Effect of Cooling Methods on the Strength of Silico-ferrite of Calcium and Aluminum of Iron Ore Sinter during the Cooling Process
Abstract
:1. Introduction
2. Materials and Methods
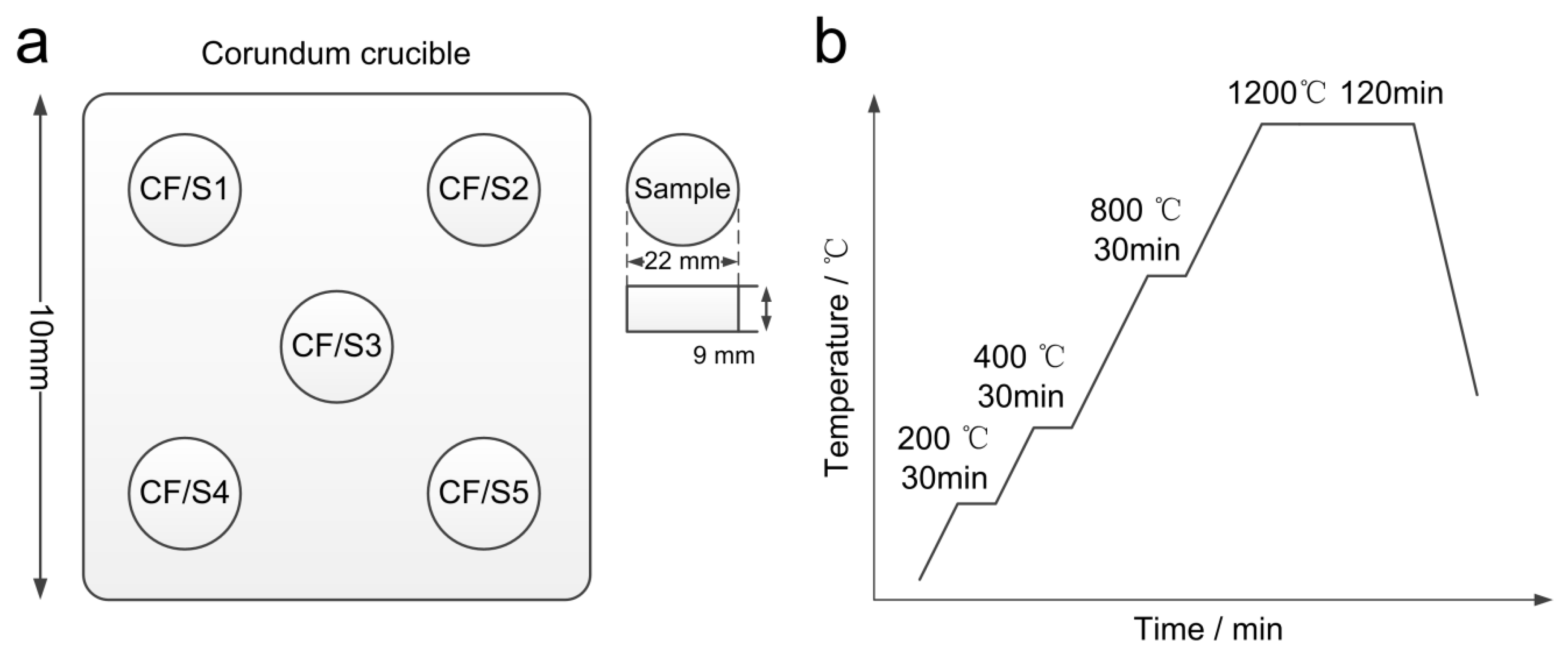
2.1. Materials and Preparation
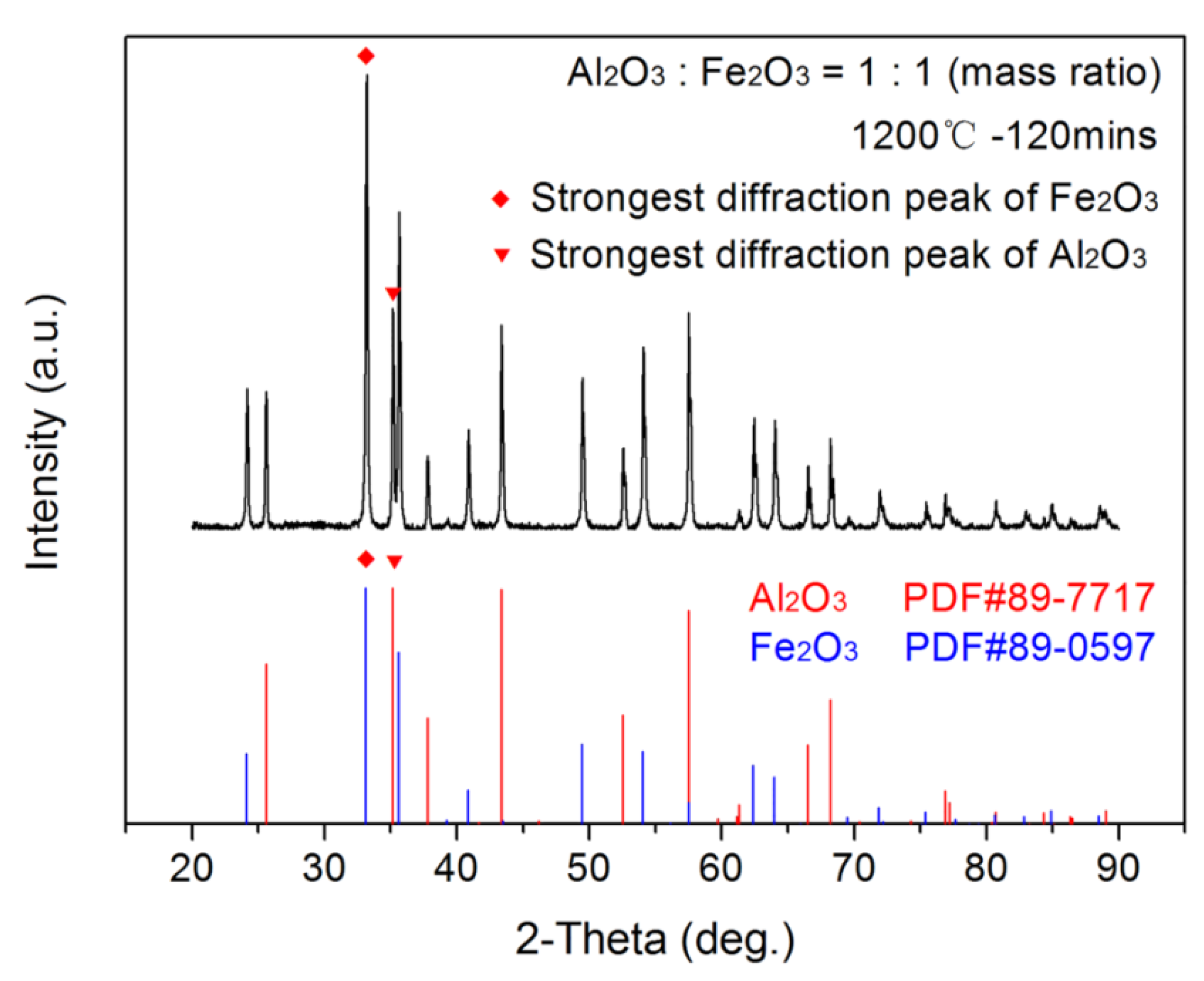
2.1.1. Synthesis and Verification of Calcium Ferrite
2.1.2. Synthesis of Silico-Ferrite of Calcium and Aluminum
2.2. Semi-Quantitative Analysis of X-ray Diffraction
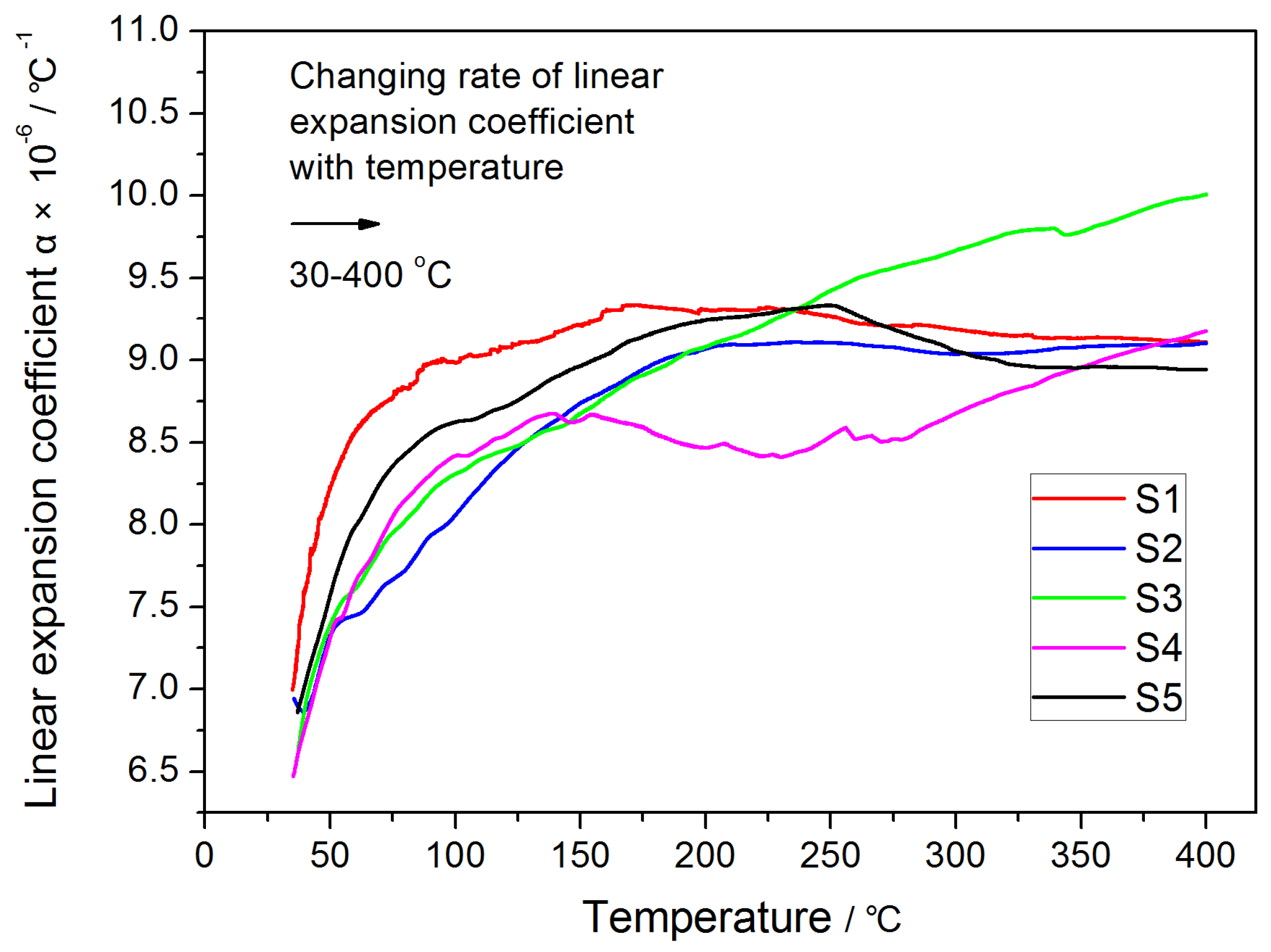
2.3. Test of Linear Expansion Coefficient
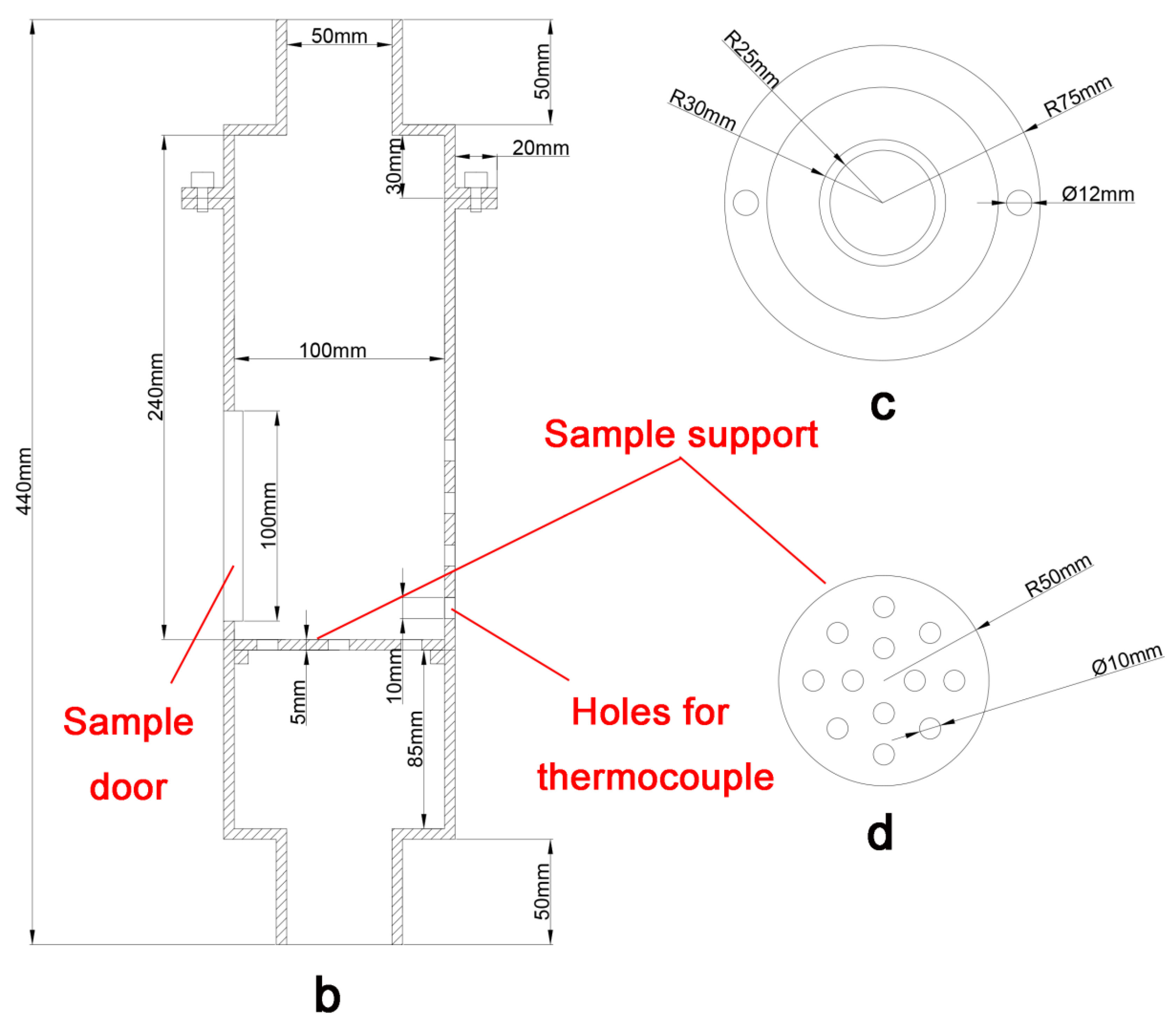
2.4. Test of Cooling and Compressive Strength
3. Results and Discussions
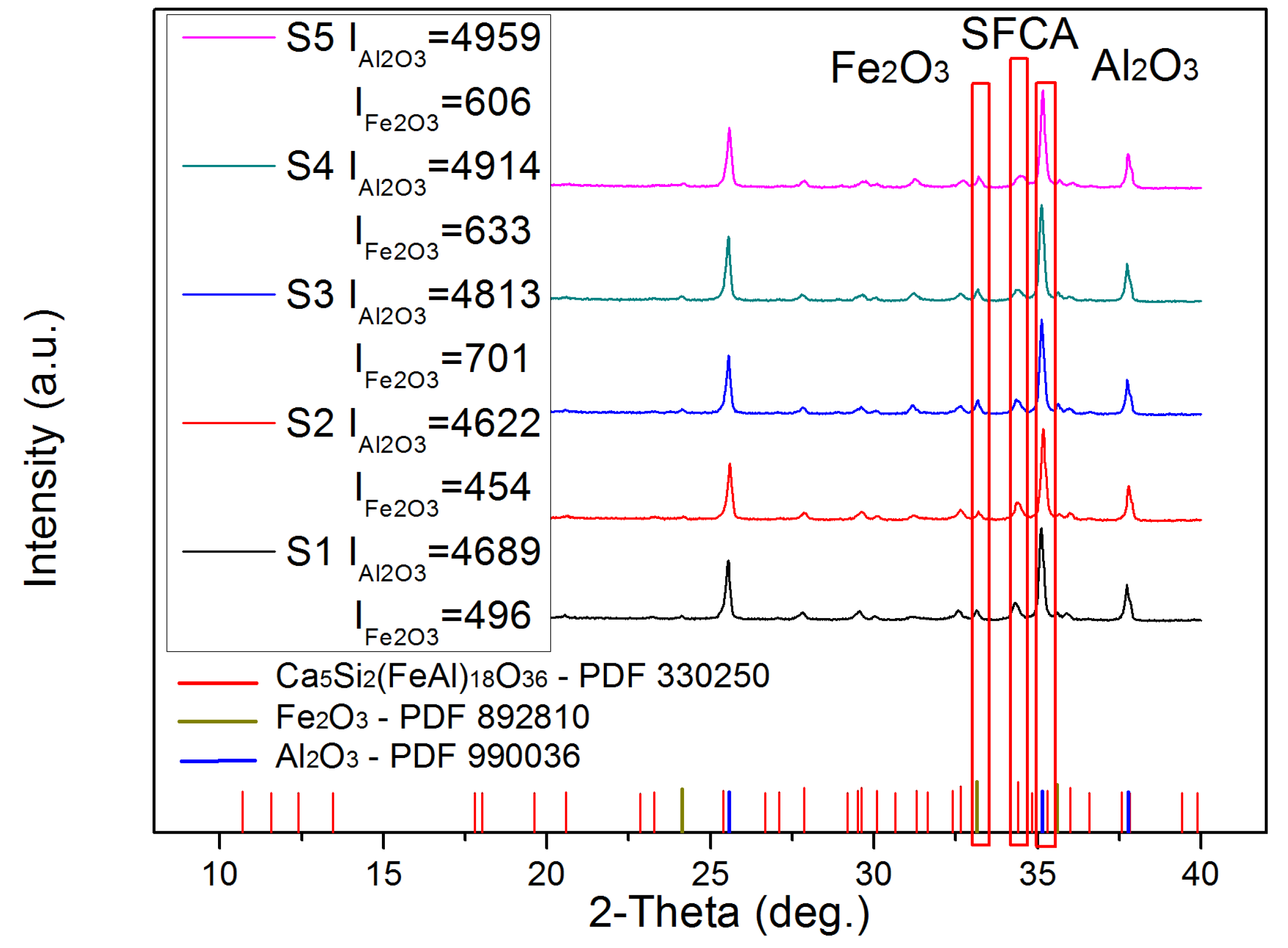
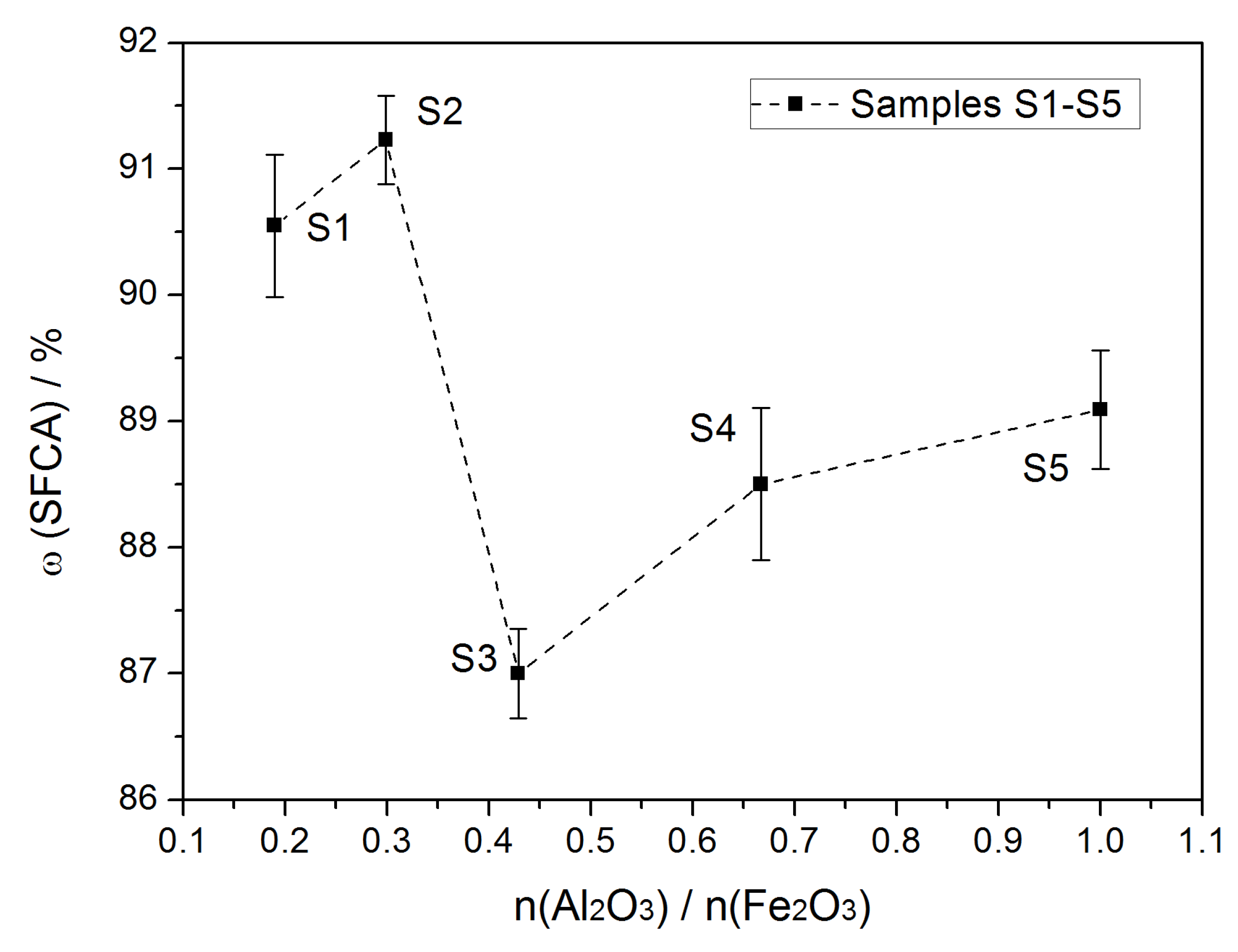
3.1. Semi-Quantitative Analysis of X-ray Diffraction
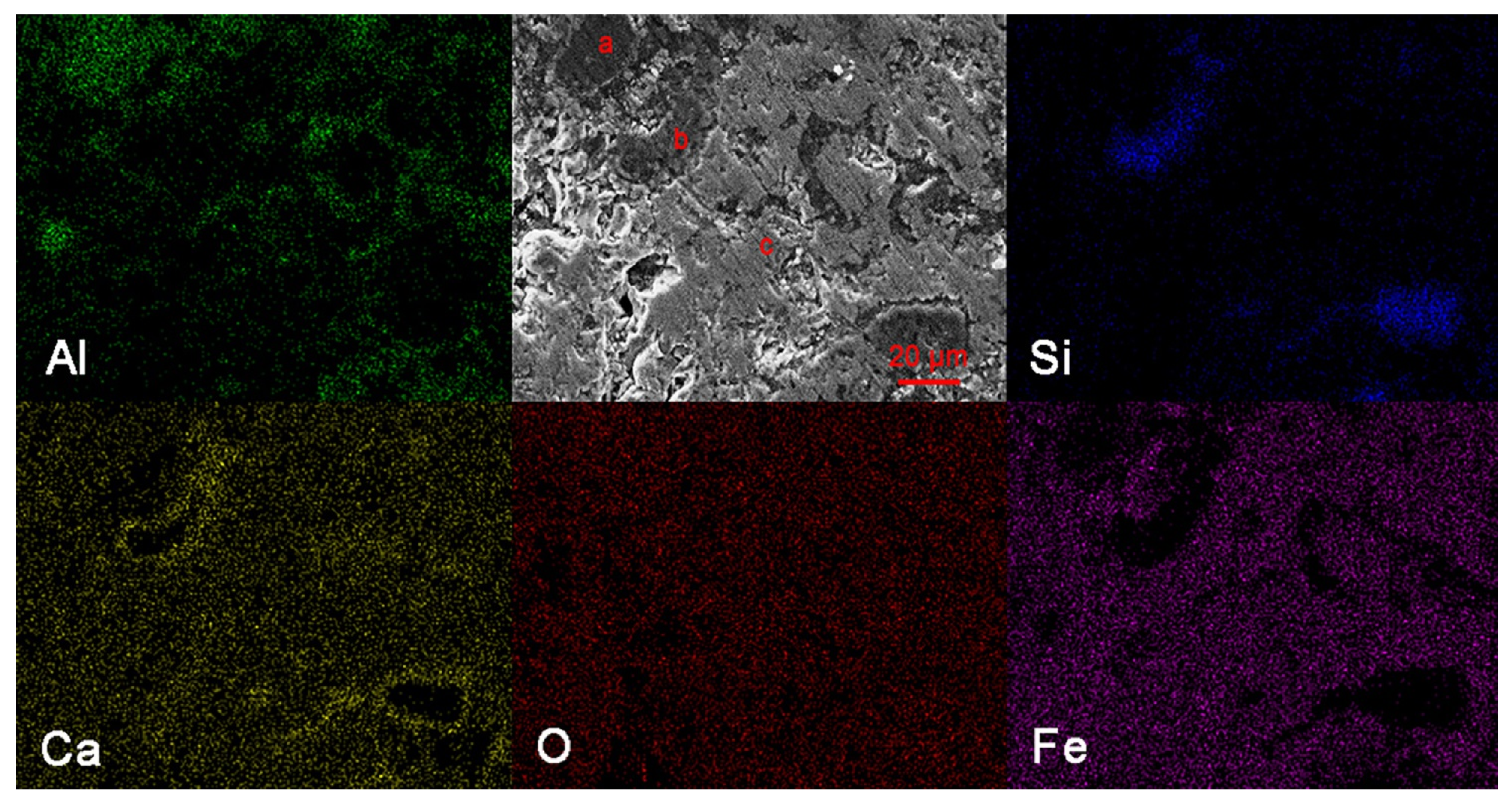
3.2. Scanning Electron Microscope and Energy Dispersive Spectrometer Analyses
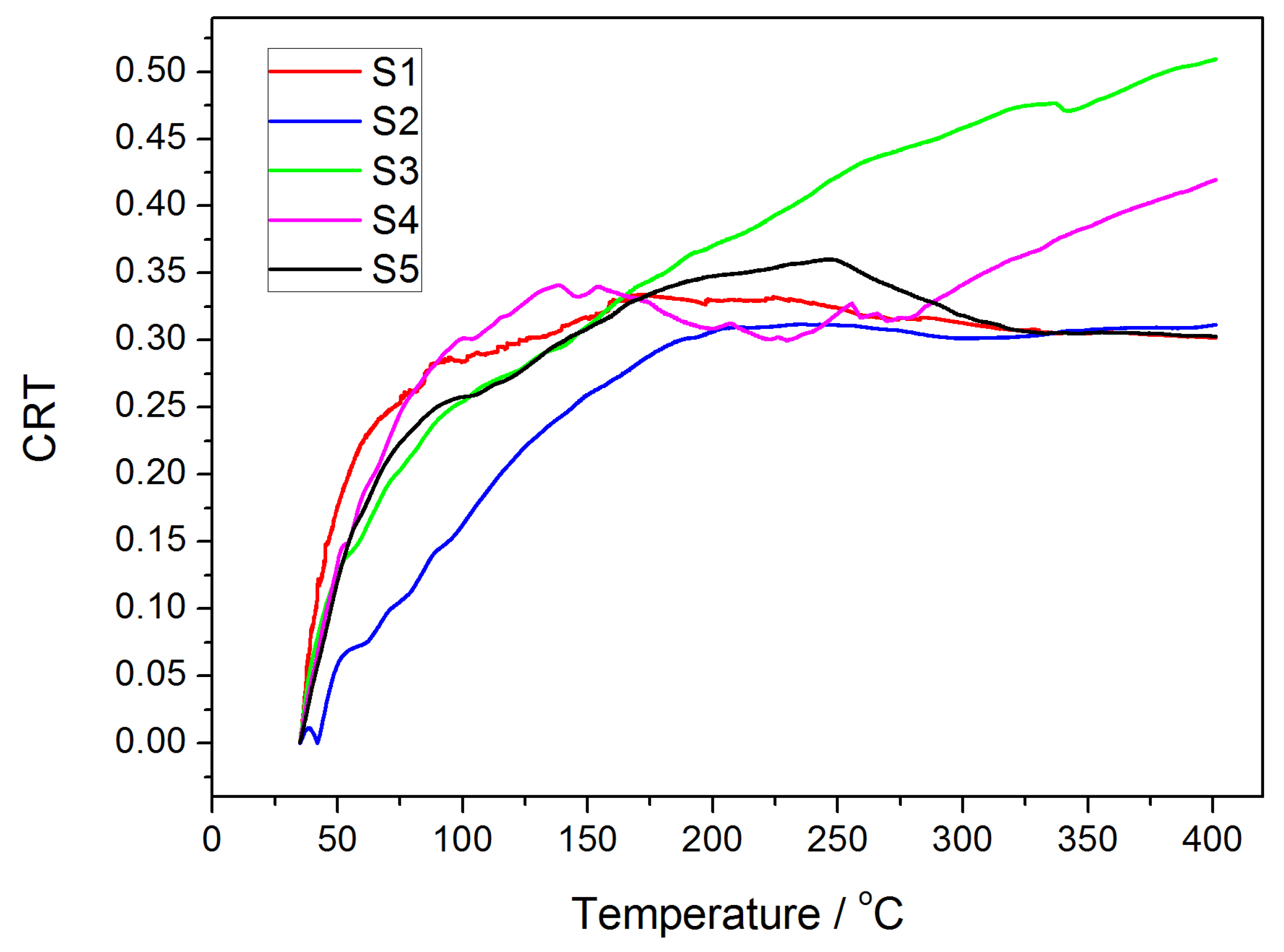
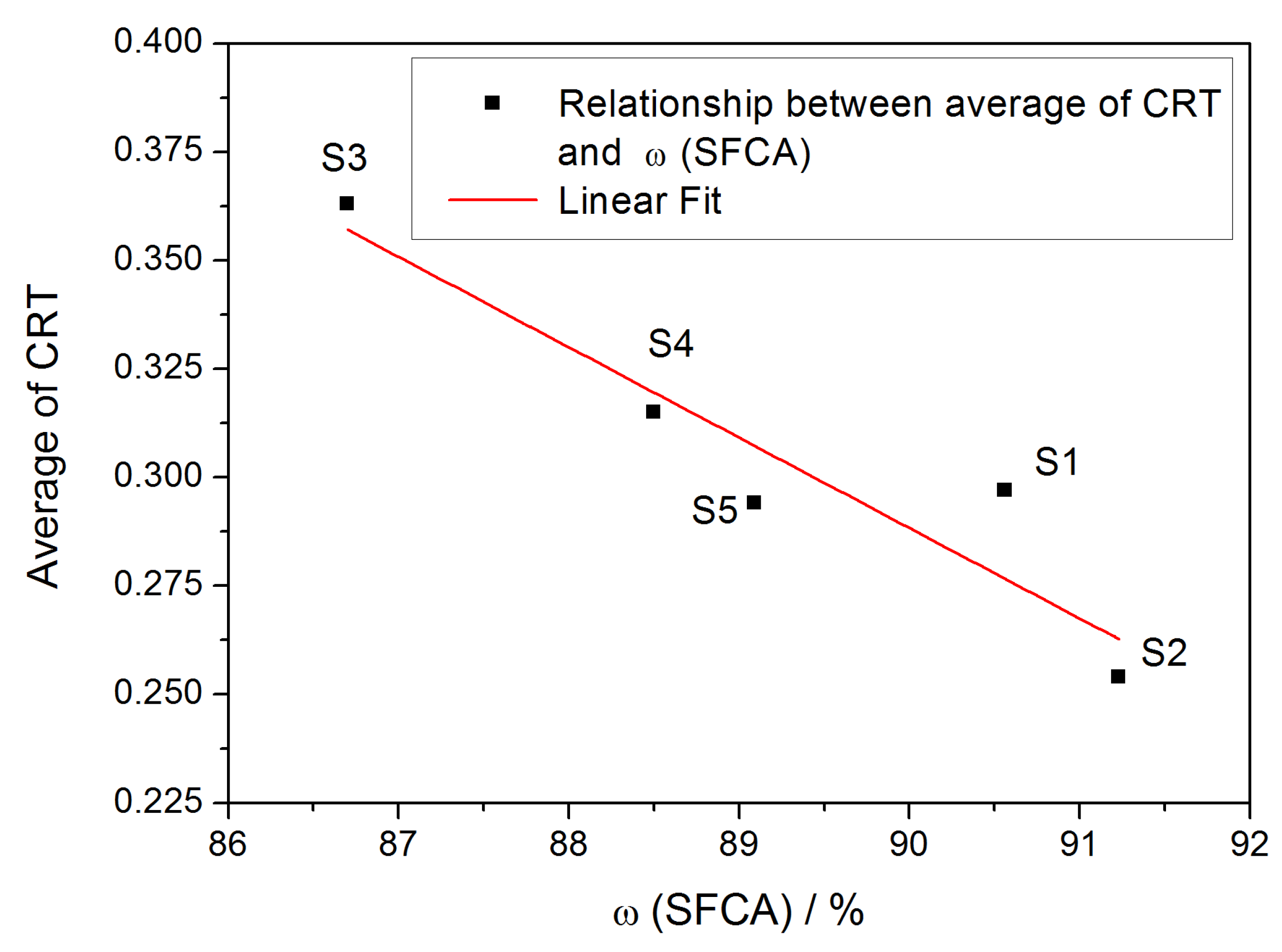
3.3. Effect of SFCA Content on Strength of Samples during Temperature Change
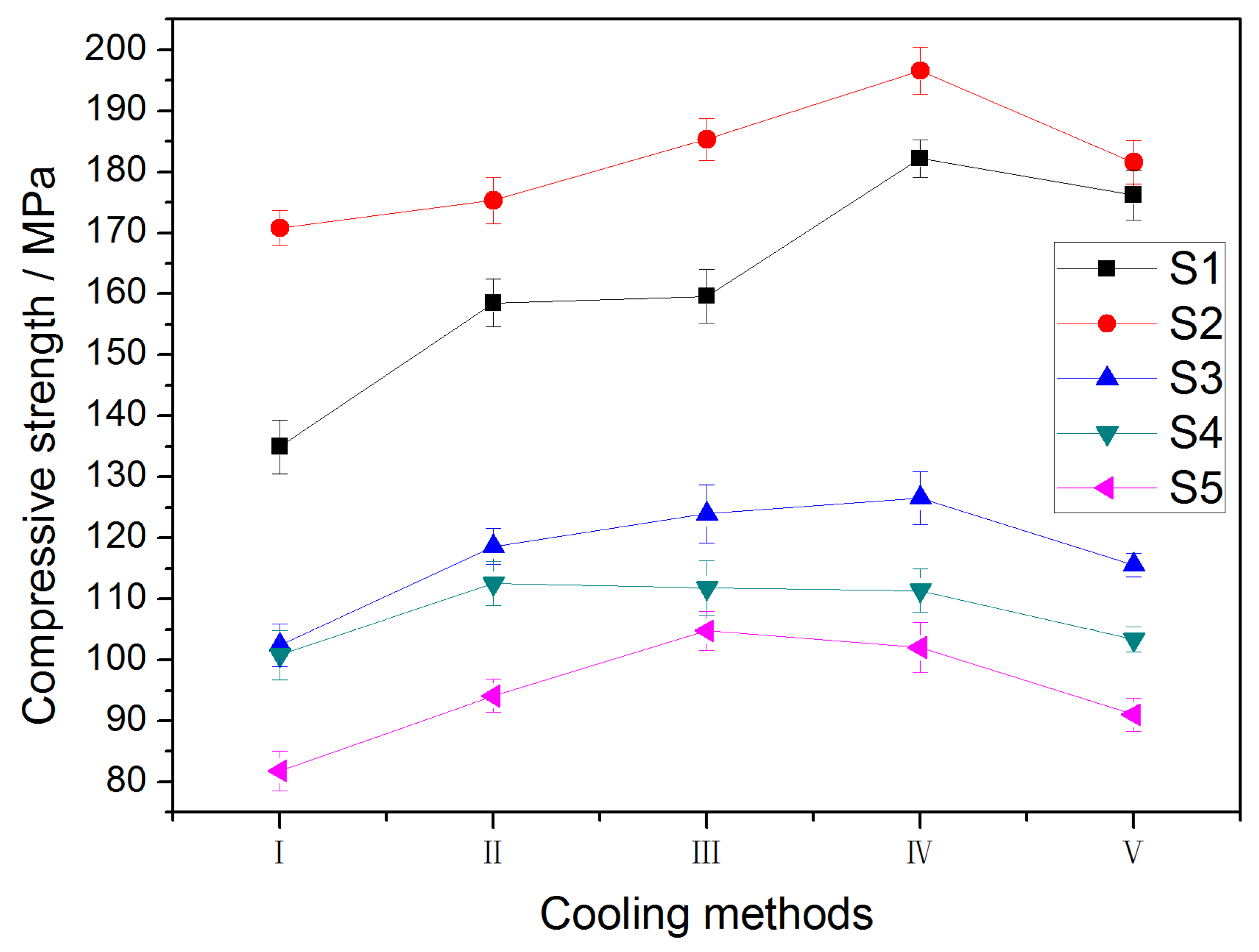
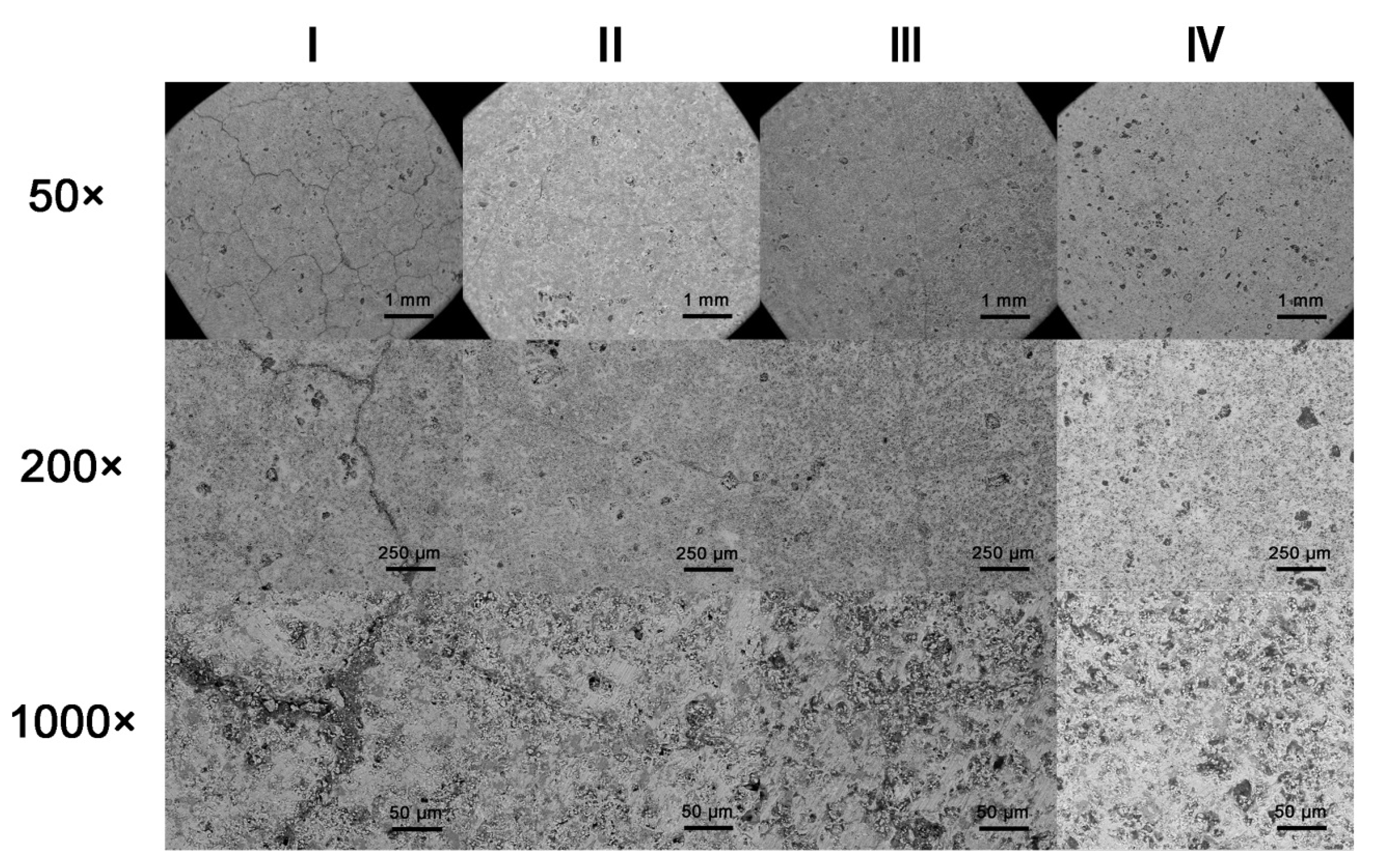
3.4. Effect of Cooling Method on Strength of Samples during Cooling Process
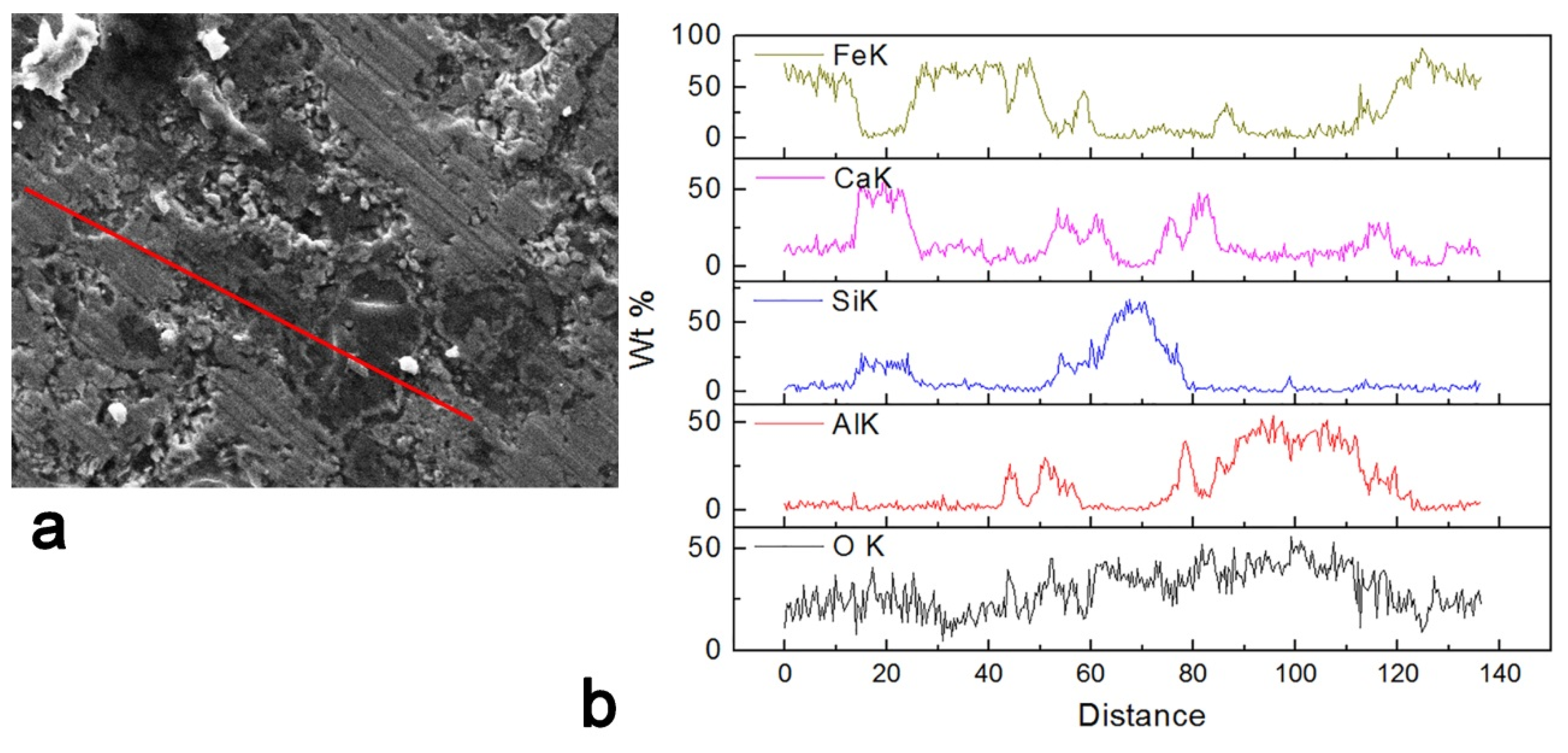
3.5. Scanning Electron Microscopy Analysis
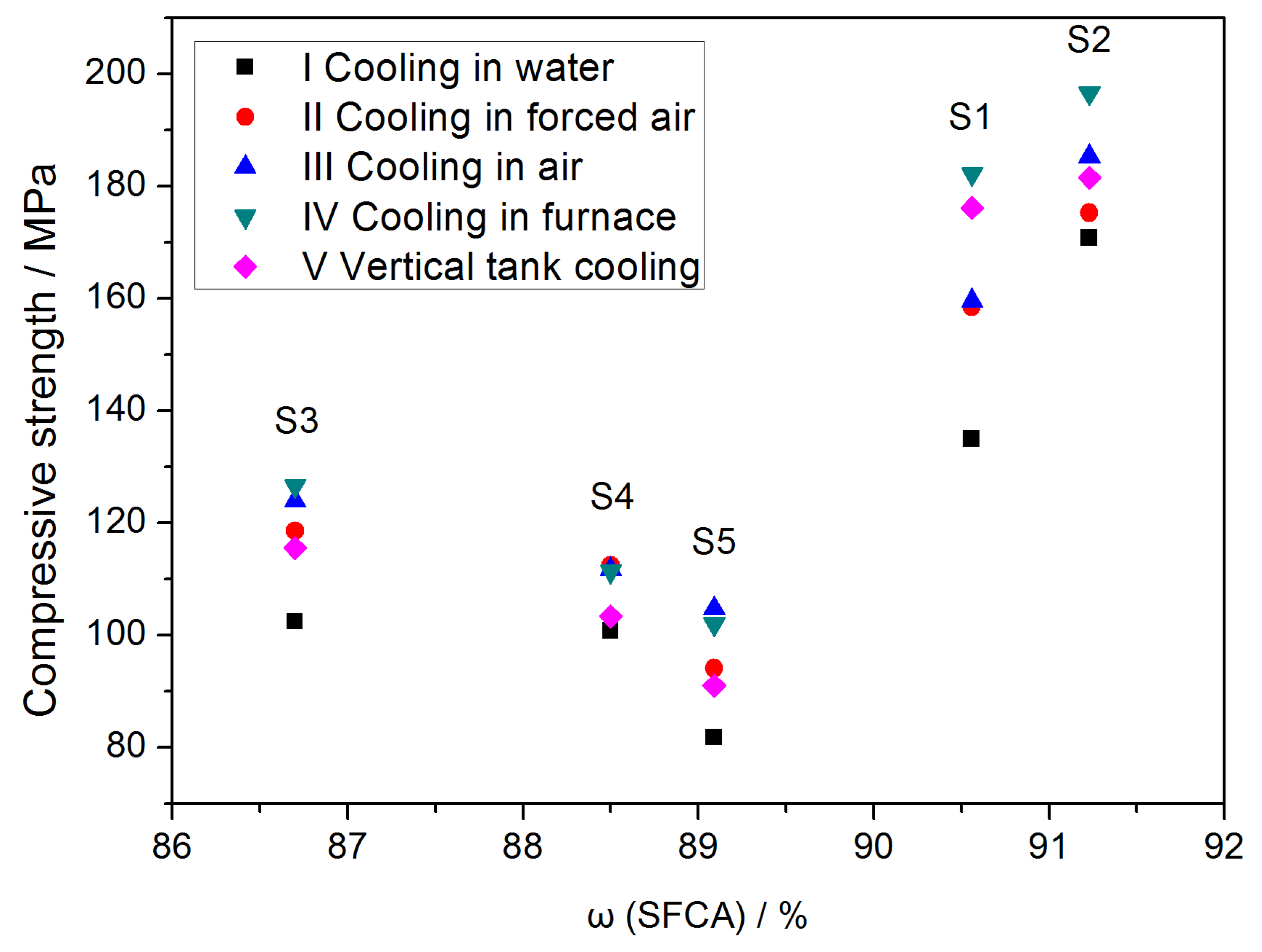
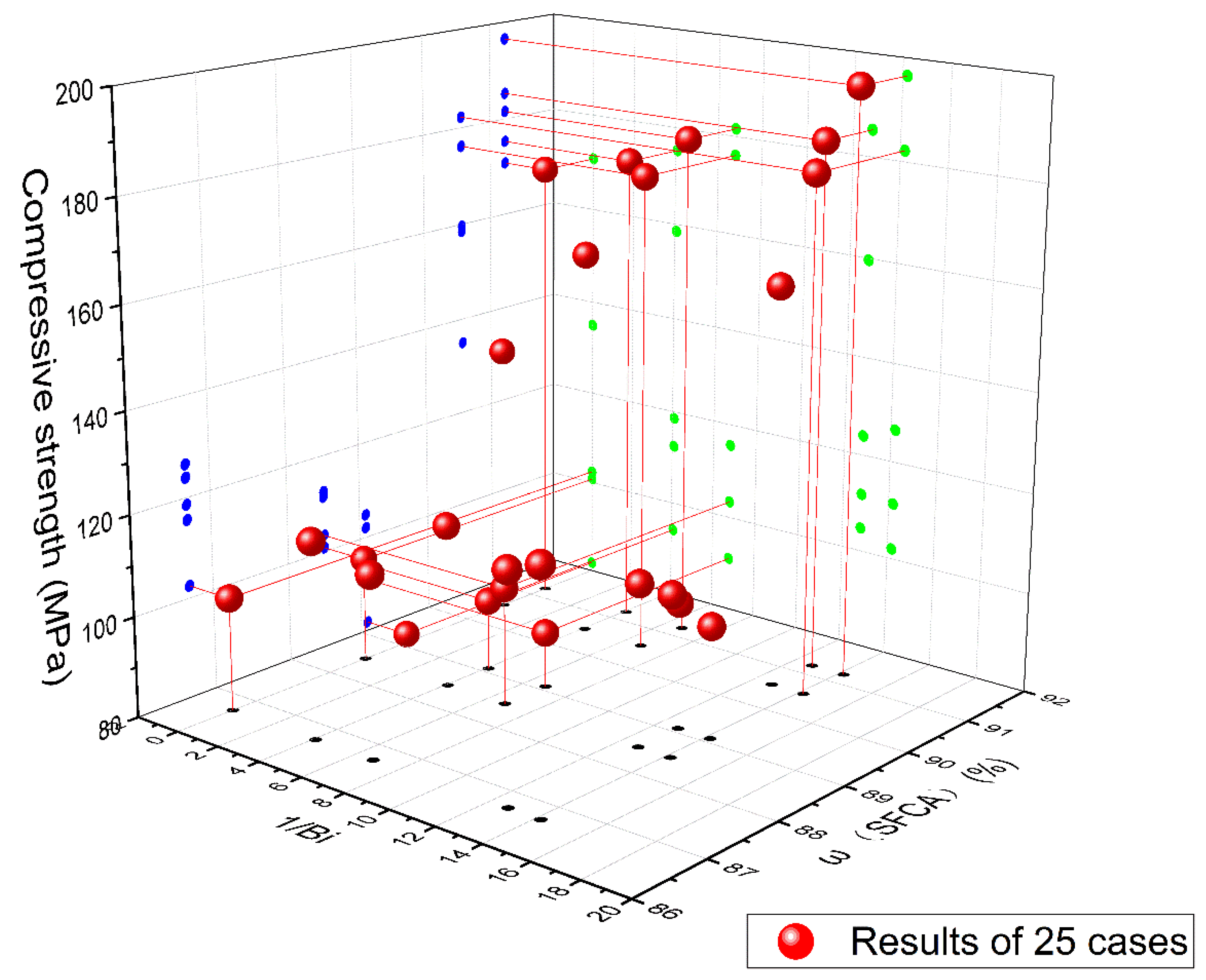
3.6. Effect of SFCA Content and Cooling Method on Strength of Iron Ore Sinter during Cooling Process
4. Conclusions
- An obvious negative correlation between the SFCA content and CRT average suggests that the SFCA in the bonding phase significantly influences the mechanical strength of the iron ore sinter.
- For a specific cooling method, the compressive strength of the SFCA samples increased with the SFCA content, suggesting that the SFCA phase has a positive effect on the mechanical strength of the iron ore sinter during the cooling process.
- The results of a compressive strength test suggested that slow cooling can prevent the generation of thermal stress which leads to a deterioration in the strength of the iron ore sinter.
- The mechanical strength of an iron ore sinter could be improved by decreasing the Biot number and increasing the SFCA content, which will guide the evaluation of mechanical strength of iron ore sinter after the cooling process in industry.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, S.; Liu, Y.; Du, J.; Mi, K.; Lin, H. New concept of iron ores sintering basic characteristics. J. Univ. Sci. Technol. Beijing 2002, 24, 254–257. [Google Scholar]
- Zhou, Q.; Kong, L. Agglomeration Theory and Process of Iron Ore; Metallurgical Industry Press: Beijing, China, 1989. [Google Scholar]
- Wu, S.; Du, J.; Ma, H.; Zhang, Z.; Chen, H. Self-intensity of binding phase in iron ores during sintering. J. Univ. Sci. Technol. Beijing 2005, 27, 169–172. [Google Scholar]
- Hancart, J.; Leroy, V.; Bragard, A. A study of the phases present in blast furnace sinter. Some considerations on the mechanism of their formation. CNRM Metall. Rep. 1967, 3–7. [Google Scholar]
- Kim, H.S.; Park, J.H.; Cho, Y.C. Crystal structure of calcium and aluminium silicoferrite in iron ore sinter. Ironmak. Steelmak. 2002, 29, 266–270. [Google Scholar] [CrossRef]
- Scarlett, N.V.Y.; Madsen, I.C.; Pownceby, M.I.; Christensen, A.N. In situ X-ray diffraction analysis of iron ore sinter phases. J. Appl. Crystallogr. 2004, 37, 362–368. [Google Scholar] [CrossRef]
- Scarlett, N.V.Y.; Pownceby, M.I.; Madsen, I.C.; Christensen, A.N. Reaction sequences in the formation of silico-ferrites of calcium and aluminum in iron ore sinter. Metall. Mater. Trans. B 2004, 35, 929–936. [Google Scholar] [CrossRef]
- Webster, N.A.S.; Pownceby, M.I.; Madsen, I.C.; Kimpton, J.A. Silico-ferrite of calcium and aluminum (sfca) iron ore sinter bonding phases: new insights into their formation during heating and cooling. Metall. Mater. Trans. B 2012, 43, 1344–1357. [Google Scholar] [CrossRef]
- Hessien, M.M.; Kashiwaya, Y.; Ishii, K.; Nasr, M.I.; EI-Geassy, A.A. Sintering and heating reduction processes of alumina containing iron ore samples. Ironmak. Steelmak. 2008, 35, 191–204. [Google Scholar] [CrossRef]
- Jeon, J.W.; Kim, S.W.; Jung, S.M. Utilization of magnetite concentrate as an additive in adhering fines of quasi-particle and its effect on assimilation behavior. ISIJ Int. 2015, 55, 513–520. [Google Scholar] [CrossRef]
- Zhang, G.L.; Wu, S.L.; Su, B.; Que, Z.G.; Hou, C.G.; Jiang, Y. Influencing factor of sinter body strength and its effects on iron ore sintering indexes. Int. J. Miner. Metall. Mater. 2015, 22, 553–561. [Google Scholar] [CrossRef]
- Liu, D.H.; Liu, H.; Zhang, J.L.; Liu, Z.J.; Xue, X.; Wang, G.W.; Kang, Q.F. Basic characteristics of Australian iron ore concentrate and its effects on sinter properties during the high-limonite sintering process. Int. J. Miner. Metall. Mater. 2017, 24, 991–998. [Google Scholar] [CrossRef] [Green Version]
- Wei, R.R.; Lv, X.W.; Yang, M.R.; You, Z.X. Solidification of calcium ferrite melt using ultrasonic vibration: effect and mechanism. Metall. Mater. Trans. B 2018, 49, 2658–2666. [Google Scholar] [CrossRef]
- Tang, W.D.; Xue, X.X.; Yang, S.T.; Zhang, L.H.; Huang, Z. Influence of basicity and temperature on bonding phase strength, microstructure, and mineralogy of high-chromium vanadium–titanium magnetite. Int. J. Miner. Metall. Mater. 2018, 25, 871–880. [Google Scholar] [CrossRef]
- Xue, M.S.; Guo, X.M. Effect of Al2O3 and SiO2 on formation and crystal structure of calcium ferrite containing Al2O3 and SiO2. J. Chin. Rare Earth Soc. 2008, 26, 205–209. [Google Scholar]
- Chung, F.H. Quantitative interpretation of X-ray diffraction patterns of mixtures. I. Matrix-flushing method for quantitative multicomponent analysis. J. Appl. Crystallogr. 1974, 7, 519–525. [Google Scholar] [CrossRef] [Green Version]
- The XRD Pattern Processing Software Jade 6 for Identification and Quantification. Available online: http://www.materialsdata.com (accessed on 28 November 2018).
- Wang, J.F.; Wang, Z.; Guo, L.L. Measurement and Analysis of Equivalent Thermal Conductivity of Sinter. Chin. J. Proc. Eng. 2017, 17, 879–882. [Google Scholar]
- Incropera, F.P.; Lavine, A.S.; Bergman, T.L. Fundamentals of Heat and Mass Transfer, 6th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]





| Sample | Composition/g | n(Al2O3)/n(Fe2O3) | |||
|---|---|---|---|---|---|
| CF | Fe2O3 | Al2O3 | SiO2 | ||
| S1 | 5.400 | 2.688 | 0.326 | 0.600 | 0.190 |
| S2 | 5.400 | 2.464 | 0.469 | 0.600 | 0.299 |
| S3 | 5.400 | 2.240 | 0.612 | 0.600 | 0.429 |
| S4 | 5.400 | 1.920 | 0.816 | 0.600 | 0.667 |
| S5 | 5.400 | 1.600 | 1.020 | 0.600 | 1.000 |
| No. | Cooling Method | Cooling Process |
|---|---|---|
| I | Cooling in water | 800 °C→water |
| II | Cooling in forced air | 800 °C→forced air in 30 m3/h |
| III | Cooling in air | 800 °C→air |
| IV | Cooling in the furnace | 800 °C→in the furnace |
| V | Vertical tank cooling | 800 °C→in tank to 600 °C→ forced air in 10 m3/h to 150 °C→in air |
| Sample | S1 | S2 | S3 | S4 | S5 |
|---|---|---|---|---|---|
| ω (SFCA)/% | 91.11 | 91.58 | 87.35 | 89.10 | 89.56 |
| 89.98 | 90.88 | 86.65 | 87.90 | 88.62 | |
| Average | 90.56 | 91.23 | 86.70 | 88.50 | 89.09 |
| Element | Weight Percent/% | Atom Percent/% | ||||
|---|---|---|---|---|---|---|
| Point a | Point b | Point c | Point a | Point b | Point c | |
| O | 38.93 | 38.93 | 23.92 | 54.56 | 53.29 | 49.23 |
| Al | 44.73 | 0.95 | 4.35 | 37.17 | 0.77 | 5.31 |
| Si | 0.39 | 57.34 | 1.16 | 0.31 | 44.72 | 1.36 |
| Ca | 9.80 | 0.86 | 10.73 | 5.48 | 0.47 | 8.82 |
| Fe | 6.15 | 1.93 | 59.83 | 2.47 | 0.76 | 35.28 |
| Cooling Method | Type of Convection | Cw,l | C | n | α/W/(m2 · °C) | Biot Number | |
|---|---|---|---|---|---|---|---|
| I | stable film boiling | - | - | - | 1973.10 | 11.36 | Average 231.30 |
| transition boiling | - | - | - | 23965.83 | 138.02 | ||
| bubble boiling | 0.0128 | - | - | 133224.6 | 767.26 | ||
| natural convection | - | 0.125 | 0.333 | 1490 | 8.57 | ||
| II | forced convection | - | - | - | 42.519 | 0.245 | |
| III | natural convection | - | 0.480 | 0.250 | 13.600 | 0.0781 | |
| IV | natural convection | - | 0.850 | 0.188 | 12.200 | 0.0705 | |
| V | forced convection | - | - | - | 25.548 | 0.147 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Bai, H.; Lu, X.; He, T.; Zhang, J.; Yuan, H.; Zhang, Z. Effect of Cooling Methods on the Strength of Silico-ferrite of Calcium and Aluminum of Iron Ore Sinter during the Cooling Process. Metals 2019, 9, 402. https://doi.org/10.3390/met9040402
Zhang X, Bai H, Lu X, He T, Zhang J, Yuan H, Zhang Z. Effect of Cooling Methods on the Strength of Silico-ferrite of Calcium and Aluminum of Iron Ore Sinter during the Cooling Process. Metals. 2019; 9(4):402. https://doi.org/10.3390/met9040402
Chicago/Turabian StyleZhang, Xu, Hao Bai, Xin Lu, Tian He, Jian Zhang, Huanmei Yuan, and Zefei Zhang. 2019. "Effect of Cooling Methods on the Strength of Silico-ferrite of Calcium and Aluminum of Iron Ore Sinter during the Cooling Process" Metals 9, no. 4: 402. https://doi.org/10.3390/met9040402




