Effect of Jet Flow between Electrodes on the Cathode Quality in Copper Electrorefining with High Current Density
Abstract
:1. Introduction
2. Experimental Details
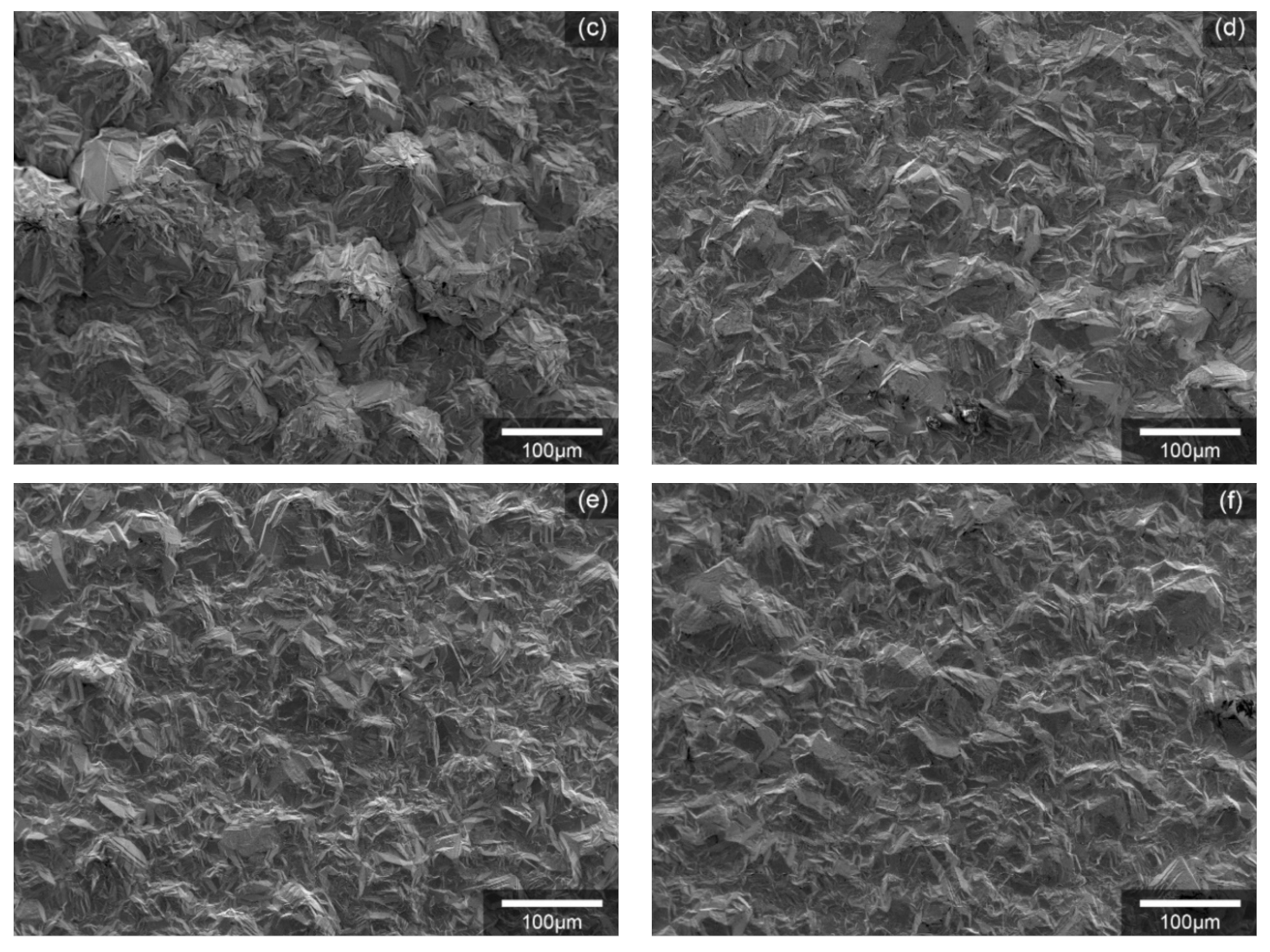
3. Results and Discussion
4. Conclusions
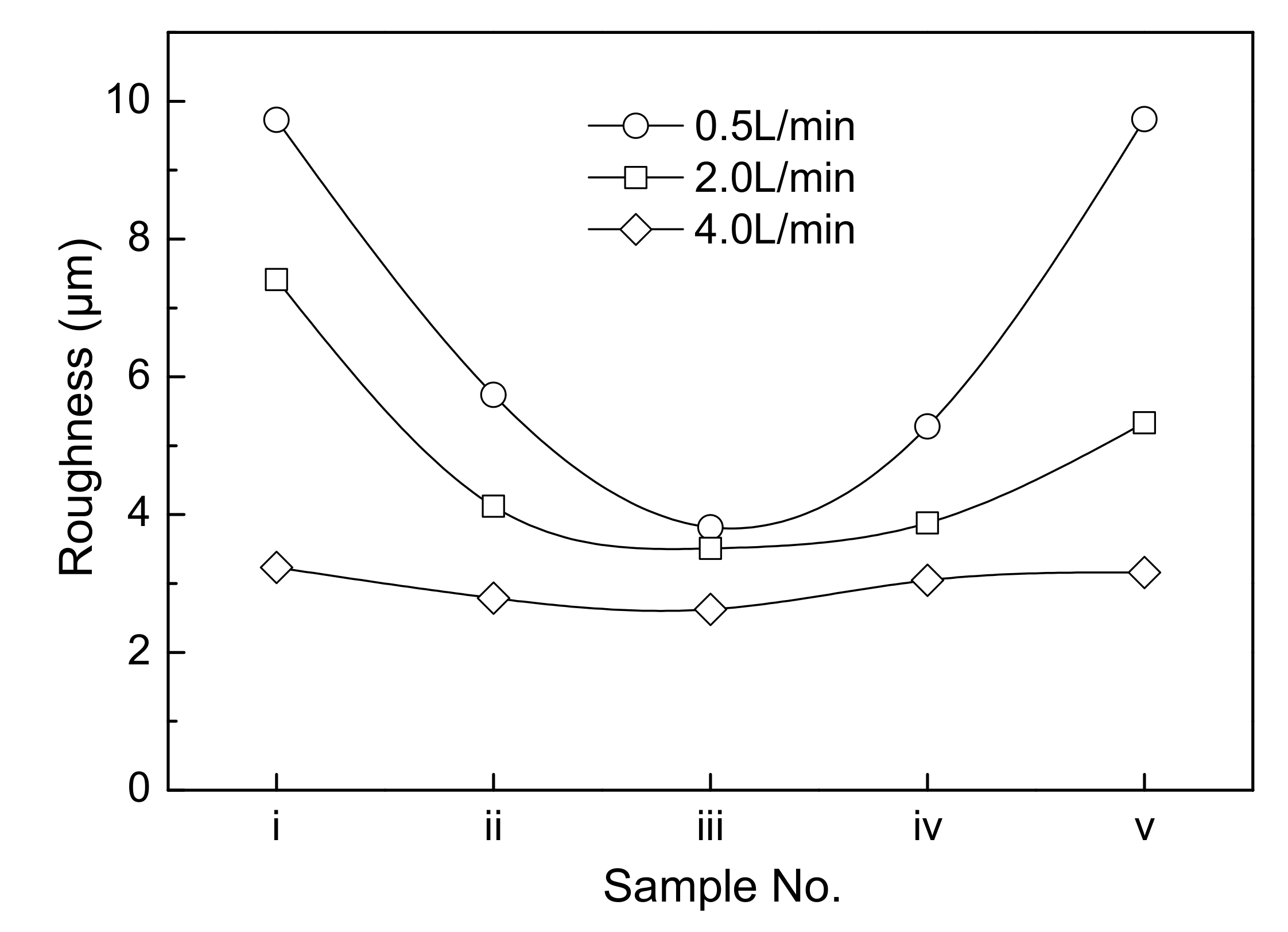
- In the conventional bottom-inlet/top-outlet mode, an increase in electrolyte circulation rate has a limited effect on the decrease of the surface roughness of cathode copper.
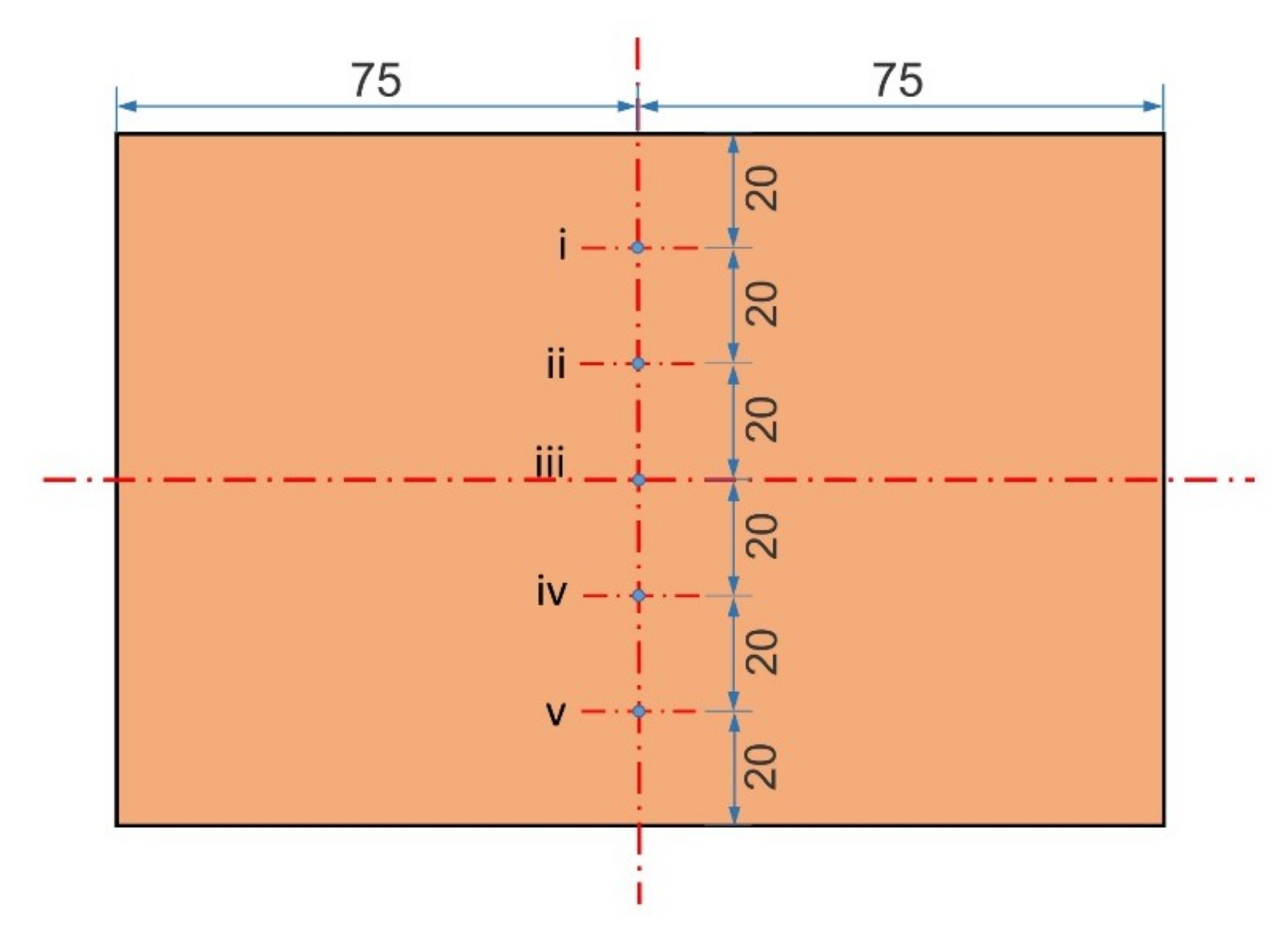
- When a jet flow is introduced between the cathode and the anode, the electrolyte circulation rate has a large influence on the surface roughness of cathode copper, and the two are negatively related. When the circulation rate is small, the surface roughness of cathode copper is not uniform. The closer the position at the surface of the cathode copper to the jet region, the flatter the surface of the cathode copper, and vice versa.
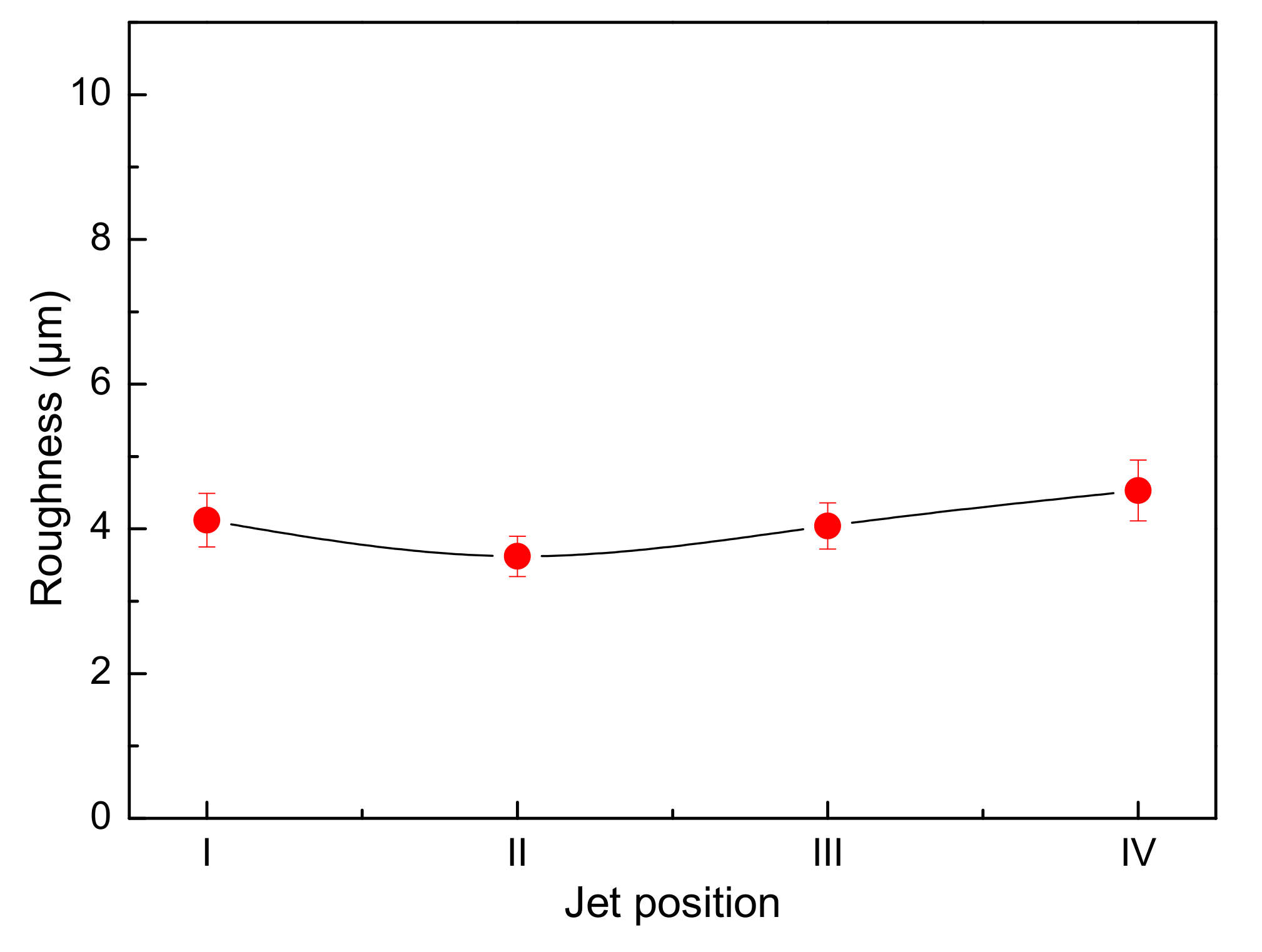
- In the single-side jet inlet mode, when the jet position is at the middle height of the cathode deposition zone, the cathode quality is the best.
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, S. Electrolytic refining technology of high strengthen copper and production practice. Nonferrous Met. (Extr. Met.) 2013, 1–4. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, P.; Wang, Y.; Lü, Z. Effect of electrorefining with high current density on cathode copper. J. Central South Univ. (Sci. Technol.) 2009, 40, 311–316. [Google Scholar]
- Kawai, S.; Miyazawa, T. CFD modelling and simulation of industrial-scale copper electrorefining process. Miner. Eng. 2014, 63, 81–90. [Google Scholar] [CrossRef]
- Zeng, W.; Free, M.L.; Wang, S. Simulation study of electrolyte flow and slime particle transport in a newly designed copper electrorefining cell. ECS Trans. 2016, 72, 23–42. [Google Scholar] [CrossRef]
- Zeng, W.; Wang, S.; Free, M.L. A comparative study of electrolyte flow and slime particle transport in a newly designed copper electrolytic cell and a laboratory-scale conventional electrolytic cell. JOM 2017, 69, 1876–1887. [Google Scholar] [CrossRef]
- Zeng, W.; Yi, G.; Wang, S.; Free, M.L. Design and analysis of direct side inflows in copper electrolytic cells by a computational method. Hydrometallurgy 2017, 169, 612–620. [Google Scholar] [CrossRef]
- Filzwieser, A.; Filzwieser, I.; Konetschnik, S. New technology for electrorefining of copper. JOM 2012, 64, 1290–1295. [Google Scholar] [CrossRef]
- Filzwieser, A.; Filzwieser, I.; Konetschnik, S.; Anzinger, A. Economic benefits of operating a copper electro-refining tankhouse at high current density using the Mettop-BRX Technology. In Proceedings of the Conference of Metallurgists (COM 2014), Vancouver, BC, Canada, 28 September–1 October 2014. [Google Scholar]
- General Administration of Quality Supervision; Inspection and Quarantine of the People’s Republic of China; Standardization Administration of the P.R.C. GB/T 665-2007 Chemical Reagent—Copper (II) Sulfate Pentahydrate; Standards Press of China: Beijing, China, 2007.
- Li, M.; Huang, J.; Tong, C.; Zhang, W.; Zhou, J.; Li, H.; Zhang, P. Numerical simulation of electrolyte flow in copper electrolytic cell. Chin. J. Nonferrous Met. 2015, 25, 2259–2267. [Google Scholar] [CrossRef]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Wang, Q.; Xia, W.; Ren, B. Effect of Jet Flow between Electrodes on the Cathode Quality in Copper Electrorefining with High Current Density. Metals 2018, 8, 833. https://doi.org/10.3390/met8100833
Wang H, Wang Q, Xia W, Ren B. Effect of Jet Flow between Electrodes on the Cathode Quality in Copper Electrorefining with High Current Density. Metals. 2018; 8(10):833. https://doi.org/10.3390/met8100833
Chicago/Turabian StyleWang, Hongdan, Qian Wang, Wentang Xia, and Bingzhi Ren. 2018. "Effect of Jet Flow between Electrodes on the Cathode Quality in Copper Electrorefining with High Current Density" Metals 8, no. 10: 833. https://doi.org/10.3390/met8100833




