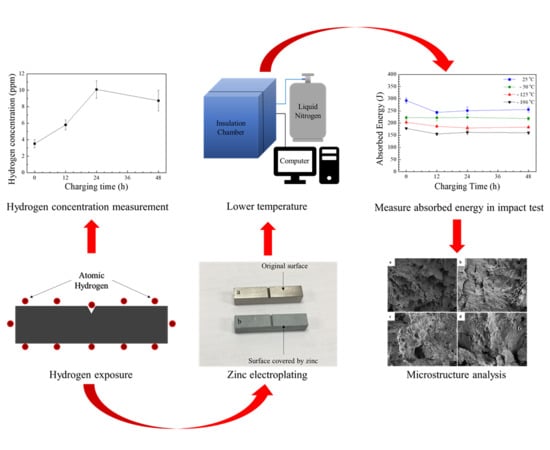
Charpy Impact Properties of Hydrogen-Exposed 316L Stainless Steel at Ambient and Cryogenic Temperatures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Specimens
2.3. Cathodic Hydrogen Charging and Zn Electroplating
2.4. Hydrogen Concentration Measurement
2.5. CVN Impact Test
2.6. Scanning Electron Microscopy (SEM)
3. Results and Discussion
3.1. Hydrogen Concentration in the Specimens
3.2. CVN Impact Absorbed Energy
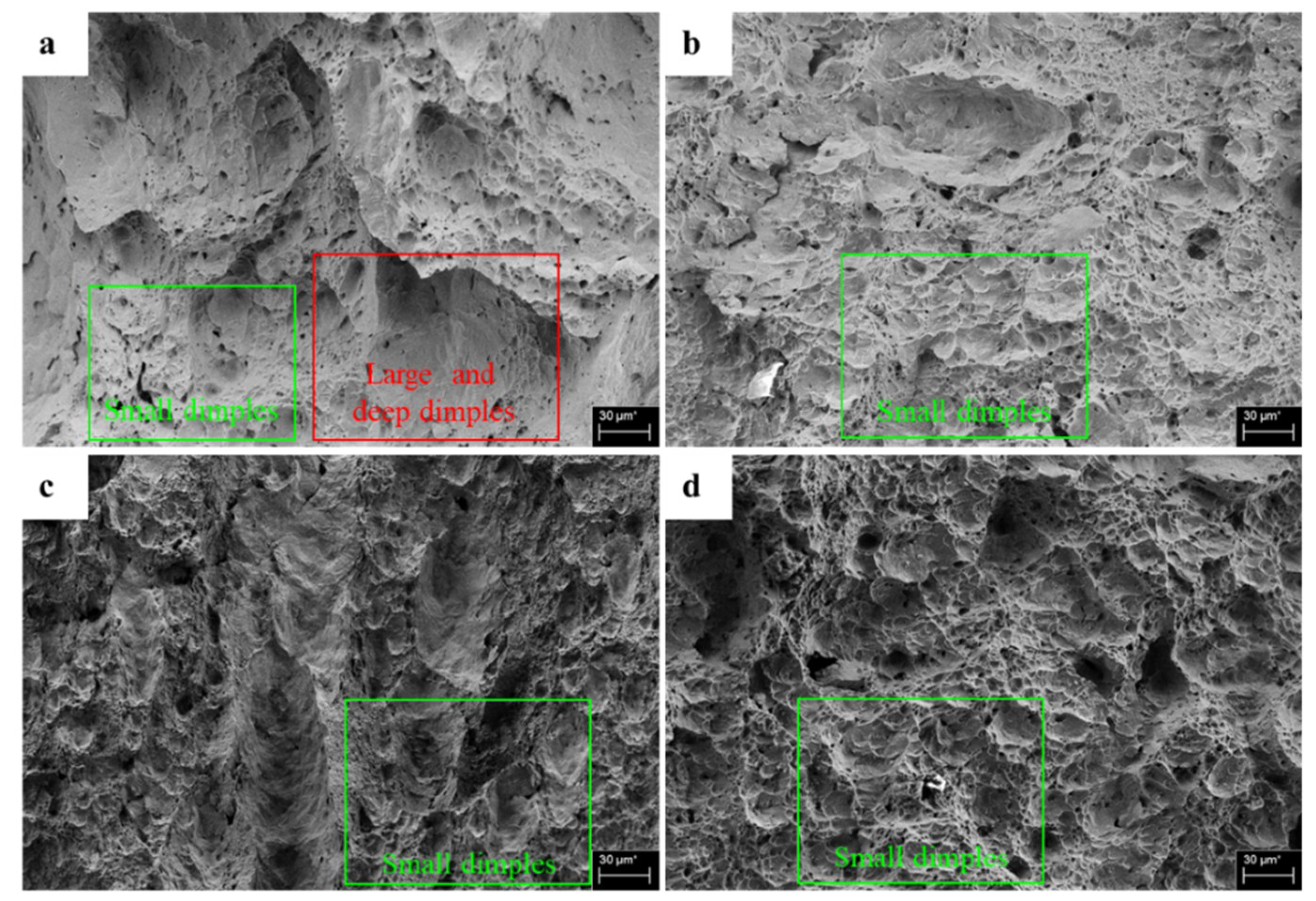
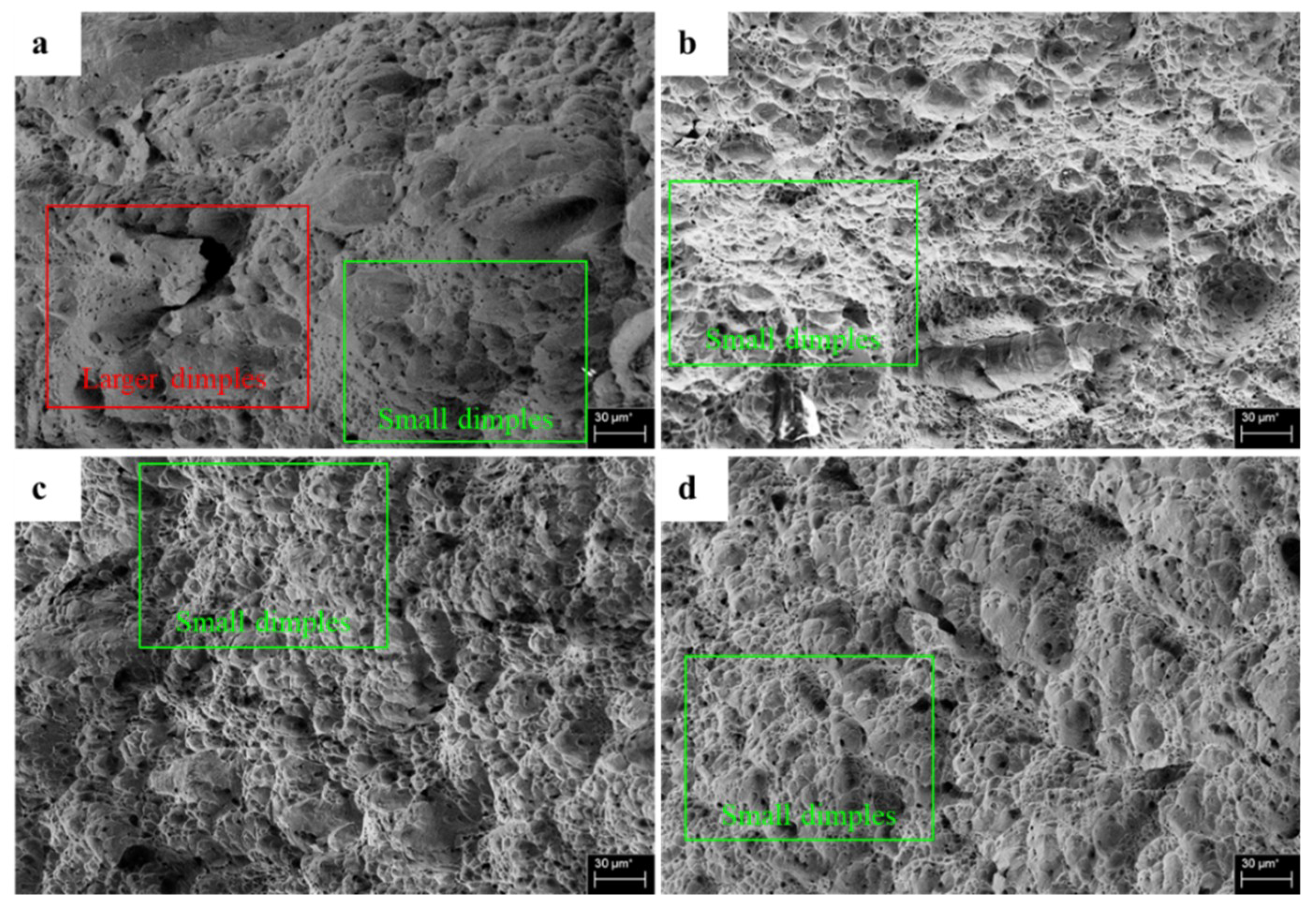
3.3. Fracture Surface Morphology
4. Conclusions
- Exposure to hydrogen increased the hydrogen concentration of the samples collected at the specimen surface. After 24 h of charging, the hydrogen concentration in the charged specimens reached a saturation point.
- Hydrogen charging resulted in a slight reduction in the absorbed energy and ductility of 316L stainless steel at most of the tested specimens. The drop in absorbed energy varied from 0.23% to 16.6%.
- The surface morphology of the uncharged specimens was more ductile than that of the pre-charged specimens impacted at ambient temperature, −125 °C and −196 °C. While the differences for specimens impacted at −50 °C were negligible.
- Another academic insight obtained herein is that low temperature decreased the ductility of the V-notched specimens in the CVN impact test. The loss of ductility caused by the ductile to brittle transformation was attributed to the lowering of the temperature in the CVN impact tests.
Author Contributions
Funding
Conflicts of Interest
References
- Marine Environment Protection Committee (MEPC), 72nd session, 9–13 April 2018. Available online: http://www.imo.org/en/MediaCentre/MeetingSummaries/MEPC/Pages/MEPC-72nd-session.aspx (accessed on 14 April 2019).
- Dwivedi, S.K.; Vishwakarma, M. Hydrogen embrittlement in different materials: A review. Int. J. Hydrogen Energy 2018, 43, 21603–21616. [Google Scholar] [CrossRef]
- Michler, T.; Lee, Y.; Gangloff, R.P.; Naumann, J. Influence of macro segregation on hydrogen environment embrittlement of SUS 316L stainless steel. Int. J. Hydrogen Energy 2009, 34, 3201–3209. [Google Scholar] [CrossRef]
- Czarkowski, P.; Krawczynska, A.T.; Slesinski, R.; Brynk, T.; Budniak, J.; Lewandowska, M.; Kurzydlowski, K.J. Low temperature mechanical properties of 316L type stainless steel after hydrostatic extrusion. Fusion Eng. Des. 2011, 86, 2517–2521. [Google Scholar] [CrossRef]
- Nagumo, M. Fundamentals of Hydrogen Embrittlement; Springer: Berlin, Germany, 2016; ISBN 9789811001611. [Google Scholar]
- Fukuyama, S.; Zhang, L.; Yokogawa, K. Development of Materials Testing Equipment in High Pressure Hydrogen and Hydrogen Environment Embrittlement of Austenitic Stainless Steels. Jpn. Inst. Met. 2004, 68, 62–65. [Google Scholar] [CrossRef] [Green Version]
- Kanezaki, T.; Narazaki, C.; Mine, Y.; Matsuoka, S.; Murakami, Y. Effects of hydrogen on fatigue crack growth behavior of austenitic stainless steels. Int. J. Hydrogen Energy 2008, 33, 2604–2619. [Google Scholar] [CrossRef]
- Weber, S.; Martin, M.; Theisen, W. Lean-alloyed austenitic stainless steel with high resistance against hydrogen environment embrittlement. Mater. Sci. Eng. A 2011, 528, 7688–7695. [Google Scholar] [CrossRef]
- Sugiyama, S.; Ohkubo, H.; Takenaka, M.; Ohsawa, K.; Ansari, M.I.; Tsukuda, N.; Kuramoto, E. The effect of electrical hydrogen charging on the strength of 316 stainless steel. J. Nucl. Mater. 2000, 283–287, 863–867. [Google Scholar] [CrossRef]
- Rozenak, P.; Eliezer, D. Phase changes related to hydrogen-induced cracking in austenitic stainless steel. Acta Metall. 1987, 35, 2329–2340. [Google Scholar] [CrossRef]
- Rozenak, P.; Robertson, I.M.; Birnbaum, H.K. HVEM studies of the effects of hydrogen on the deformation and fracture of AISI type 316 austenitic stainless steel. Acta Metall. Mater. 1990, 38, 2031–2040. [Google Scholar] [CrossRef]
- Garber, R.; Bernstein, I.M.; Thompson, A.W. Effect of hydrogen on ductile fracture of spheroidized steel. Scr. Metall. 1976, 10, 341–345. [Google Scholar] [CrossRef]
- Venezuela, J.; Tapia-Bastidas, C.; Zhou, Q.; Depover, T.; Verbeken, K.; Gray, E.; Liu, Q.; Liu, Q.; Zhang, M.; Atrens, A. Determination of the equivalent hydrogen fugacity during electrochemical charging of 3.5NiCrMoV steel. Corros. Sci. 2018, 132, 90–106. [Google Scholar] [CrossRef] [Green Version]
- Matsuo, T.; Yamabe, J.; Matsuoka, S. Effects of hydrogen on tensile properties and fracture surface morphologies of Type 316L stainless steel. Int. J. Hydrogen Energy 2014, 39, 3542–3551. [Google Scholar] [CrossRef]
- Yamabe, J.; Takakuwa, O.; Matsunaga, H.; Itoga, H.; Matsuoka, S. Hydrogen diffusivity and tensile-ductility loss of solution-treated austenitic stainless steels with external and internal hydrogen. Int. J. Hydrogen Energy 2017, 42, 13289–13299. [Google Scholar] [CrossRef]
- Momotani, Y.; Shibata, A.; Terada, D.; Tsuji, N. Effect of strain rate on hydrogen embrittlement in low-carbon martensitic steel. Int. J. Hydrogen Energy 2017, 42, 3371–3379. [Google Scholar] [CrossRef]
- ASTM Standard Test Methods for Notched Bar Impact Testing of Metallic Materials; ASTM International: West Conshohocken, PA, USA, 2016.
- Rossoll, A.; Berdin, C.; Forget, P.; Prioul, C.; Marini, B. Mechanical aspects of the Charpy impact test. Nucl. Eng. Des. 1999, 188, 217–229. [Google Scholar] [CrossRef] [Green Version]
- Fassina, P.; Bolzoni, F.; Fumagalli, G.; Lazzari, L.; Vergani, L.; Sciuccati, A. Influence of hydrogen and low temperature on pipeline steels mechanical behaviour. Procedia Eng. 2011, 10, 3226–3234. [Google Scholar] [CrossRef]
- Mori, K.; Lee, E.W.; Frazier, W.E.; Niji, K.; Battel, G.; Tran, A.; Iriarte, E.; Perez, O.; Ruiz, H.; Choi, T.; et al. Effect of Tempering and Baking on the Charpy Impact Energy of Hydrogen-Charged 4340 Steel. J. Mater. Eng. Perform. 2015, 24, 329–337. [Google Scholar] [CrossRef]
- Rosenberg, G.; Sinaiova, I. Evaluation of hydrogen induced damage of steels by different test methods. Mater. Sci. Eng. A 2017, 682, 410–422. [Google Scholar] [CrossRef]
- ISO 16573:2015. Steel—Measurement Method for the Evaluation of Hydrogen Embrittlement Resistance of High Strength Steels; International Organization for Standardization: Geneva, Switzerland, 2015. [Google Scholar]
- Jeon, H.H.; Lee, S.M.; Han, J.; Park, I.J.; Lee, Y.K. The effect of Zn coating layers on the hydrogen embrittlement of hot-dip galvanized twinning-induced plasticity steel. Corros. Sci. 2016, 111, 267–274. [Google Scholar] [CrossRef]
- Brass, A.M.; Chêne, J. Hydrogen uptake in 316L stainless steel: Consequences on the tensile properties. Corros. Sci. 2006, 48, 3222–3242. [Google Scholar] [CrossRef]
- Byun, T.S.; Lach, T.G. Mechanical Properties of 304L and 316L Austenitic Stainless Steels after Thermal Aging for 1500 Hours; U.S. Department of Energy/Office of Nuclear Energy: District of Columbia, WA, USA, 2016; pp. 1–19.
- Kim, J.H.; Choi, S.W.; Park, D.H.; Lee, J.M. Charpy impact properties of stainless steel weldment in liquefied natural gas pipelines: Effect of low temperatures. Mater. Des. 2015, 65, 914–922. [Google Scholar] [CrossRef]
- Djukic, M.B.; Zeravcic, V.S.; Bakic, G.; Sedmak, A.; Rajicic, B. Hydrogen Embrittlement of Low Carbon Structural Steel. Procedia Mater. Sci. 2014, 3, 1167–1172. [Google Scholar] [CrossRef] [Green Version]
- Godoi, W.; Kuromoto, N.K.; Guimaraes, A.S.; Lepienski, C.M. Effect of the hydrogen outgassing time on the hardness of austenitic stainless steels welds. Mater. Sci. Eng. A 2003, 354, 251–256. [Google Scholar] [CrossRef]











| Specimen | Chemical Elements (wt. %) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C | Si | S | P | Mn | Mo | Ni | Cr | Fe | |
| 316L | 0.020 | 0.56 | 0.003 | 0.028 | 1.33 | 2.1 | 10.19 | 16.4 | Bal. |
| No | Charging Current Density (A/m2) | Charging Duration (h) | Electroplating Current Density (A/m2) | Temperature of CVN Impact Test (°C) |
|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 25 |
| 2 | 20 | 12 | 300 | |
| 3 | 24 | |||
| 4 | 48 | |||
| 5 | 0 | 0 | 0 | −50 |
| 6 | 20 | 12 | 300 | |
| 7 | 24 | |||
| 8 | 48 | |||
| 9 | 0 | 0 | 0 | −125 |
| 10 | 20 | 12 | 300 | |
| 11 | 24 | |||
| 12 | 48 | |||
| 13 | 0 | 0 | 0 | −196 |
| 14 | 20 | 12 | 300 | |
| 15 | 24 | |||
| 16 | 48 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, L.T.H.; Hwang, J.-S.; Kim, M.-S.; Kim, J.-H.; Kim, S.-K.; Lee, J.-M. Charpy Impact Properties of Hydrogen-Exposed 316L Stainless Steel at Ambient and Cryogenic Temperatures. Metals 2019, 9, 625. https://doi.org/10.3390/met9060625
Nguyen LTH, Hwang J-S, Kim M-S, Kim J-H, Kim S-K, Lee J-M. Charpy Impact Properties of Hydrogen-Exposed 316L Stainless Steel at Ambient and Cryogenic Temperatures. Metals. 2019; 9(6):625. https://doi.org/10.3390/met9060625
Chicago/Turabian StyleNguyen, Le Thanh Hung, Jae-Sik Hwang, Myung-Sung Kim, Jeong-Hyeon Kim, Seul-Kee Kim, and Jae-Myung Lee. 2019. "Charpy Impact Properties of Hydrogen-Exposed 316L Stainless Steel at Ambient and Cryogenic Temperatures" Metals 9, no. 6: 625. https://doi.org/10.3390/met9060625