Successes and Issues in the Growth of Moad and MoSe2 on Ag(111) by the E-ALD Method
Abstract
:1. Introduction
2. Materials and Methods
3. Results
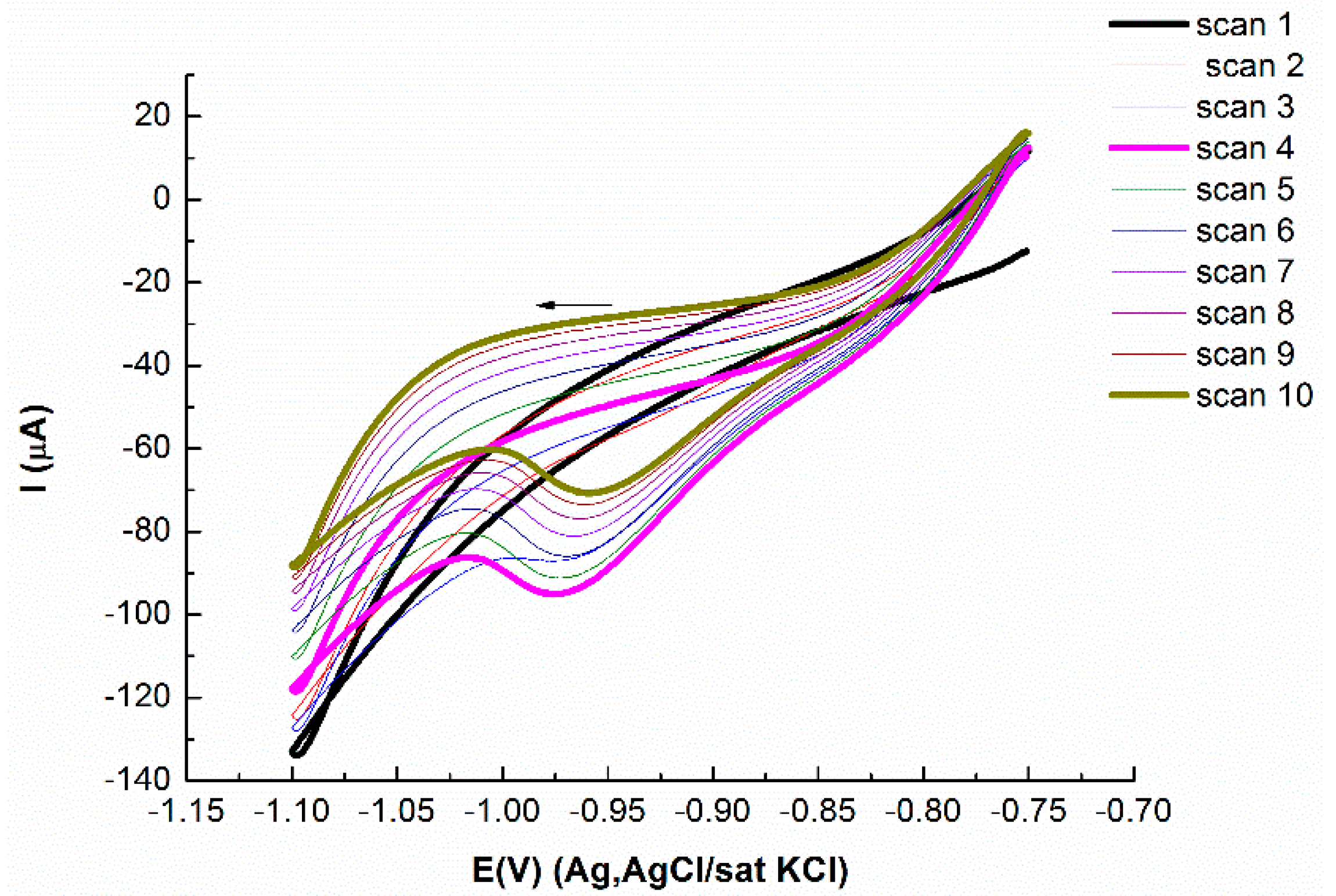
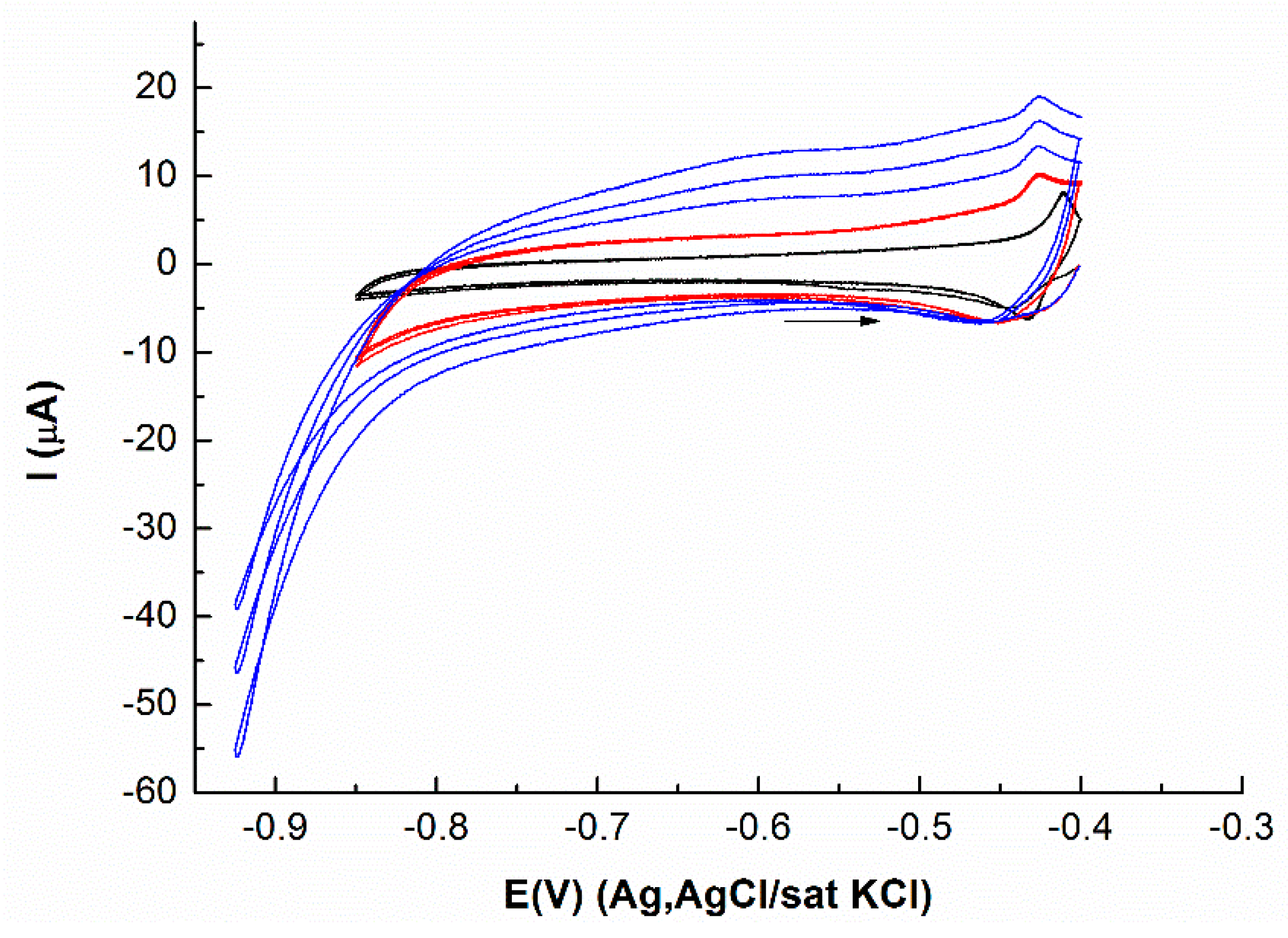
3.1. Deposition of Mo Atomic Layers on Ag(111)
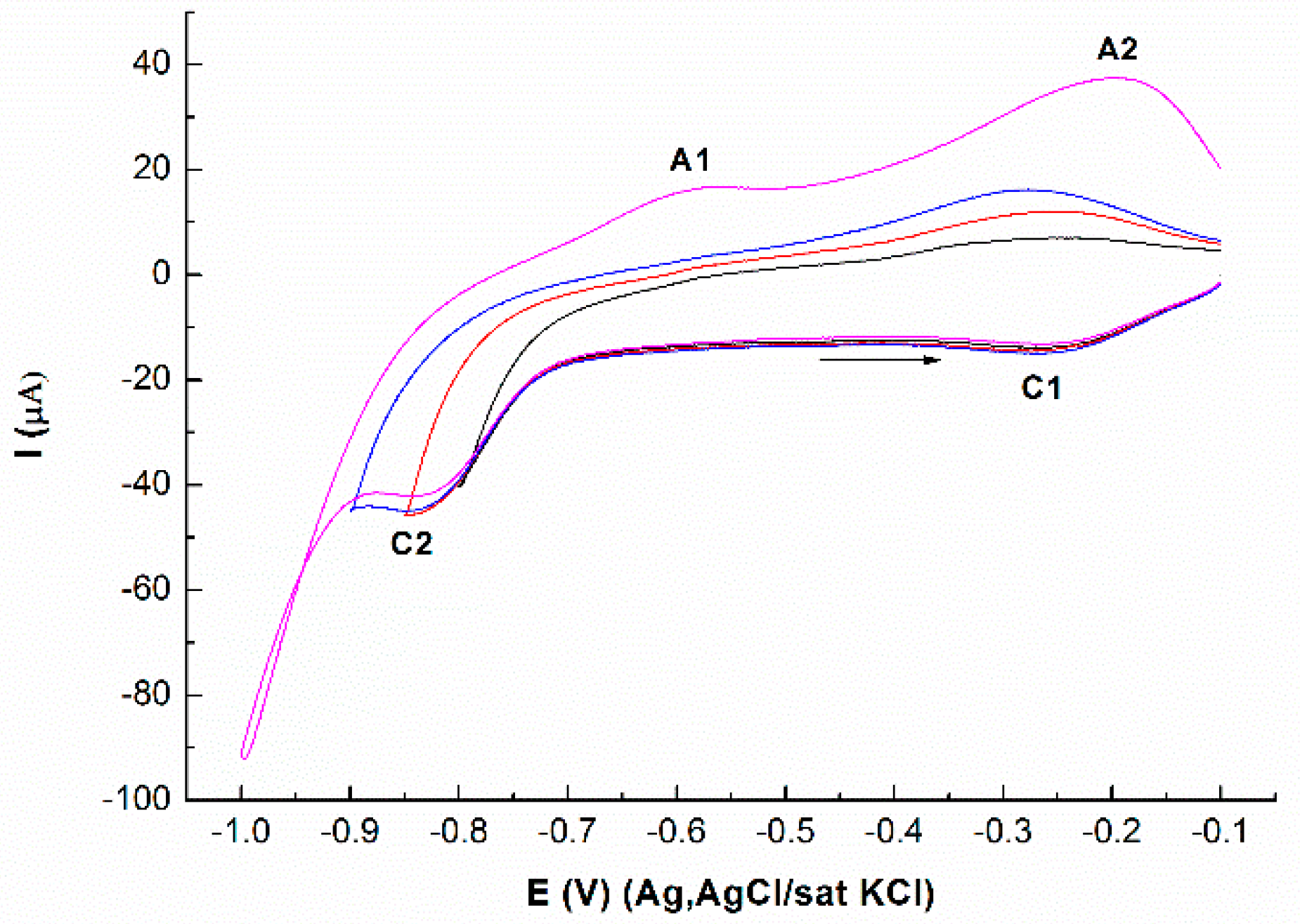
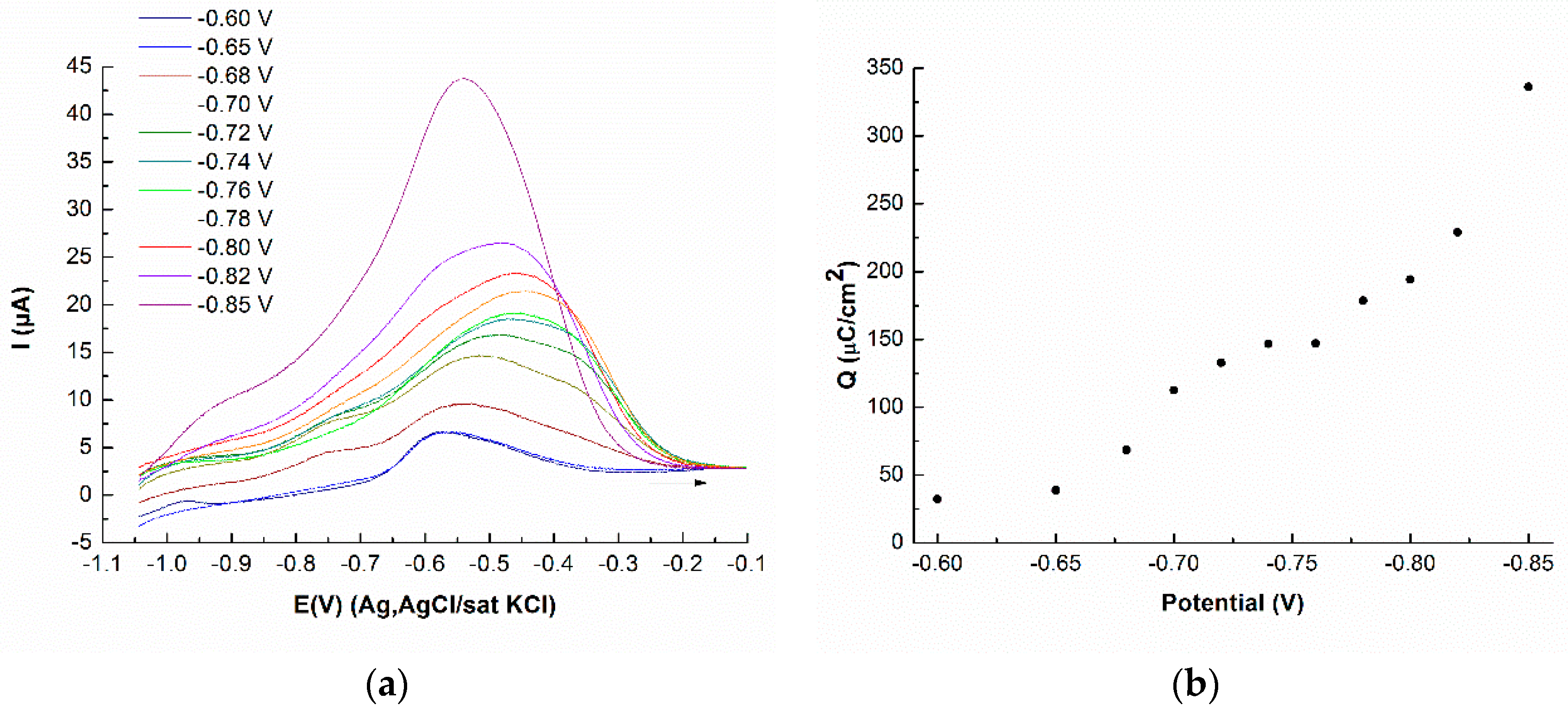
3.2. Deposition of Se on Atomic Layer of Mo on Ag(111)
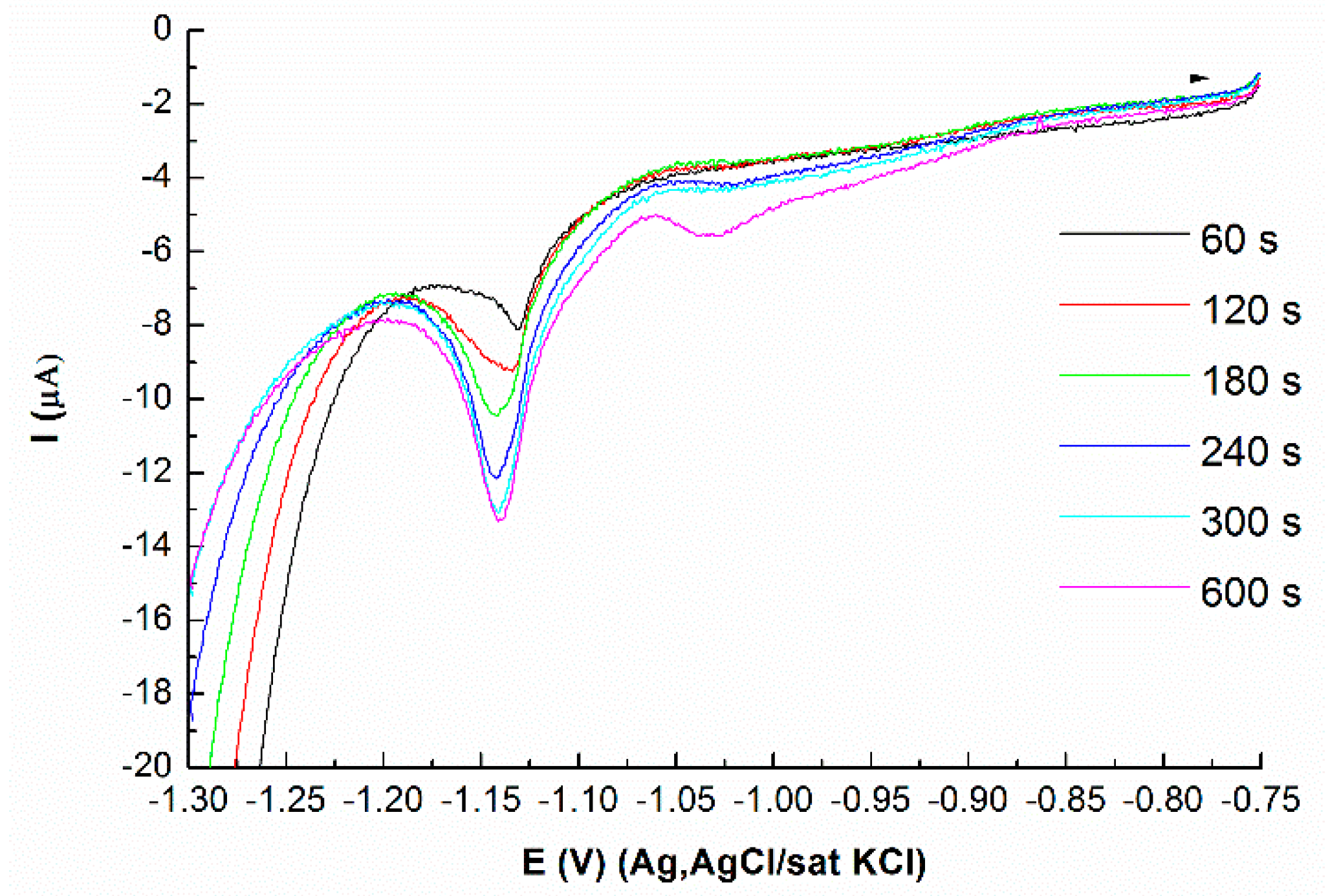
3.3. Deposition of Mo on Atomic Layer of Se on Ag(111)
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Fabricating Energy Devices with Low Environmental Impacts. Available online: http://www.spie.org/newsroom/6249-fabricating-energy-devices-with-low-environmental-impacts?SSO=1 (accessed on 11 January 2016).
- Lu, W.; Lieber, C.M. Nanoelectronics from the bottom up. Nat. Mater. 2007, 6, 841–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stickney, B.W.; Gregory, J.L. Electrochemical atomic layer epitaxy (ECALE). J. Electroanal. Chem. 1991, 300, 543–561. [Google Scholar]
- Lay, M.D.; Varazo, K.; Stickney, J.L. Formation of Sulfur Atomic Layers on Gold from Aqueous Solutions of Sulfide and Thiosulfate: Studies Using EC-STM, UHV-EC, and TLEC. Langmuir 2003, 19, 8416–8427. [Google Scholar] [CrossRef]
- Cavallini, M.; Aloisi, G.; Guidelli, R. In situ STM study of selenium electrodeposition on Ag(111). Langmuir 1999, 15, 2993–2995. [Google Scholar] [CrossRef]
- Aloisi, G.D.; Cavallini, M.; Innocenti, M.; Foresti, M.L.; Pezzatini, G.; Guidelli, R. In situ STM and electrochemical investigation of sulfur oxidative underpotential deposition on Ag(111). J. Phys. Chem. B 1997, 101, 4774–4780. [Google Scholar] [CrossRef]
- Innocenti, M.; Bencista, I.; Di Benedetto, F.; Cinotti, S.; De Luca, A.; Bellandi, S.; Lavacchi, A.; Muniz Miranda, M.; Vizza, F.; Marinelli, F.; et al. Underpotential Deposition of Sn on S-Covered Ag(111). ECS Trans. 2013, 50, 1–7. [Google Scholar] [CrossRef]
- Oviedo, O.A.; Reinaudi, L.; Garciía, S.G.; Leiva, E.P.M. Underpotential Deposition: From Fundamentals and Theory to Applications at the Nanoscale; Springer: Berlin, Germany, 2016; ISBN 9783319243948. [Google Scholar]
- Giaccherini, A.; Felici, R.; Innocenti, M. Operando structural characterization of the E-ALD process ultra-thin films growth. In X-ray Characterization of Nanostructured Energy Materials by Synchrotron Radiation; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar]
- Salvietti, E.; Giaccherini, A.; Gambinossi, F.; Foresti, M.L.; Passaponti, M.; Di Benedetto, F.; Innocenti, M. E-ALD: Tailoring the Optoeletronic Properties of Metal Chalcogenides on Ag Single Crystals. Semicond. Growth Charact. 2018. [Google Scholar] [CrossRef] [Green Version]
- Di Benedetto, F.; Bencistà, I.; Caporali, S.; Cinotti, S.; De Luca, A.; Lavacchi, A.; Vizza, F.; Muniz Miranda, M.; Foresti, M.L.; Innocenti, M. Electrodeposition of ternary CuxSnySz thin films for photovoltaic applications. Prog. Photovolt. Res. Appl. 2014, 22, 97–106. [Google Scholar] [CrossRef]
- Bencistà, I.; Di Benedetto, F.; Innocenti, M.; De Luca, A.; Fornaciai, G.; Lavacchi, A.; Montegrossi, G.; Oberhauser, W.; Pardi, L.A.; Romanelli, M.; et al. Phase composition of CuxS thin films: Spectroscopic evidence of covellite formation. Eur. J. Mineral. 2012, 24, 879–884. [Google Scholar] [CrossRef]
- Lastraioli, E.; Loglio, F.; Cavallini, M.; Simeone, F.C.; Innocenti, M.; Carlaà, F.; Foresti, M.L. In Situ Scanning Tunneling Microscopy Investigation of Sulfur Oxidative Underpotential Deposition on Ag(100) and Ag(110). Langmuir 2010, 26, 17679–17685. [Google Scholar] [CrossRef]
- Innocenti, M.; Bencistà, I.; Bellandi, S.; Bianchini, C.; Di Benedetto, F.; Lavacchi, A.; Vizza, F.; Foresti, M.L. Electrochemical layer by layer growth and characterization of copper sulfur thin films on Ag(111). Electrochim. Acta 2011, 58, 599–605. [Google Scholar] [CrossRef]
- Innocenti, M.; Bellandi, S.; Lastraioli, E.; Loglio, F.; Foresti, M.L. Selective electrodesorption based atomic layer deposition (SEBALD): A novel electrochemical route to deposit metal clusters on Ag(111). Langmuir 2011, 27, 11704–11709. [Google Scholar] [CrossRef] [PubMed]
- Innocenti, M.; Cattarin, S.; Cavallini, M.; Loglio, F.; Foresti, M.L. Characterisation of thin films of CdS deposited on Ag(111) by ECALE. A morphological and photoelectrochemical investigation. J. Electroanal. Chem. 2002, 532, 219–225. [Google Scholar] [CrossRef]
- Innocenti, M.; Becucci, L.; Bencistà, I.; Carretti, E.; Cinotti, S.; Dei, L.; Di Benedetto, F.; Lavacchi, A.; Marinelli, F.; Salvietti, E.; et al. Electrochemical growth of Cu-Zn sulfides. J. Electroanal. Chem. 2013, 710, 17–21. [Google Scholar] [CrossRef]
- Di Benedetto, F.; Cinotti, S.; Guerri, A.; De Luca, A.; Lavacchi, A.; Montegrossi, G.; Carlà, F.; Felici, R.; Innocenti, M. Physical Characterization of Thin Films of CuxZnySz For Photovoltaic Applications. ECS Trans. 2013, 58, 59–65. [Google Scholar] [CrossRef]
- Giaccherini, A.; Russo, F.; Carlà, F.; Guerri, A.; Picca, R.A.; Cioffi, N.; Cinotti, S.; Montegrossi, G.; Passaponti, M.; Di Benedetto, F.; et al. Operando SXRD of E-ALD deposited sulphides ultra-thin films: Crystallite strain and size. Appl. Surf. Sci. 2018, 432, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Giaccherini, A.; Montegrossi, G.; Di Benedetto, F.; Innocenti, M. Thermochemistry of the E-ALD process for the growth of CuxZnyS on Ag(111): Interpretation of experimental data. Electrochim. Acta 2018, 262. [Google Scholar] [CrossRef]
- Berretti, E.; Cinotti, S.; Caporali, S.; Cioffi, N.; Giaccherini, A.; Di Benedetto, F.; Foresti, M.L.; Montegrossi, G.; Lavacchi, A.; Vizza, F.; et al. Electrodeposition and Characterization of p and n Sulfide Semiconductors Composite Thin Film. J. Electrochem. Soc. 2016, 163, D3034–D3039. [Google Scholar] [CrossRef]
- Pezzatini, G.; Caporali, S.; Innocenti, M.; Foresti, M.L. Formation of ZnSe on Ag(111) by electrochemical atomic layer epitaxy. J. Electroanal. Chem. 1999, 475, 164–170. [Google Scholar] [CrossRef]
- Foresti, M.L.; Milani, S.; Loglio, F.; Innocenti, M.; Pezzatini, G.; Cattarin, S. Ternary CdSxSe1−x deposited on Ag(111) by ECALE: Synthesis and characterization. Langmuir 2005, 21, 6900–6907. [Google Scholar] [CrossRef] [PubMed]
- Gregory, B.W.; Norton, M.L.; Stickney, J.L. Thin-layer electrochemical studies of the underpotential deposition of cadmium and tellurium on polycrystalline Au, Pt and Cu electrodes. J. Electroanal. Chem. 1990, 293, 85–101. [Google Scholar] [CrossRef]
- Loglio, F.; Innocenti, M.; Jarek, A.; Caporali, S.; Pasquini, I.; Foresti, M.L. Nickel sulfur thin films deposited by ECALE: Electrochemical, XPS and AFM characterization. J. Electroanal. Chem. 2010, 638, 15–20. [Google Scholar] [CrossRef]
- Russo, F.; Giaccherini, A.; Salvietti, E.; Berretti, E.; Passaponti, M.; Lavacchi, A.; Montegrossi, G.; Piciollo, E.; Di Benedetto, F.; Innocenti, M. Morphology and composition of Cu2S ultra-thin films deposited by E-ALD. ECS Trans. 2017, 80, 749–756. [Google Scholar] [CrossRef]
- Innocenti, M.; Cinotti, S.; Bencista, I.; Carretti, E.; Becucci, L.; Di Benedetto, F.; Lavacchi, A.; Foresti, M.L. Electrochemical Growth of Cu-Zn Sulfides of Various Stoichiometries. ECS Trans. 2014, 161, D14–D17. [Google Scholar] [CrossRef]
- Caporali, S.; Tolstogouzov, A.; Teodoro, O.M.N.D.N.D.; Innocenti, M.; Di Benedetto, F.; Cinotti, S.; Picca, R.A.; Sportelli, M.C.; Cioffi, N. Sn-deficiency in the electrodeposited ternary CuxSnySz thin films by ECALE. Sol. Energy Mater. Sol. Cells 2015, 138, 9–16. [Google Scholar] [CrossRef]
- Cecconi, T.; Atrei, A.; Bardi, U.; Forni, F.; Innocenti, M.; Loglio, F.; Foresti, M.L.; Rovida, G. X-ray photoelectron diffraction (XPD) study of the atomic structure of the ultrathin CdS phase deposited on Ag(111) by electrochemical atomic layer epitaxy (ECALE). J. Electron Spectrosc. Relat. Phenom. 2001, 114–116, 563–568. [Google Scholar] [CrossRef]
- Loglio, F.; Innocenti, M.; D’Acapito, F.; Felici, R.; Pezzatini, G.; Salvietti, E.; Foresti, M.L. Cadmium selenide electrodeposited by ECALE: Electrochemical characterization and preliminary results by EXAFS. J. Electroanal. Chem. 2005, 575, 161–167. [Google Scholar] [CrossRef]
- Cavallini, M.; Facchini, M.; Albonetti, C.; Biscarini, F.; Innocenti, M.; Loglio, F.; Salvietti, E.; Pezzatini, G.; Foresti, M.L. Two-Dimensional Self-Organization of CdS Ultra Thin Films by Confined Electrochemical Atomic Layer Epitaxy Growth. J. Phys. Chem. C 2007, 111, 1061–1064. [Google Scholar] [CrossRef]
- Huang, B.M.; Colletti, L.P.; Gregory, B.W.; Anderson, J.L.; Stickney, J.L. Preliminary studies of the use of an automated flow-cell electrodeposition system for the formation of CdTe thin films by electrochemical atomic layer epitaxy. J. Electrochem. Soc. 1995, 142, 3007–3016. [Google Scholar] [CrossRef]
- Innocenti, M.; Pezzatini, G.; Forni, F.; Foresti, M.L.F. CdS and ZnS Deposition on Ag(111) by Electrochemical Atomic Layer Epitaxy. J. Electrochem. Soc. 2001, 148, C357–C362. [Google Scholar] [CrossRef]
- Keyshar, K.; Berg, M.; Zhang, X.; Vajtai, R.; Gupta, G.; Chan, C.K.; Beechem, T.E.; Ajayan, P.M.; Mohite, A.D.; Ohta, T. Experimental Determination of the Ionization Energies of MoSe2, WS2, and MoS2 on SiO2 Using Photoemission Electron Microscopy. ACS Nano 2017, 11, 8223–8230. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, F.; Wang, Q.; Yang, P.; Lin, H.; Qu, F. Hierarchical MoSe2 nanoflowers as novel nanocarriers for NIR-light-mediated synergistic photo-thermal/dynamic and chemo-therapy. Nanoscale 2018, 10, 14534–14545. [Google Scholar] [CrossRef] [PubMed]
- Wilcoxon, J.P.; Samara, G.A. Strong quantum-size effects in a layered semiconductor: MoS2 nanoclusters. Phys. Rev. B 1995, 51, 7299–7302. [Google Scholar] [CrossRef]
- Yuan, R.-Y.; Yang, Q.-J.; Guo, Y. Enhanced spin polarization and valley polarization in monolayer MoS2 junctions. J. Phys. Condens. Matter 2018, 30. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-Z.; Yao, W.; Xiao, D.; Shen, S.-Q. Intervalley Scattering and Localization Behaviors of Spin-Valley Coupled Dirac Fermions. Phys. Rev. Lett. 2013, 110, 016806. [Google Scholar] [CrossRef] [PubMed]
- Schaibley, J.R.; Yu, H.; Clark, G.; Rivera, P.; Ross, J.S.; Seyler, K.L.; Yao, W.; Xu, X. Valleytronics in 2D materials. Nat. Rev. Mater. 2016, 1, 16055. [Google Scholar] [CrossRef]
- Mueed, M.A.; Hossain, M.S.; Jo, I.; Pfeiffer, L.N.; West, K.W.; Baldwin, K.W.; Shayegan, M. Realization of a Valley Superlattice. Phys. Rev. Lett. 2018, 121, 036802. [Google Scholar] [CrossRef]
- Mak, K.F.; Xiao, D.; Shan, J. Light–valley interactions in 2D semiconductors. Nat. Photonics 2018, 12, 451–460. [Google Scholar] [CrossRef]
- Wang, X.; Gong, Y.; Shi, G.; Chow, W.L.; Keyshar, K.; Ye, G.; Vajtai, R.; Lou, J.; Liu, Z.; Ringe, E.; et al. Chemical Vapor Deposition Growth of Crystalline Monolayer MoSe2. ACS Nano 2014, 8, 5125–5131. [Google Scholar] [CrossRef]
- Boscher, N.D.; Carmalt, C.J.; Palgrave, R.G.; Gil-Tomas, J.J.; Parkin, I.P. Atmospheric Pressure CVD of Molybdenum Diselenide Films on Glass. Chem. Vap. Depos. 2006, 12, 692–698. [Google Scholar] [CrossRef]
- Chang, Y.-H.; Zhang, W.; Zhu, Y.; Han, Y.; Pu, J.; Chang, J.-K.; Hsu, W.-T.; Huang, J.-K.; Hsu, C.-L.; Chiu, M.-H.; et al. Monolayer MoSe2 Grown by Chemical Vapor Deposition for Fast Photodetection. ACS Nano 2014, 8, 8582–8590. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.F.; Ledina, M.A.; Stickney, J.L. Molybdenum diselenide formation using electrochemical atomic layer deposition (E-ALD). J. Electroanal. Chem. 2017, 793, 242–249. [Google Scholar] [CrossRef]
- Hamelin, A. Modern Aspects of Electrochemistry; Conway, B.E., White, R.E., Bockris, J.O., Eds.; Plenum Press: New York, NY, USA, 1985; Volume 16, pp. 1–101. [Google Scholar]
- Foresti, M.L.; Capolupo, F.; Innocenti, M.; Loglio, F. Visual Detection of Crystallographic Orientations of Face-Centered Cubic Single Crystals. Cryst. Growth Des. 2002, 2, 73–77. [Google Scholar] [CrossRef]
- Hamelin, A.; Stoicoviciu, L.; Doubova, L.; Trasatti, S. Influence of the crystallographic orientation of the surface on the potential of zero charge of silver electrodes. Surf. Sci. 1988, 201, L498–L506. [Google Scholar] [CrossRef]
- Aveston, J.; Anacker, E.W.; Johnson, J.S. Hydrolysis of Molybdenum (VI). Ultracentrifugation, Acidity Measurements, and Raman Spectra of Polymolybdates. Inorg. Chem. 1964, 3, 735–746. [Google Scholar] [CrossRef]
- Foresti, M.; Pezzatini, G.; Cavallini, M.; Aloisi, G.; Innocenti, M.; Guidelli, R. Electrochemical atomic layer epitaxy deposition of CdS on Ag (111): An electrochemical and STM investigation. J. Phys. Chem. B 1998, 102, 7413–7420. [Google Scholar] [CrossRef]
- Pezzatini, G.; Loglio, F.; Innocenti, M.; Foresti, M.L. Selenium (IV) Electrochemistry on Silver: A Combined Electrochemical Quartz-Crystal Microbalance and Cyclic Voltammetric Investigation. Collect. Czechoslov. Chem. Commun. 2003, 68, 1579–1595. [Google Scholar] [CrossRef]
- Morley, T.J.; Penner, L.; Schaffer, P.; Ruth, T.J.; Bénard, F.; Asselin, E. The deposition of smooth metallic molybdenum from aqueous electrolytes containing molybdate ions. Electrochem. Commun. 2012, 15, 78–80. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vizza, M.; Giaccherini, A.; Giurlani, W.; Passaponti, M.; Cioffi, N.; Picca, R.A.; De Luca, A.; Fabbri, L.; Lavacchi, A.; Gambinossi, F.; et al. Successes and Issues in the Growth of Moad and MoSe2 on Ag(111) by the E-ALD Method. Metals 2019, 9, 122. https://doi.org/10.3390/met9020122
Vizza M, Giaccherini A, Giurlani W, Passaponti M, Cioffi N, Picca RA, De Luca A, Fabbri L, Lavacchi A, Gambinossi F, et al. Successes and Issues in the Growth of Moad and MoSe2 on Ag(111) by the E-ALD Method. Metals. 2019; 9(2):122. https://doi.org/10.3390/met9020122
Chicago/Turabian StyleVizza, Martina, Andrea Giaccherini, Walter Giurlani, Maurizio Passaponti, Nicola Cioffi, Rosaria Anna Picca, Antonio De Luca, Lorenzo Fabbri, Alessandro Lavacchi, Filippo Gambinossi, and et al. 2019. "Successes and Issues in the Growth of Moad and MoSe2 on Ag(111) by the E-ALD Method" Metals 9, no. 2: 122. https://doi.org/10.3390/met9020122






