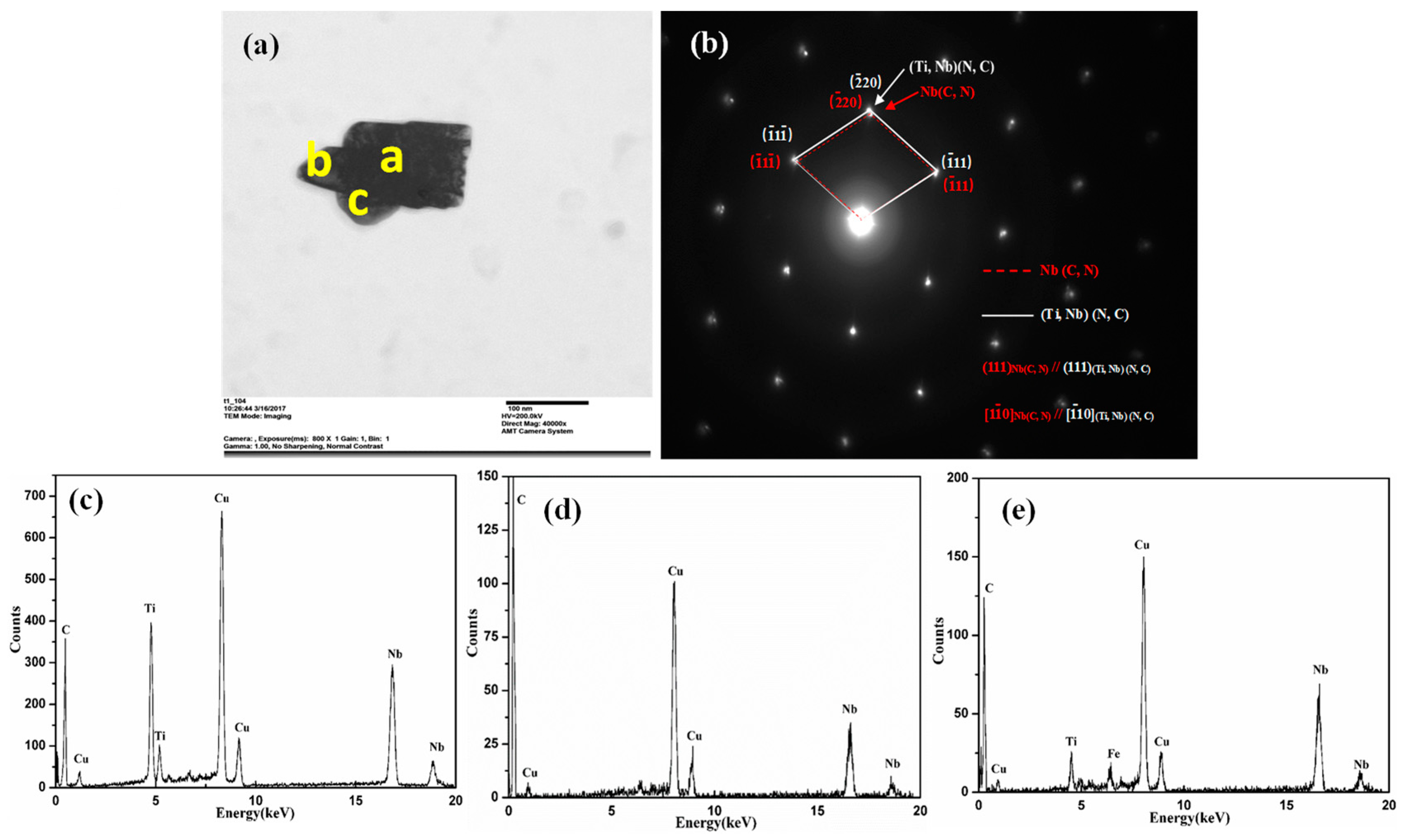
3.3.3. TEM Observation
3.4. Analysis of Heterogeneous Nucleation
4. Conclusions
- (1)
- For the NbC and TiC crystals, the surface energy of the (100) plane is lower than that of the (110) plane, that is, the stability of the (100) surface is better than that of the (110) surface. However, the surface energy of the (111) plane is dependent on the chemical potential of Ti, Nb, or C.
- (2)
- As for the NbC (100)/TiC (100) and NbC (110)/TiC (110) interfaces, interface-II is more stable than interface-I. For the NbC (111)/TiC (111) interface, the interface with the Nb–C bond is the most stable, and the stability of interfaces with the C–Ti bond, Nb–Ti bond, and C–C bond decreases in turn. The NCTL interface has the largest work of adhesion (10.15 J/m2) and the smallest equilibrium interface spacing (1.290 Å).
- (3)
- The strength and stability of (100), (110), and (111) interfaces are mainly attributed to the orbital hybridization of interfacial atoms in the range of −7.5–2.5 eV. The most stable (100) and (110) interfaces are mainly composed of covalent bonds, while the most stable (111) interface (NCTL interface) is mainly composed of covalent bonds and metallic bonds.
- (4)
- For the NbC/TiC interface, in the range of low Nb chemical potential and high C chemical potential, NbC nucleation on the surface of TiC precipitates preferentially occurs with the coherent relationship: , which is in good agreement with our experimental results from TEM observation. However, in the range of high Nb chemical potential and low C chemical potential, NbC nucleation on the surface of TiC precipitates preferentially occurs with the coherent relationship: .
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Z.Q.; Chen, H.; Yang, Z.G.; Jiang, F.C. Decelerated coarsening of (Ti, Mo)C particles with a core-shell structure in austenite of a Ti-Mo-bearing steel. Metall. Mater. Trans. 2018, A49, 1455–1459. [Google Scholar] [CrossRef]
- Yao, M.J.; Welsch, E.; Ponge, D.; Haghighat, S.M.H. Strengthening and strain hardening mechanisms in a precipitation-hardened high-Mn lightweight steel. Acta Mater. 2017, 140, 258–273. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Zhang, H.; Guo, C.H.; Liu, W.B. Effect of molybdenum addition on the precipitation of carbides in the austenite matrix of titanium micro-alloyed steels. J. Mater. Sci. 2016, 51, 4996–5007. [Google Scholar] [CrossRef]
- Jang, J.H.; Lee, C.H.; Han, H.N.; Bhadeshia, H.K.D.H. Modelling coarsening behaviour of TiC precipitates in high strength, low alloy steels. Mater. Sci. Technol. 2013, 29, 1074–1079. [Google Scholar] [CrossRef]
- Ming, L.; Wang, Q.F.; Wang, H.B.; Zhang, Y.; Guo, C.A. A remarkable role of niobium precipitation in refining microstructure and improving toughness of a QT-treated 20CrMo47NbV steel with ultrahigh strength. Mater. Sci. Eng. 2014, A613, 240–249. [Google Scholar] [CrossRef]
- Pérez Escobar, D.; Batista Castro, C.S.; Cavichioli Borba, E.; Oliveira, A.P. Correlation of the solidification path with As-cast microstructure and precipitation of Ti,Nb(C,N) on a high-temperature processed steel. Metall. Mater. Trans. 2018, A49, 3358–3372. [Google Scholar] [CrossRef]
- Subramanian, S.V.; Xiaoping, M.; Rehman, K.; Zurob, H. On control of grain coarsening of austenite by nano-scale precipitate engineering of TiN-NbC composite in Ti-Nb microalloyed steel. In Proceedings of the 6th International Conference on Recrystallization and Grain Growth (ReX&GG 2016); Holm, E.A., Farjami, S., Manohar, P., Rohrer, G.S., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 119–124. [Google Scholar]
- Chen, Z.; Loretto, M.H.; Cochrane, R.C. Nature of large precipitates in titanium-containing HSLA steels. Mater. Sci. Technol. 1987, 3, 836–844. [Google Scholar] [CrossRef]
- Craven, A.J.; He, K.; Garvie, L.A.J.; Baker, T.N. Complex heterogeneous precipitation in titanium-niobium microalloyed Al-killed HSLA steels-I. (Ti,Nb)(C, N) particles. Acta Mater. 2000, 48, 3857–3868. [Google Scholar] [CrossRef]
- Li, Y.; Wilson, J.A.; Crowther, D.N.; Mitchell, P.S. The effects of vanadium, niobium, titanium and zirconium on the microstructure and mechanical properties of thin slab cast steels. ISIJ Int. 2004, 44, 1093–1102. [Google Scholar] [CrossRef]
- Wang, R.Z.; Garcia, C.I.; Hua, M.; Cho, K. Microstructure and precipitation behavior of Nb, Ti complex microalloyed steel produced by compact strip processing. ISIJ Int. 2006, 46, 1345–1353. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, G.Y.; Tian, H.Y.; Li, F.P. Microstructures and mechanical properties of Nb-Ti bearing hot-rolled TRIP steels. J. Mater. Sci. Technol. 2006, 22, 759–762. [Google Scholar]
- Ma, X.P.; Miao, C.L.; Langelier, B.; Subramanian, S. Suppression of strain-induced precipitation of NbC by epitaxial growth of NbC on pre-existing TiN in Nb-Ti microalloyed steel. Mater. Des. 2017, 132, 244–249. [Google Scholar] [CrossRef]
- Yang, J.; Huang, J.H.; Fan, D.Y.; Chen, S.H. First-principles investigation on the electronic property and bonding configuration of NbC (111)/NbN (111) interface. J. Alloy. Compd. 2016, 689, 874–884. [Google Scholar] [CrossRef]
- Fan, X.W.; Chen, B.; Zhang, M.M.; Li, D. First-principles calculations on bonding characteristic and electronic property of TiC (111)/TiN (111) interface. Mater. Des. 2016, 112, 282–289. [Google Scholar] [CrossRef]
- Shi, Z.J.; Liu, S.; Zhou, Y.F.; Xing, X.L. Structure and properties of YAlO3/NbC heterogeneous nucleation interface: First principles calculation and experimental research. J. Alloy. Compd. 2019, 773, 264–276. [Google Scholar] [CrossRef]
- Zhuo, Z.M.; Mao, H.K.; Xu, H.; Fu, Y.Z. Density functional theory study of Al/NbB2 heterogeneous nucleation interface. Appl. Surf. Sci. 2018, 456, 37–42. [Google Scholar] [CrossRef]
- Xiong, H.H.; Zhang, H.N.; Dong, J.H. Adhesion strength and stability of TiB2/TiC interface in composite coatings by first principles calculation. Comp. Mater. Sci. 2017, 127, 244–250. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, Y.F.; Xing, X.L.; Wang, J.B. Refining effect of TiC on primary M7C3 in hypereutectic FeCrC harden-surface welding coating: Experimental research and first-principles calculation. J. Alloy. Compd. 2017, 691, 239–249. [Google Scholar] [CrossRef]
- Yang, J.; Huang, J.H.; Fan, D.Y.; Chen, S.H. Comparative investigation on RE(La,Ce)AlO3(100)/γ-Fe(100) interfaces: A first-principles calculation. Appl. Surf. Sci. 2016, 384, 207–216. [Google Scholar] [CrossRef]
- Xiong, H.H.; Zhang, H.H.; Zhang, H.N.; Zhou, Y. Effects of alloying elements X (X = Zr, V, Cr, Mn, Mo, W, Nb, Y) on ferrite/TiC heterogeneous nucleation interface: First-principles study. J. Iron Steel Res. Int. 2017, 24, 328–334. [Google Scholar] [CrossRef]
- Bramfitt, B.L. The effect of carbide and nitride additions on the heterogeneous nucleation behavior of liquid iron. Metall. Trans. 1970, 1, 1987–1995. [Google Scholar] [CrossRef]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J. First principles methods using CASTEP. Z. Krist.-Cryst. Mater. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J.; Pickard, C.J. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter 2002, 14, 2717. [Google Scholar] [CrossRef]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. 1990, B41, 7892–7895. [Google Scholar] [CrossRef]
- White, J.A.; Bird, D.M. Implementation of gradient-corrected exchange-correlation potentials in Car-Parrinello total-energy calculations. Phys. Rev. 1994, B50, 4954–4957. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. 1976, B13, 5188–5192. [Google Scholar] [CrossRef]
- Liu, P.; Han, X.L.; Sun, D.L.; Chen, Z.H. Adhesion, stability and electronic properties of Ti2AlN (0001)/TiAl (111) coherent interface from first-principles calculation. Intermetallics 2018, 96, 49–57. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, D.H.; Park, J.H. TEM characterization of a TiN-MgAl2O4 epitaxial interface. J. Alloy. Compd. 2017, 695, 476–481. [Google Scholar] [CrossRef]
- Lv, Z.; Xiao, S.; Xiao, Z.; Qian, L. Structural properties and bonding characteristic of interfaces between VN and VC from density functional calculations. J. Alloy. Compd. 2017, 718, 139–149. [Google Scholar] [CrossRef]
- Xiong, H.H.; Liu, Z.; Zhang, H.H.; Du, Z. First principles calculation of interfacial stability, energy and electronic properties of SiC/ZrB2 interface. J. Phys. Chem. Solids 2017, 107, 162–169. [Google Scholar] [CrossRef]
- Zhao, X.B.; Zhuo, Y.G.; Liu, S.; Zhou, Y.F. Investigation on WC/TiC interface relationship in wear-resistant coating by first-principle. Surf. Coat. Technol. 2016, 305, 200–207. [Google Scholar] [CrossRef]
- Zhao, X.B.; Yuan, X.M.; Liu, S.; Zhao, C.C. Investigation on WC/LaAlO3 heterogeneous nucleation interface by first-principles. J. Alloy. Compd. 2017, 695, 1753–1762. [Google Scholar] [CrossRef]
- Ma, L.; Lu, Y.; Li, S.Y.; Zuo, W. First-principles investigation of Sn9Zn (0001)/α-Al2O3 (0001) interfacial adhesion. Appl. Surf. Sci. 2018, 435, 863–869. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Xing, J.D.; Li, Y.F.; Sun, L. A first principles study of adhesion and electronic structure at Fe (110)/graphite (0001) interface. Appl. Surf. Sci. 2017, 405, 497–502. [Google Scholar] [CrossRef]
- Ono, K.; Moriyama, J. The phase relationships in the Nb-Ti-C system. J. Less Com. Met. 1981, 79, 255–260. [Google Scholar] [CrossRef]
- Dunand, A.; Flack, H.D.; Yvon, K. Bonding study of TiC and TiN. I. high-precision x-ray-diffraction determination of the valence-electron density distribution, Debye-Waller temperature factors, and atomic static displacements in TiC0.94 and TiN0.99. Phys. Rev. 1985, B31, 2299–2315. [Google Scholar] [CrossRef]
- Christensen, M.; Dudiy, S.; Wahnström, G. First-principles simulations of metal-ceramic interface adhesion: Co/WC versus Co/TiC. Phys. Rev. 2002, B65, 405–408. [Google Scholar] [CrossRef]



| Layer Number | NbC (100) | NbC (110) | TiC (100) | TiC (110) |
|---|---|---|---|---|
| 3 | 1.60 | 3.13 | 1.79 | 3.58 |
| 5 | 1.55 | 3.06 | 1.77 | 3.53 |
| 7 | 1.56 | 2.98 | 1.77 | 3.51 |
| 9 | 1.48 | 2.97 | 1.76 | 3.51 |
| 11 | 1.48 | 2.97 | 1.75 | 3.52 |
| 13 | 1.47 | 2.97 | 1.76 | 3.51 |
| Models | d0 (Å) | Wad (J/m2) |
|---|---|---|
| (100) interface-I | 4.155 | −0.96 |
| (100) interface-II | 2.221 | 2.46 |
| (110) interface-I | 2.626 | −0.17 |
| (110) interface-II | 2.085 | 4.47 |
| Termination | Stacking Sequence | |||
|---|---|---|---|---|
| NbC | TiC | OT | SL | TL |
| Nb | C | 5.55 | 8.62 | 10.15 |
| Nb | Ti | 2.01 | 2.10 | 3.48 |
| C | C | 3.21 | −0.32 | −0.68 |
| C | Ti | 4.76 | 6.18 | 8.38 |
| Phase | Weight Ratio | Atomic Ratio | ||||
|---|---|---|---|---|---|---|
| Nb | Ti | Ti/Nb | Nb | Ti | Ti/Nb | |
| a | 76.8 | 23.2 | 0.3021 | 63.05 | 36.95 | 0.5861 |
| b | 99.79 | 0.21 | 0.0021 | 99.6 | 0.4 | 0.004 |
| c | 93.22 | 6.78 | 0.0727 | 87.63 | 12.37 | 0.1412 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, J.; Hou, D.; Li, J.-Y.; Huang, R. Investigation of NbC/TiC Heterogeneous Nucleation Interface by First-Principles and Experimental Methods. Metals 2019, 9, 1265. https://doi.org/10.3390/met9121265
Dong J, Hou D, Li J-Y, Huang R. Investigation of NbC/TiC Heterogeneous Nucleation Interface by First-Principles and Experimental Methods. Metals. 2019; 9(12):1265. https://doi.org/10.3390/met9121265
Chicago/Turabian StyleDong, Jianhong, Dejian Hou, Jin-Yan Li, and Rui Huang. 2019. "Investigation of NbC/TiC Heterogeneous Nucleation Interface by First-Principles and Experimental Methods" Metals 9, no. 12: 1265. https://doi.org/10.3390/met9121265
APA StyleDong, J., Hou, D., Li, J.-Y., & Huang, R. (2019). Investigation of NbC/TiC Heterogeneous Nucleation Interface by First-Principles and Experimental Methods. Metals, 9(12), 1265. https://doi.org/10.3390/met9121265





