Desiliconisation and Dephosphorisation Behaviours of Various Oxygen Sources in Hot Metal Pre-Treatment
Abstract
:1. Introduction
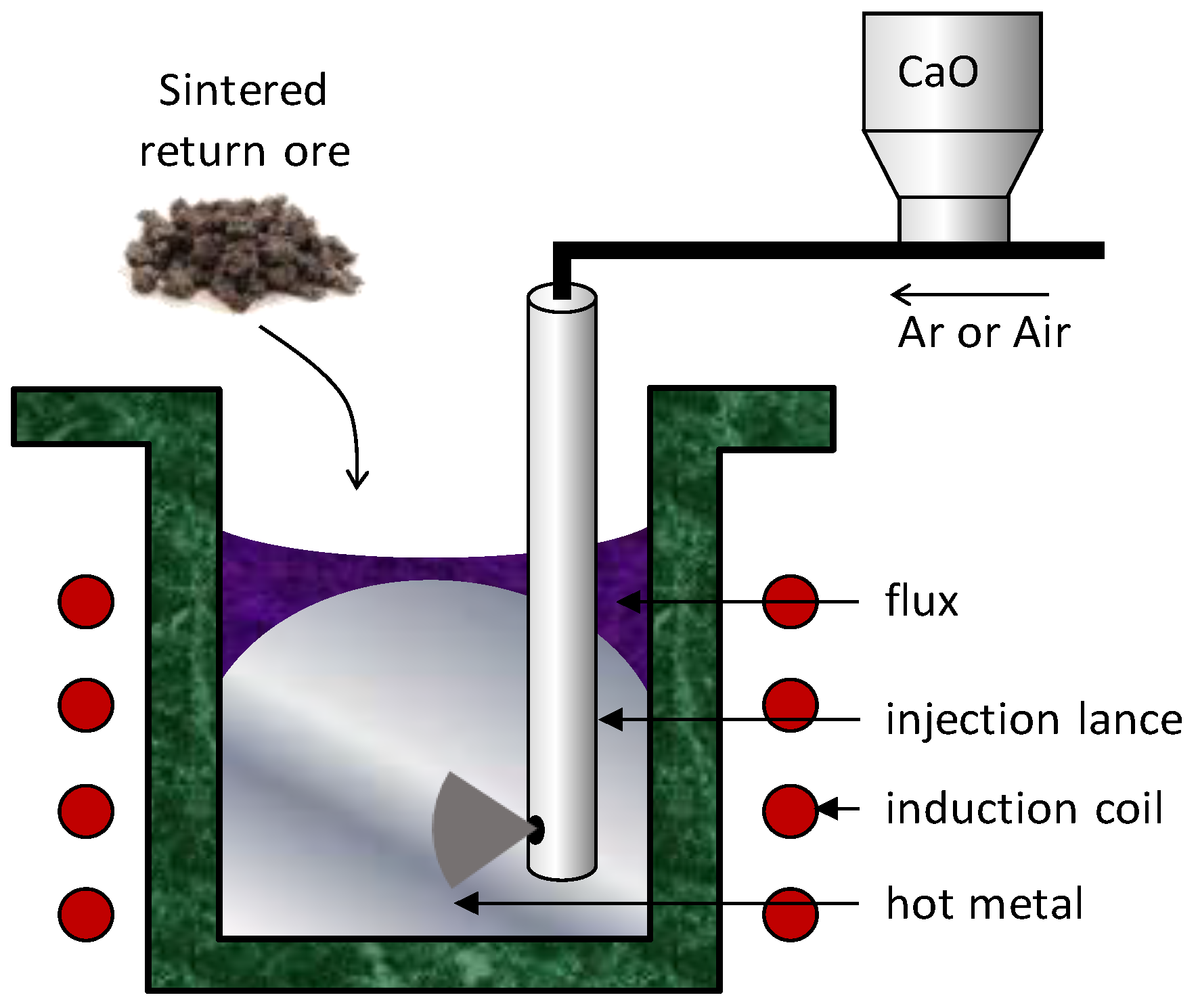
2. Materials and Methods
3. Results and Discussion
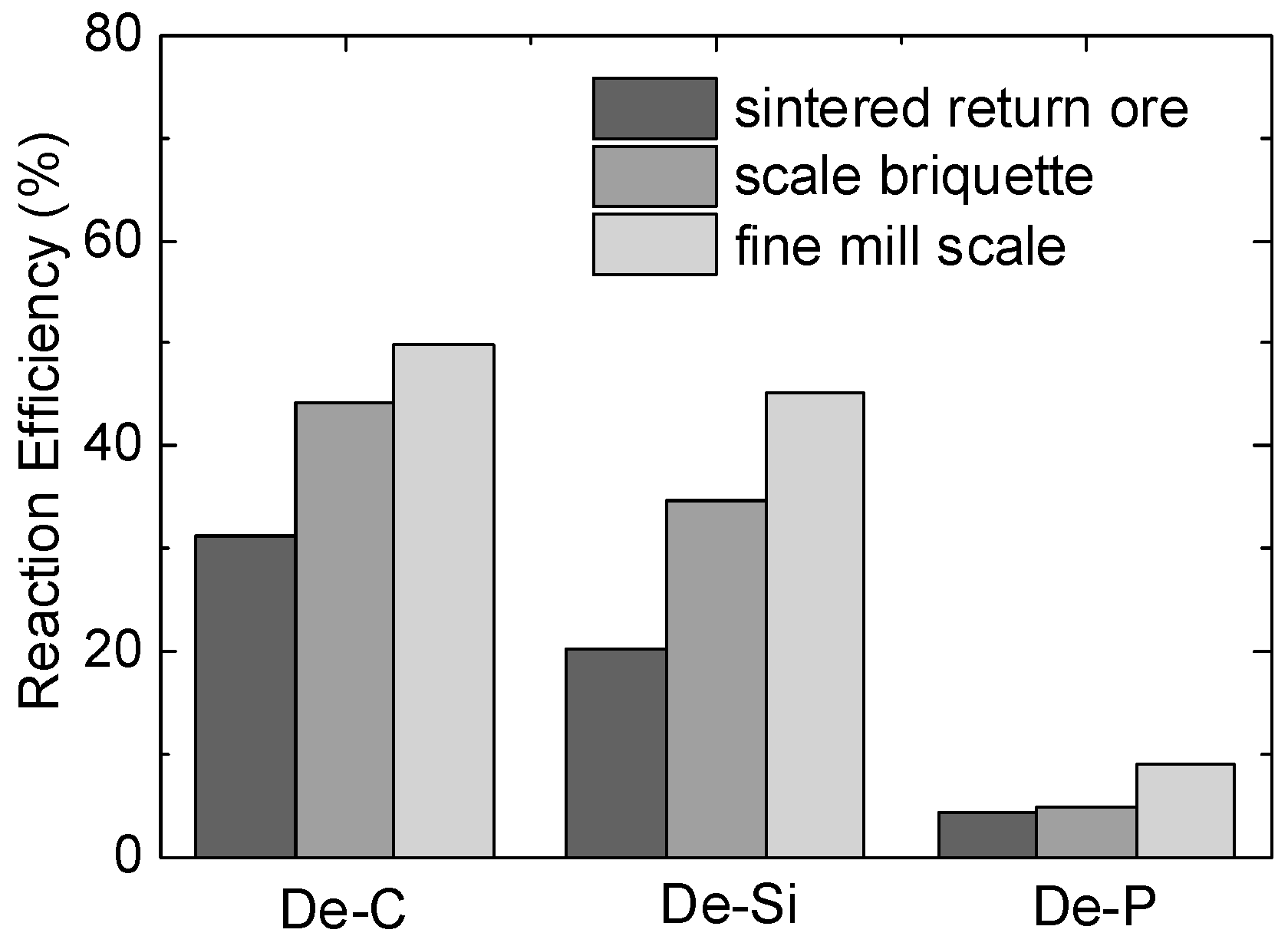
3.1. Desiliconisation and Dephosphorisation Behaviours of Various Solid Oxygen Materials
3.2. Effect of Gaseous Oxygen Supply Method on Desiliconisation and Dephosphorisation of Hot Metal
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Ogawa, Y.; Maruoka, N. Progress of Hot Metal Treatment Technology and Future Outlook. Tetsu Hagane J. Iron Steel Inst. Jpn. 2014, 100, 434–444. [Google Scholar] [CrossRef] [Green Version]
- Iwasaki, M.; Matsuo, M. Change and Development of Steelmaking Technology. Nippon Steel Tech. Rep. 2012, 101, 89–94. [Google Scholar]
- Guo, S.; Dong, Y.; Chen, E.; Wang, H. Dephosphorization and rephosphorization of liquid steel by lime based fluxes. Gangtie 2000, 3, 5. [Google Scholar]
- Yue, K.; Li, J. Dephosphorization of Hot Metal With Slag Containing CaO-Fe2O3. J. Iron Steel Res. 2006, 9, 2. [Google Scholar]
- Yang, X.; Li, J.; Chai, J.; Duan, D.; Zhang, J. A Thermodynamic Model for Predicting Phosphorus Partition between CaO-based Slags and Hot Metal during Hot Metal Dephosphorization Pretreatment Process Based on the Ion and Molecule Coexistence Theory. Metall. Mater. Trans. B 2016, 47, 2279–2301. [Google Scholar] [CrossRef]
- Pak, J.J.; Fruehan, R.J. Dynamics of the hot metal dephosphorization with Na2O slags. Metall. Trans. B 1987, 18B, 687–693. [Google Scholar] [CrossRef]
- Nakamura, Y.; Harashima, K.; Fukuda, Y. Dephosphorization and Desulfurization of Molten 4%C-Fe Alloy with CaO-based flux Containing Halide. Tetsu-to-Hagané 1981, 67, 2138–2144. [Google Scholar] [CrossRef]
- Inoue, H.; Shigeno, Y.; Tokuda, M.; Ohtani, M. On Simultaneous Dephosphorization and Desulphurization of Pig Iron with CaO-CaCl2 Fluxes. Tetsu-to-Hagané 1983, 69, 210–219. [Google Scholar] [CrossRef]
- Taskinen, A.; Janke, D. Slag Pretreatment for Simultaneous Dephosphorization and Desulphurization of Hot Metal. Stahl Eisen 1983, 103, 491–496. [Google Scholar]
- Umezawa, K.; Matsunaga, H.; Arima, R.; Tonomura, S.; Furugaki, I. The Influence of Operating Condition on Dephosphorization and Desulphurization Reactions of Hot Metal with Lime-based Flux. Tetsu-to-Hagané 1983, 69, 1810–1817. [Google Scholar] [CrossRef]
- Hernandez, A.; Romero, A.; Chavez, F.; Angeles, M. Dephosphorization and desulfurization pretreatment of molten iron with CaO-SiO2-CaF2-FeO-Na2O slags. ISIJ Int. 1998, 38, 126–131. [Google Scholar] [CrossRef]
- Nozaki, T.; Nakanishi, K.; Morishita, H.; Yamada, S.; Sudo, F. Characteristics of Dephosphorization in a Bottom Blown Converter and Its Application to the Preliminary Treatment of Hot Metal. Tetsu-to-Hagané 1982, 68, 1737–1743. [Google Scholar] [CrossRef] [Green Version]
- Ono, H.; Masui, T.; Mori, H. Dephosphorization Kinetics of Hot Metal by Lime Injection with Oxygen Gas. Tetsu-to-Hagané 1983, 69, 1763–1770. [Google Scholar] [CrossRef]
- Ogawa, Y.; Yano, M.; Kitamura, S.; Hirata, H. Development of the Continuous Dephosphorization and Decarburization Process Using BOF. Tetsu-to-Hagané 2001, 87, 21–28. [Google Scholar] [CrossRef]
- Mukawa, S.; Mizukami, Y. Effect of Stirring Energy and Rate of Oxygen Supply on the Rate of Hot Metal Dephosphorization. ISIJ Int. 1995, 35, 1374–1380. [Google Scholar] [CrossRef]
- Haida, O.; Takeuchi, S.; Nozaki, T.; Emi, T.; Sudo, F. Mechanism of Hot Metal Dephosphorization by Injecting Lime Base Fluxes into Bottom Blown Converter. Tetsu-to-Hagané 1982, 68, 1744–1753. [Google Scholar] [CrossRef]
- Kawauchi, Y.; Maede, H.; Kamisaka, E.; Satoh, S.; Inoue, T.; Naki, M. Metallurgical Characteristics of Hot Metal Desiliconization by Injecting Gaseous Oxygen. Tetsu-to-Hagané 1983, 69, 1730–1737. [Google Scholar] [CrossRef]
- Saito, K.; Nakanishi, K.; Misaki, N.; Nakai, K.; Onishi, M. Dephosphorization of Hot Metal with Injection of Lime Bearing Fluxes in a Ladle. Tetsu-to-Hagané 1983, 69, 1802–1809. [Google Scholar] [CrossRef] [Green Version]
- Inoue, T.; Yoshida, M.; Sato, H.; Yonenaka, E. De-siliconization and de-phosphorization treatment of hot metal by gaseous oxygen injection in TPC. Tetsu-to-Hagané 1985, 71, S943. [Google Scholar]
- Roy, G.G.; Chaudhary, P.N.; Minj, R.K.; Goel, R.P. Dephosphorization of ferromanganese using BaCO3-based fluxes by submerged injection of powders: A preliminary kinetic study. Metall. Mater. Trans. B 2001, 32B, 558–561. [Google Scholar] [CrossRef]





| Oxygen Source | Composition (wt. %) | Original Size (mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T-Fe* | M-Fe* | O | FeO | Fe2O3 | SiO2 | CaO | P | Others | ||
| Sintered return ore | 58.3 | - | 24.1 | 7.8 | 74.7 | 5.51 | 9.57 | 0.05 | 2.46 | 2–10 |
| Scale briquette | 73.6 | 12.8 | 22.4 | 33.7 | 49.4 | 0.80 | 0.10 | 0.02 | 0.21 | 40–50 |
| Fine mill scale | 74.5 | - | 24.9 | 63.7 | 35.8 | 0.15 | 0.18 | 0.01 | 0.12 | 5–8 |
| Blowing Conditions | Top Lance | Bottom Lance |
|---|---|---|
| 1 | O2 0.4 SLPM* | Ar 0.4 SLPM |
| 2 | O2 0.2 SLPM, Ar 0.2 SLPM | O2 0.2 SLPM, Ar 0.2 SLPM |
| 3 | Ar 0.4 SLPM | O2 0.4 SLPM |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, Y. Desiliconisation and Dephosphorisation Behaviours of Various Oxygen Sources in Hot Metal Pre-Treatment. Metals 2019, 9, 251. https://doi.org/10.3390/met9020251
Kang Y. Desiliconisation and Dephosphorisation Behaviours of Various Oxygen Sources in Hot Metal Pre-Treatment. Metals. 2019; 9(2):251. https://doi.org/10.3390/met9020251
Chicago/Turabian StyleKang, Youngjo. 2019. "Desiliconisation and Dephosphorisation Behaviours of Various Oxygen Sources in Hot Metal Pre-Treatment" Metals 9, no. 2: 251. https://doi.org/10.3390/met9020251





