Formation of Surface Depression during Continuous Casting of High-Al TRIP Steel
Abstract
:1. Introduction
2. Experimental Materials and Methods
3. Results
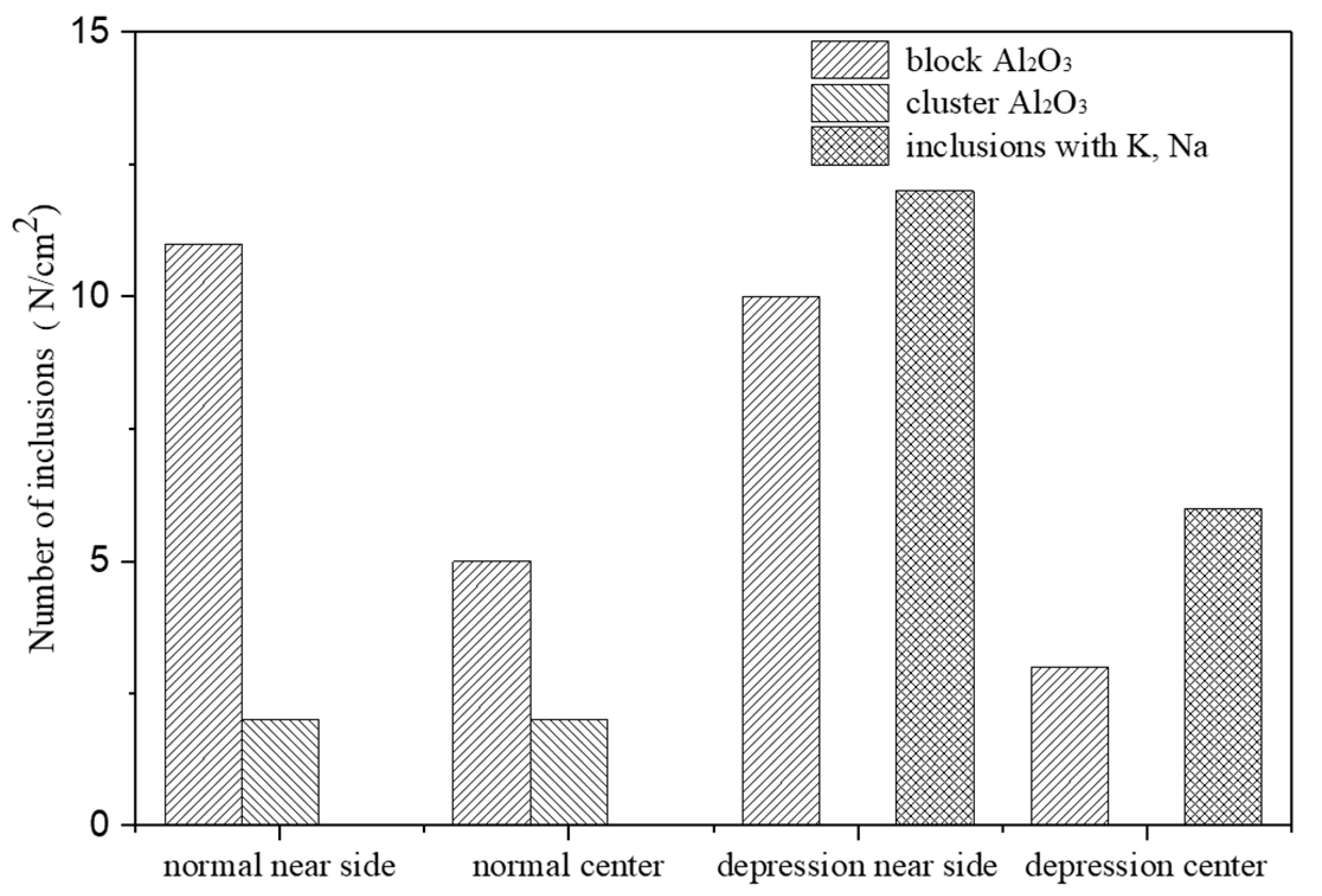
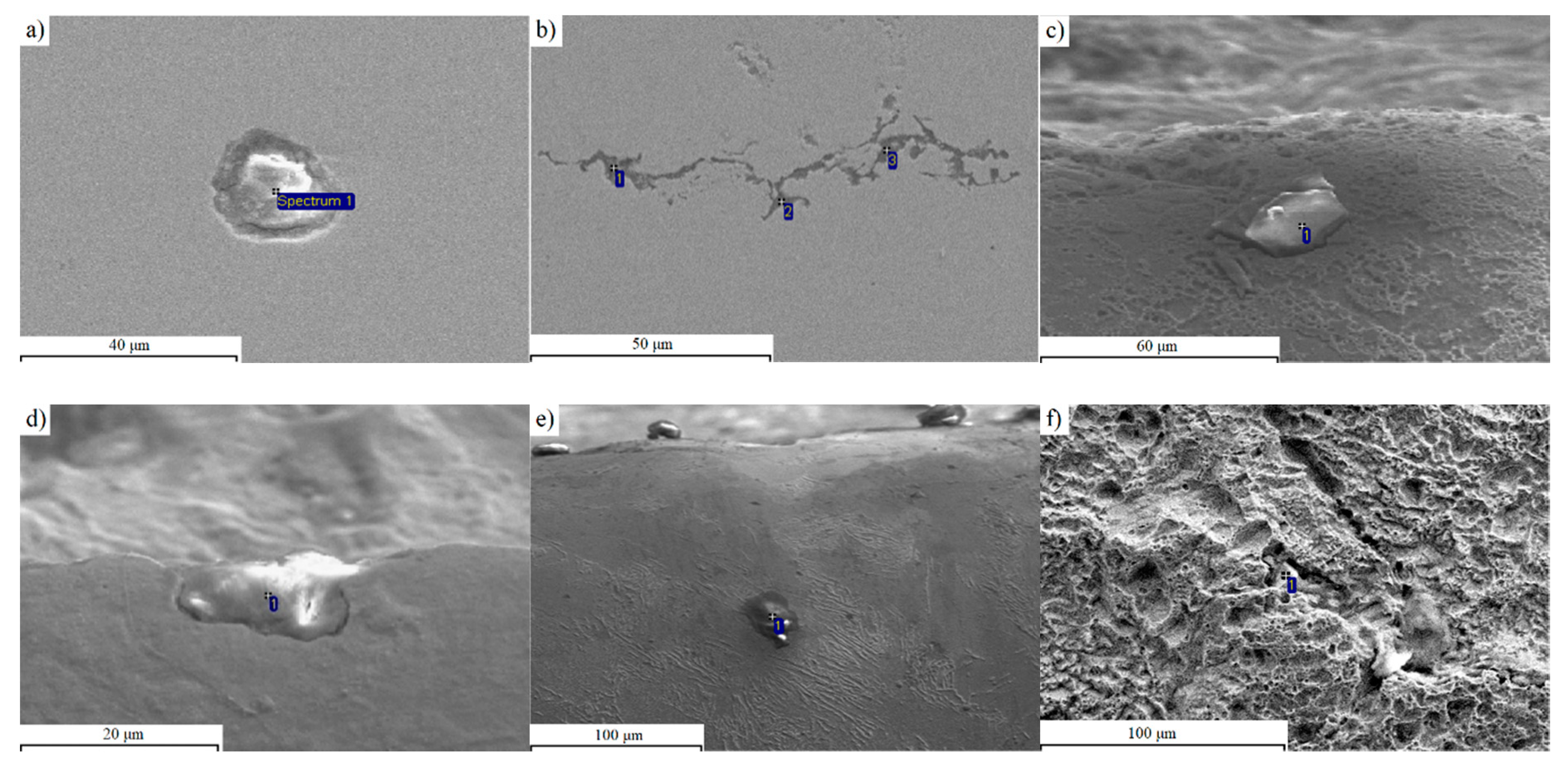
3.1. Metallographic Analysis
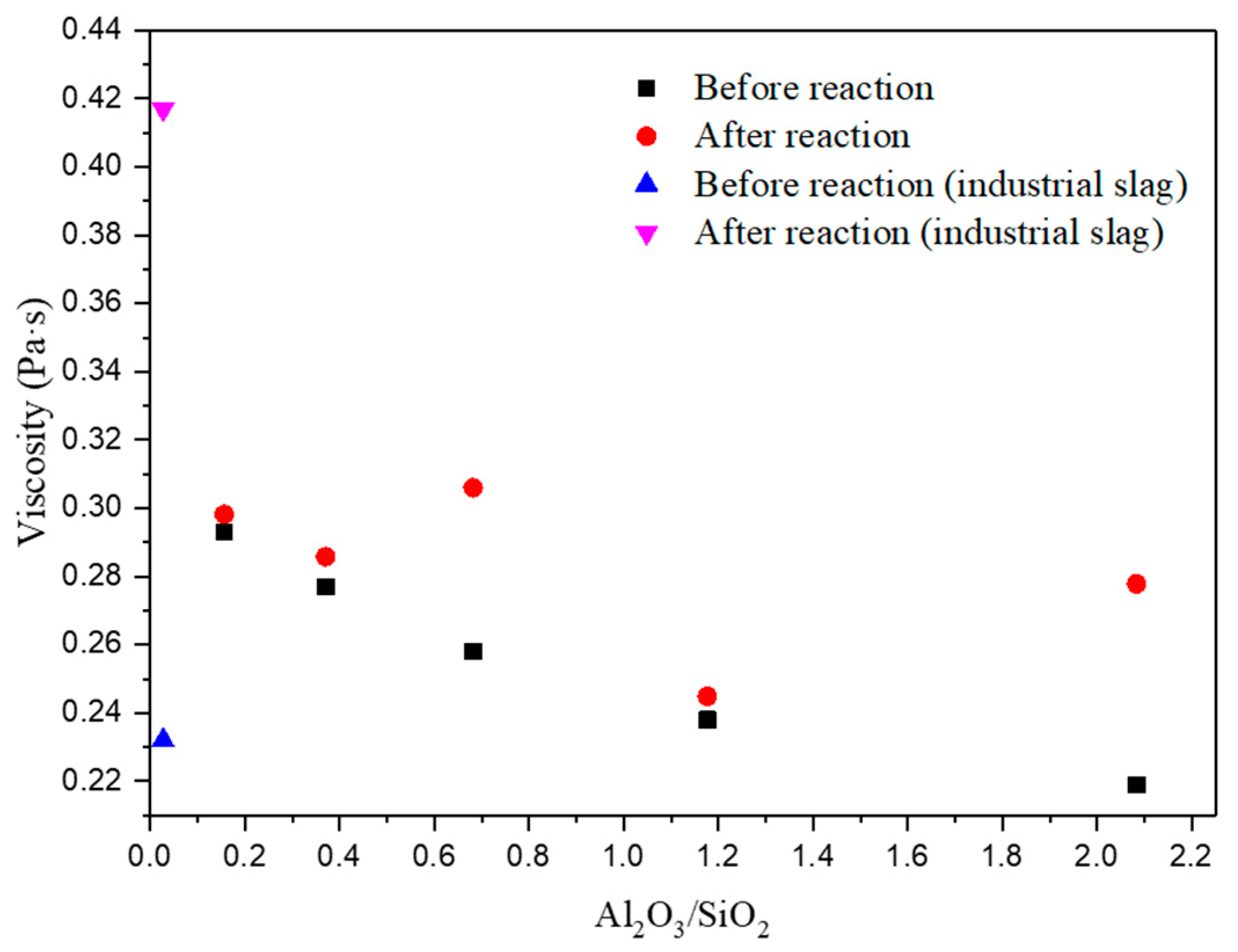
3.2. Reactivity of Mold Fluxes for Al-TRIP Steel
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fonstein, N.; Pottore, N.; Lalam, S.H.; Bhattacharya, D. Phase transformation behavior during continuous cooling and isothermal holding of aluminum and silicon bearing TRIP steels. In Proceedings of the Materials Science and Technology, Chicago, IL, USA, 9–12 November 2003. [Google Scholar]
- Tuling, A.; Banerjee, J.R.; Mintz, B. Influence of peritectic phase transformation on hot ductility of high aluminium TRIP steels containing Nb. Mater. Sci. Tech. 2011, 27, 1724–1731. [Google Scholar] [CrossRef]
- Bellhouse, E.M.; Mertens, A.I.M.; McDermid, J.R. Development of the surface of TRIP steels prior to hot-dip galvanizing. Mat. Sci. Eng. A 2007, 436, 147–156. [Google Scholar] [CrossRef]
- Bhattacharyya, T.; Singh, S.B.; Bhattacharyya, S.; Ray, R.K.; Bleck, W.; Bhattacharjee, D. An assessment on coatability of trans formation induced plasticity (TRIP)-aided steel. Surf. Coat. Tech. 2013, 235, 226–234. [Google Scholar] [CrossRef]
- Shi, C.B.; Seo, M.D.; Cho, J.W.; Kim, S.H. Crystallization Characteristics of CaO- Al2O3-Based Mold Flux and Their Effects on In-Mold Performance during High-Aluminum TRIP Steels Continuous Casting. Metall. Mater. Trans. B 2014, 45B, 1081–1097. [Google Scholar] [CrossRef]
- Ji, C.X.; Cui, Y.; Zeng, Z.; Tian, Z.H.; Zhao, C.L.; Zhu, G.S. Continuous casting of high-Al steel in Shougang Jingtang steel works. J. Iron Steel Res. Int. 2015, 22, 53–56. [Google Scholar] [CrossRef]
- Bernhard, C.; Xia, G. Influence of alloying elements on the thermal contraction of peritectic steels during initial solidification. Ironmak. Steelmak. 2006, 33, 52–56. [Google Scholar] [CrossRef]
- Pierer, R.; Bernhard, C. High temperature behavior during solidification of peritectic steels under continuous casting conditions. In Proceedings of the Materials Science and Technology, Cincinnati, OH, USA, 15–19 October 2006. [Google Scholar]
- Presoly, P.; Pierer, R.; Bernhard, C. Identification of defect prone peritectic steel grades by analyzing high-temperature phase transformations. Metall. Mater. Trans. A 2013, 44A, 5377–5388. [Google Scholar] [CrossRef]
- Wang, W.L.; Blazek, K.; Cramb, A. A study of the crystallization behavior of a new mold flux used in the casting of transformation-induced-plasticity steels. Metall. Mater. Trans. B 2008, 39B, 66–74. [Google Scholar] [CrossRef]
- Cho, J.W.; Blazek, K.; Frazee, M.; Yin, H.B.; Park, J.H.; Moon, S.W. Assessment of CaO-Al2O3 based mold flux system for high aluminum TRIP casting. ISIJ Int. 2013, 53, 62–70. [Google Scholar] [CrossRef]
- Liu, Q.; Wen, G.; Li, J.; Fu, X.; Tang, P.; Li, W. Development of mould fluxes based on lime-alumina slag system for casting high aluminium TRIP steel. Ironmak. Steelmak. 2014, 41, 292–297. [Google Scholar] [CrossRef]
- Cui, H.; Wu, H.J.; Yue, F.; Wu, W.S.; Wang, M.; Bao, Y.P.; Chen, B.; Ji, C.X. Surface defects of cold-rolled Ti-IF steel sheets due to non-metallic inclusions. J. Iron Steel Res. Int. 2011, 18, 335–340. [Google Scholar]
- Solek, K.; Korolczuk-Hejnak, M.; Slezak, W. Viscosity measurements for modeling of continuous steel casting. Arch. Metall. Mater. 2012, 57, 333–338. [Google Scholar]
- Lu, B.X.; Chen, K.; Wang, W.L.; Jiang, B.B. Effects of Li2O and Na2O on the crystallization behavior of lime-alumina-based mold flux for casting high-Al steels. Metall. Mater. Trans. B 2014, 45B, 1496–1509. [Google Scholar] [CrossRef]
- Thomas, B.G.; Storkman, W.R. Mathematical models of continuous slab casting to optimize mold taper. In Proceedings of the Modeling and Control of Casting and Welding Processes, Warrendale, PA, USA, 17–22 April 1988. [Google Scholar]
- Cho, J.W.; Yoo, S.; Park, M.S.; Park, J.K.; Moon, K.H. Improvement of castability and surface quality of continuously cast TWIP slabs by molten mold flux feeding technology. Metall. Mater. Trans. B 2017, 48B, 187–196. [Google Scholar] [CrossRef]



| C | Si | Mn | P | S | Alt | N |
|---|---|---|---|---|---|---|
| 0.16 | 0.16 | 1.49 | 0.008 | 0.001 | 1.35 | 0.0016 |
| Samples | O | Na | Mg | Al | Si | Mn | P | S | K | Ca | Ti | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a | 38.19 | - | - | 57.21 | - | - | - | - | - | - | - | 4.60 |
| b-1 | 55.90 | - | 1.90 | 42.21 | - | - | - | - | - | - | - | - |
| b-2 | 51.09 | - | 4.30 | 42.55 | - | 2.07 | - | - | - | - | - | - |
| b-3 | 55.25 | - | 5.93 | 38.82 | - | - | - | - | - | - | - | - |
| c | 62.14 | - | - | 37.31 | - | - | - | - | - | - | - | 0.55 |
| d | 43.26 | 2.51 | 0.64 | 0.53 | 1.30 | - | 0.42 | 5.39 | 0.57 | 2.49 | - | 42.89 |
| e | 7.56 | 4.55 | 0.64 | 1.76 | 0.59 | 1.60 | - | 0.57 | 0.62 | 0.56 | - | 81.56 |
| f | 36.43 | - | - | 5.26 | 0.99 | 0.54 | - | 1.15 | 0.48 | - | 0.79 | 54.37 |
| Slag Sample | CaO | SiO2 | Al2O3 | CaF2 | MnO | BaO | Na2O | Fe2O3 | MgO | CaO/SiO2 | Viscosity (Pa·s) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Before reaction | 36.30 | 40.35 | 1.07 | 13.66 | 0.21 | 0.50 | 6.64 | 0.58 | 0.68 | 0.90 | 0.232 |
| After reaction | 39.60 | 28.90 | 12.58 | 12.73 | 1.57 | 0.59 | 1.52 | 1.33 | 1.17 | 1.37 | 0.417 |
| Samples | CaO | SiO2 | Al2O3 | Al2O3/SiO2 | a(SiO2) | a(Al2O3) | ΔG (kJ/mol) | Viscosity (Pa·s) |
|---|---|---|---|---|---|---|---|---|
| A-1 | 30 | 32 | 5 | 0.16 | 3.50 × 10−2 | 1.10 × 10−3 | −154.4 | 0.293 |
| A-2 | 30 | 27 | 10 | 0.37 | 2.24 × 10−3 | 2.41 × 10−2 | −99.7 | 0.277 |
| A-3 | 30 | 22 | 15 | 0.68 | 4.36 × 10−4 | 1.55 × 10−2 | −84.5 | 0.258 |
| A-4 | 30 | 17 | 20 | 1.18 | 2.76 × 10−4 | 1.40 × 10−2 | −80.1 | 0.238 |
| A-5 | 30 | 12 | 25 | 2.08 | 2.16 × 10−4 | 1.06 × 10−2 | −79.4 | 0.219 |
| Sample | CaO | SiO2 | Al2O3 | ΔSiO2 (%) | ΔAl2O3 (%) | CaO/SiO2 | Viscosity (Pa·s) |
|---|---|---|---|---|---|---|---|
| A-1 | 34.25 | 20.14 | 17.53 | −37.50 | 250.51 | 1.70 | 0.298 |
| A-2 | 34.49 | 18.41 | 23.93 | −31.82 | 139.29 | 1.87 | 0.286 |
| A-3 | 31.94 | 15.18 | 25.42 | −31.00 | 69.49 | 2.10 | 0.306 |
| A-4 | 33.17 | 12.00 | 28.37 | −29.41 | 41.85 | 2.76 | 0.245 |
| A-5 | 32.33 | 8.58 | 34.00 | −29.17 | 36.02 | 3.77 | 0.278 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, H.; Zhang, K.; Wang, Z.; Chen, B.; Liu, B.; Qing, J.; Li, Z. Formation of Surface Depression during Continuous Casting of High-Al TRIP Steel. Metals 2019, 9, 204. https://doi.org/10.3390/met9020204
Cui H, Zhang K, Wang Z, Chen B, Liu B, Qing J, Li Z. Formation of Surface Depression during Continuous Casting of High-Al TRIP Steel. Metals. 2019; 9(2):204. https://doi.org/10.3390/met9020204
Chicago/Turabian StyleCui, Heng, Kaitian Zhang, Zheng Wang, Bin Chen, Baisong Liu, Jing Qing, and Zhijun Li. 2019. "Formation of Surface Depression during Continuous Casting of High-Al TRIP Steel" Metals 9, no. 2: 204. https://doi.org/10.3390/met9020204




