Influence of SiO2 on the Compressive Strength and Reduction-Melting of Pellets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Experiment Process
3. Results
3.1. Effect of SiO2 on the Characteristics of Pellets
3.1.1. Compressive Strength
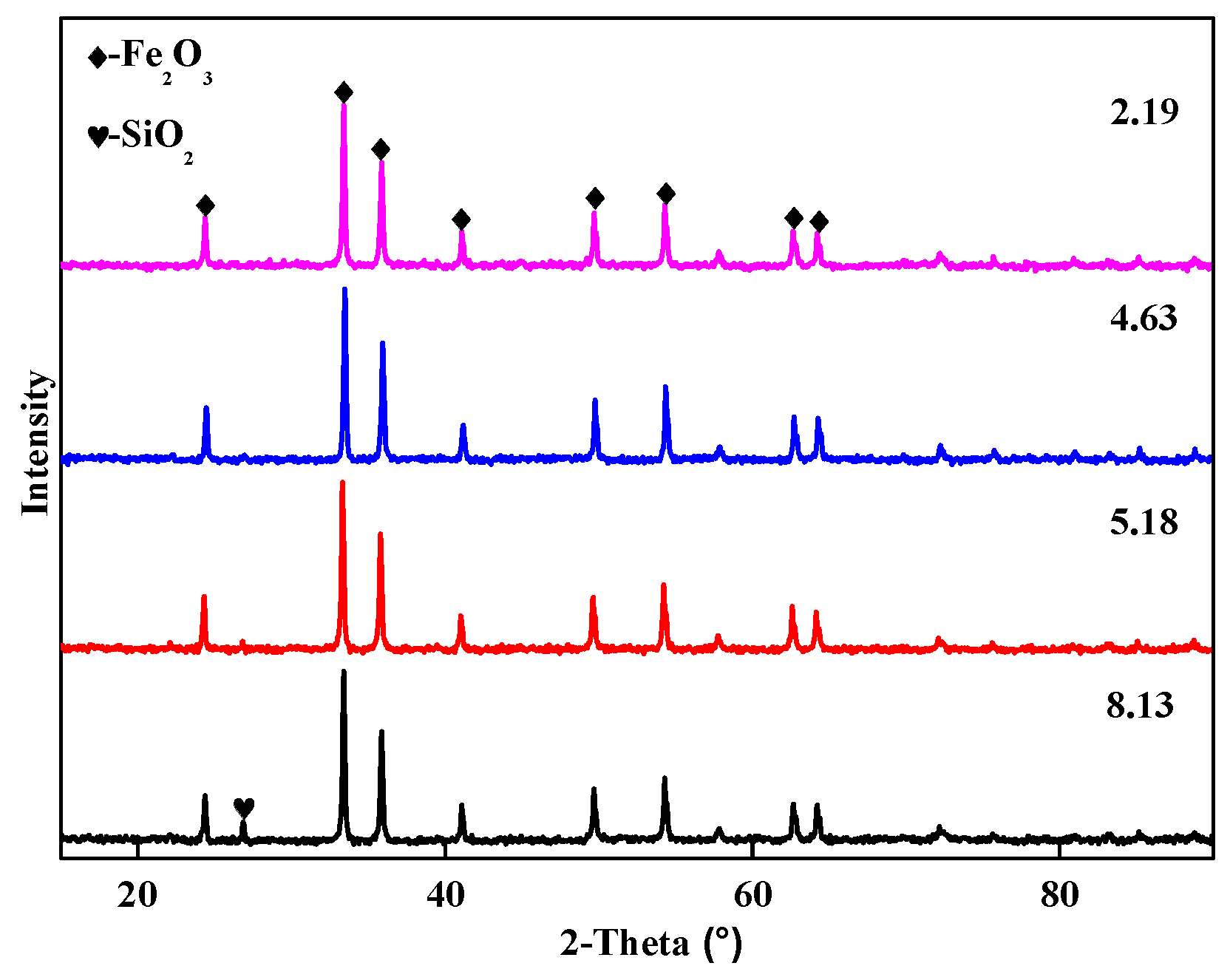
3.1.2. Phase Composition
3.1.3. Morphology Change
3.2. Effect of SiO2 on the Reduction Behavior of Pellets
3.2.1. Reduction Degree
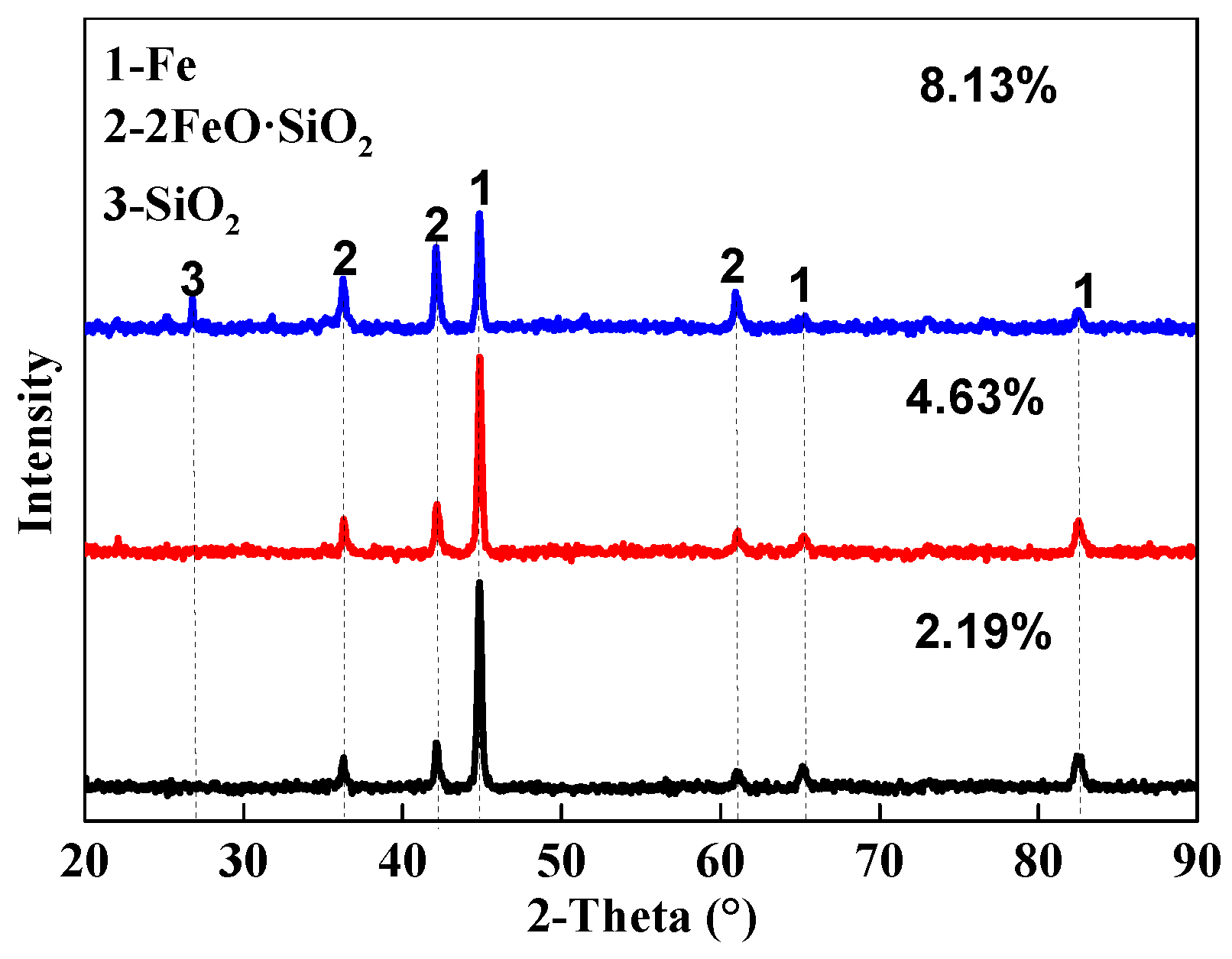
3.2.2. Phase Compositions
3.2.3. Morphology Change
3.3. Effect of SiO2 and Burden Composition on Melting Properties
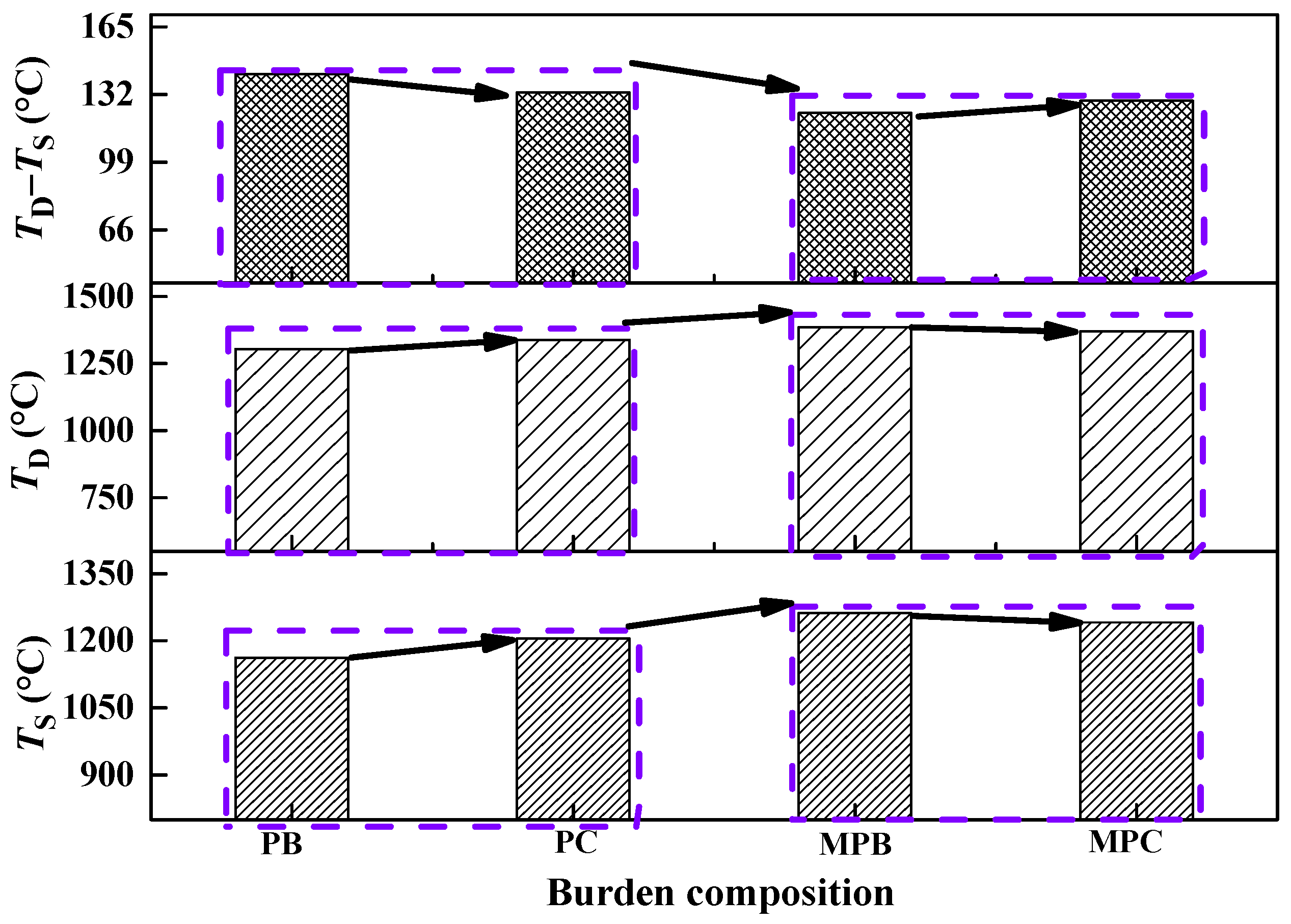
3.3.1. Melting-Dripping Properties
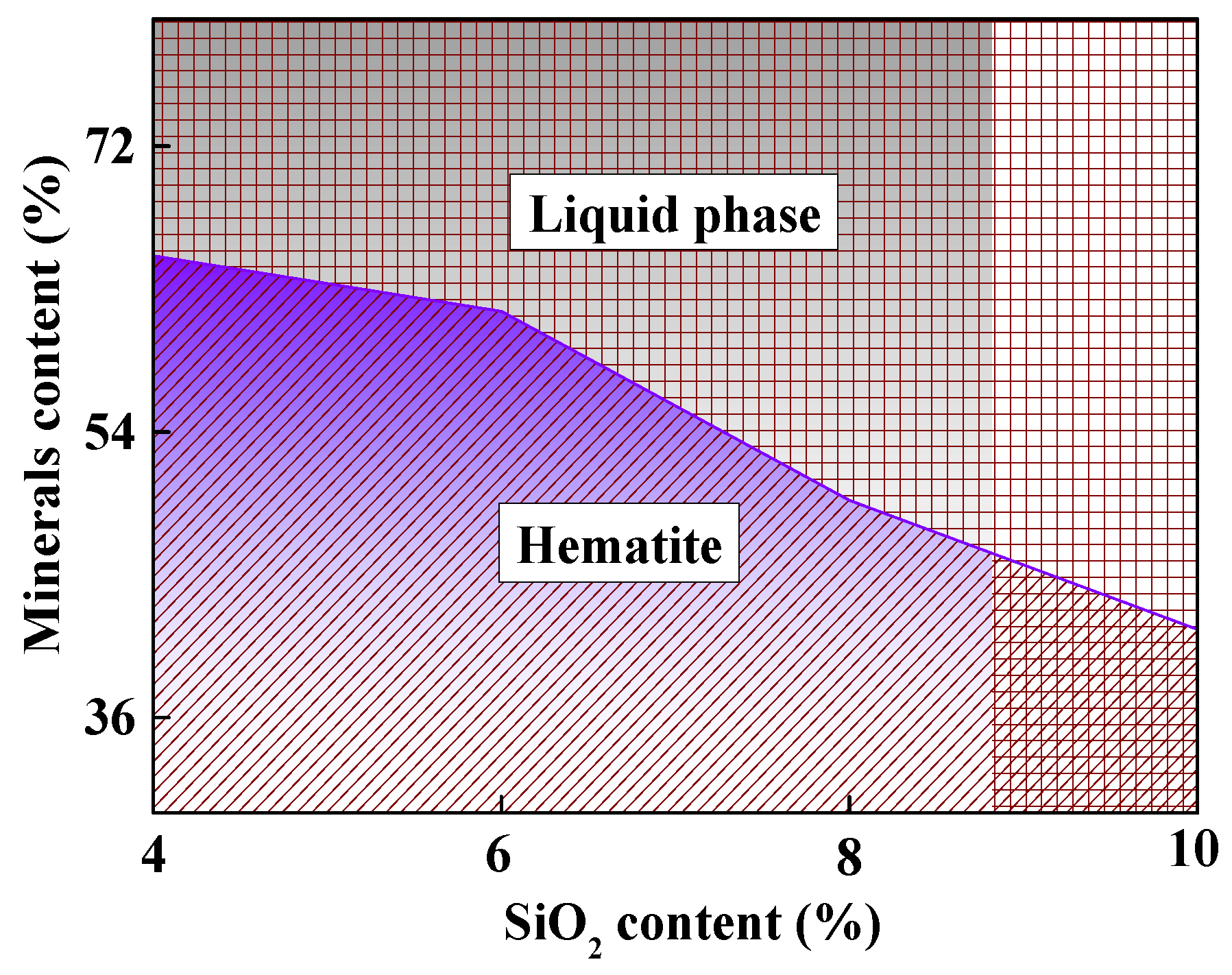
3.3.2. The Permeability of Different Burden Compositions
4. Discussion
4.1. Influence Mechanism of SiO2 on Compressive Strength of Pellets
4.2. Effect of Burden Compositions on Melting Start Temperature
4.3. Dripping Behavior of Different Burden Composition
5. Conclusions
- (1)
- When the SiO2 content in pellets increased from 2.19% to 8.13%, the compressive strength decreased from 3.39 kN to 2.20 kN, because more silicate melts formed by the reaction between SiO2 and wustite. These silicate melts inhibited the contact between the solid Fe2O3 particles and resulted in the poor recrystallization of Fe2O3 in pellets.
- (2)
- The reduction degree decreased with increasing SiO2 contents in pellets. When the SiO2 content in pellets increased from 2.19% to 8.13%, the reduction degree decreased from 75.22% to 46.19%. The main reasons were as follows: First, FeO content in pellets increased during the reduction process; Then, the FeO reacted with SiO2 to generate 2FeO·SiO2 (a substance with lower melting point and worse reductivity) and a little of liquid phase. Finally, the liquid phase inhibited the diffusion of reducing gas towards the inside of pellets.
- (3)
- Increasing the SiO2 content had negative effects on the melting-dripping properties of pellets. The melting-dripping properties can be improved by adding some sinter with high basicity in the mixed burden, because more akermanite (higher melting point) formed in the mixed burden during the reduction and melting process.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Friel, J.J.; Erickson, E.S. Chemistry, Microstructure, and Reduction Characteristics of Dolomite-Fluxed Magnetite Pellets. Metall. Mater. Trans. B 1980, 11, 233–243. [Google Scholar] [CrossRef]
- Pal, J.; Ghoral, S.; Agarwal, S.; Nandi, B.; Chakraborty, T.; Das, G.; Prakash, S. Effect of Blaine Fineness on the Quality of Hematite Iron Ore Pellets for Blast Furnace. Miner. Process Extr. Metall. Rev. 2014, 36, 83–91. [Google Scholar] [CrossRef]
- Gao, Q.J.; Shen, F.M.; Wei, G.; Jiang, X.; Zheng, H.Y. Effects of MgO Containing Additive on Low-Temperature Metallurgical Properties of Oxidized Pellet. J. Iron Steel Res. Int. 2013, 20, 25–28. [Google Scholar] [CrossRef]
- Tang, J.; Chu, M.S.; Xue, X.X. Optimized use of MgO flux in the agglomeration of high-chromium vanadium-titanium magnetite. Int. J. Miner. Metall. Mater. 2015, 22, 371–380. [Google Scholar] [CrossRef]
- Gao, Z.X.; Cheng, G.J.; Xue, X.X.; Yang, H.; Duan, P.N. Property Investigations of Low-Grade Vanadium-Titanium Magnetite Pellets with Different MgO Contents. Steel Res. Int. 2018, 89, 1700543. [Google Scholar] [CrossRef]
- Long, H.M.; Li, J.X.; Wang, P.; Shi, S.Q. Reduction kinetics of carbon containing pellets made from metallurgical dust. Ironmak. Steelmak. 2012, 39, 585–592. [Google Scholar] [CrossRef]
- Guo, Y.H.; Xie, J.; Gao, J.J.; Xu, H.J.; Qie, J.M. Study on the Production and Metallurgical Properties of Fluxed Pellets with High Hematite Content. Metallurgist 2017, 61, 638–645. [Google Scholar]
- Iljana, M.; Kemppainen, A.; Paananen, T.; Mattila, O.; Pisila, E.; Kondrakov, M.; Fabritius, T. Effect of adding limestone on the metallurgical properties of iron ore pellets. Int. J. Miner. Process. 2015, 141, 34–43. [Google Scholar] [CrossRef]
- Umadevi, T.; Kumar, P.; Lobo, N.F.; Prabhu, M.; Mahapatra, P.C.; Ranjan, M. Influence of Pellet Basicity (CaO/SiO2) on Iron Ore Pellet Properties and Microstructure. ISIJ Int. 2011, 51, 14–20. [Google Scholar] [CrossRef]
- Qing, G.; Wu, K.; Tian, Y.; An, G.; Yuan, X.; Xu, D.; Huang, W. Effect of the firing temperature and the added MgO on the reduction swelling index of the pellet with low SiO2 content. Ironmak. Steelmak. 2018, 45, 83–89. [Google Scholar] [CrossRef]
- Wang, H.T.; Sohn, H.Y. Effects of Reducing Gas on Swelling and Iron Whisker Formation during the Reduction of Iron Oxide Compact. Steel Res. Int. 2012, 83, 903–909. [Google Scholar] [CrossRef]
- Umadevi, T.; Kumar, A.; Karthik, P.; Srinidhi, R.; Manjini, S. Characterisation studies on swelling behaviour of iron ore pellets. Ironmak. Steelmak. 2018, 45, 157–165. [Google Scholar] [CrossRef]
- Dwarapudi, S.; Ghosh, T.K.; Shankar, A.; Tathavadkar, V.; Bhattacharjee, D.; Venugopal, R. Effect of pellet basicity and MgO content on the quality and microstructure of hematite pellets. Int. J. Miner. Process. 2011, 99, 43–53. [Google Scholar] [CrossRef]
- Dwarapudi, S.; Devi, T.U.; Rao, S.M.; Ranjan, M. Influence of pellet size on quality and microstructure of iron ore pellets. ISIJ Int. 2008, 48, 768–776. [Google Scholar] [CrossRef]
- Mohanty, M.K.; Mishra, S.; Mishra, B.; Sarkar, S. Effect of Basicity on the Reduction Behavior of Iron Ore Pellets. Arab. J. Sci. Eng. 2018, 43, 5989–5998. [Google Scholar] [CrossRef]
- Shen, F.M.; Gao, Q.J.; Jiang, X.; Wei, G.; Zheng, H.Y. Effect of magnesia on the compressive strength of pellets. Int. J. Miner. Metall. Mater. 2014, 21, 431–437. [Google Scholar] [CrossRef]
- Zhang, J.L.; Wang, Z.Y.; Xing, X.D.; Liu, Z.J. Effect of aluminum oxide on the compressive strength of pellets. Int. J. Miner. Metall. Mater. 2014, 21, 339–344. [Google Scholar] [CrossRef]
- Wang, S.; Guo, Y.F.; Yang, L.; Fu, G.H.; Zheng, F.Q.; Chen, F.; Yang, L.Z.; Jiang, T. Investigation of solidification mechanism of fluorine-bearing magnetite concentrate pellets. Powder Technol. 2018, 332, 188–196. [Google Scholar] [CrossRef]
- Guo, H.W.; Bai, J.L.; Zhang, J.L.; Li, H.G. Mechanism of Strength Improvement of Magnetite Pellet by Adding Boron-bearing Iron Concentrate. J. Iron Steel Res. Int. 2014, 21, 9–15. [Google Scholar] [CrossRef]
- Chen, J.W.; Chen, W.B.; Mi, L.; Jiao, Y.; Wang, X.D. Kinetic Studies on Gas-Based Reduction of Vanadium Titano-Magnetite Pellet. Metals 2019, 9, 95. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.S.; Xue, Q.G. Kinetics of the Volume Shrinkage of a Magnetite/Carbon Composite Pellet during Solid-State Carbothermic Reduction. Metals 2018, 8, 1050. [Google Scholar] [CrossRef]
- Gao, Q.J.; Jiang, X.; Zheng, H.Y.; Shen, F.M. Induration Process of MgO Flux Pellet. Minerals 2018, 8, 389. [Google Scholar] [CrossRef]













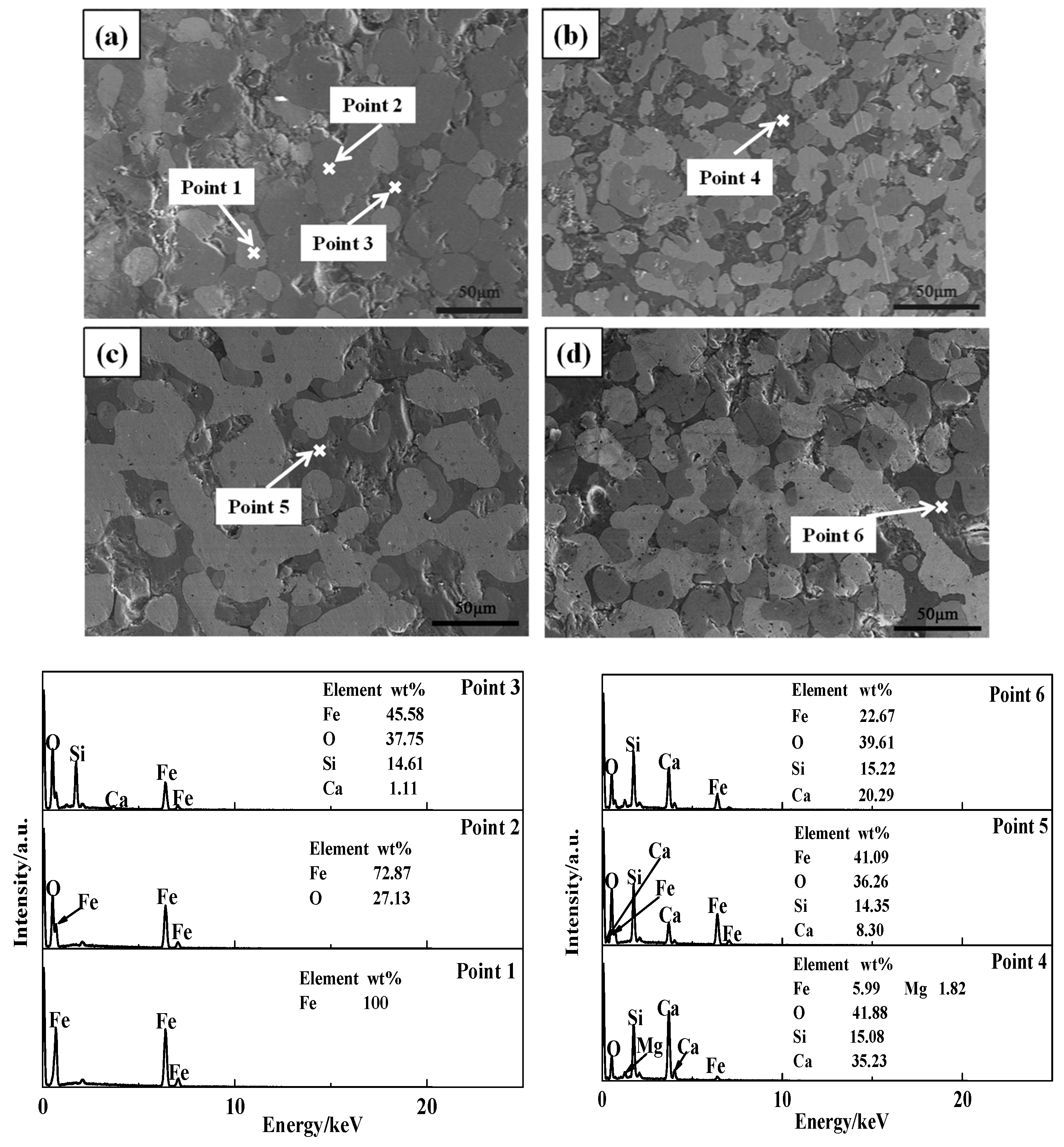


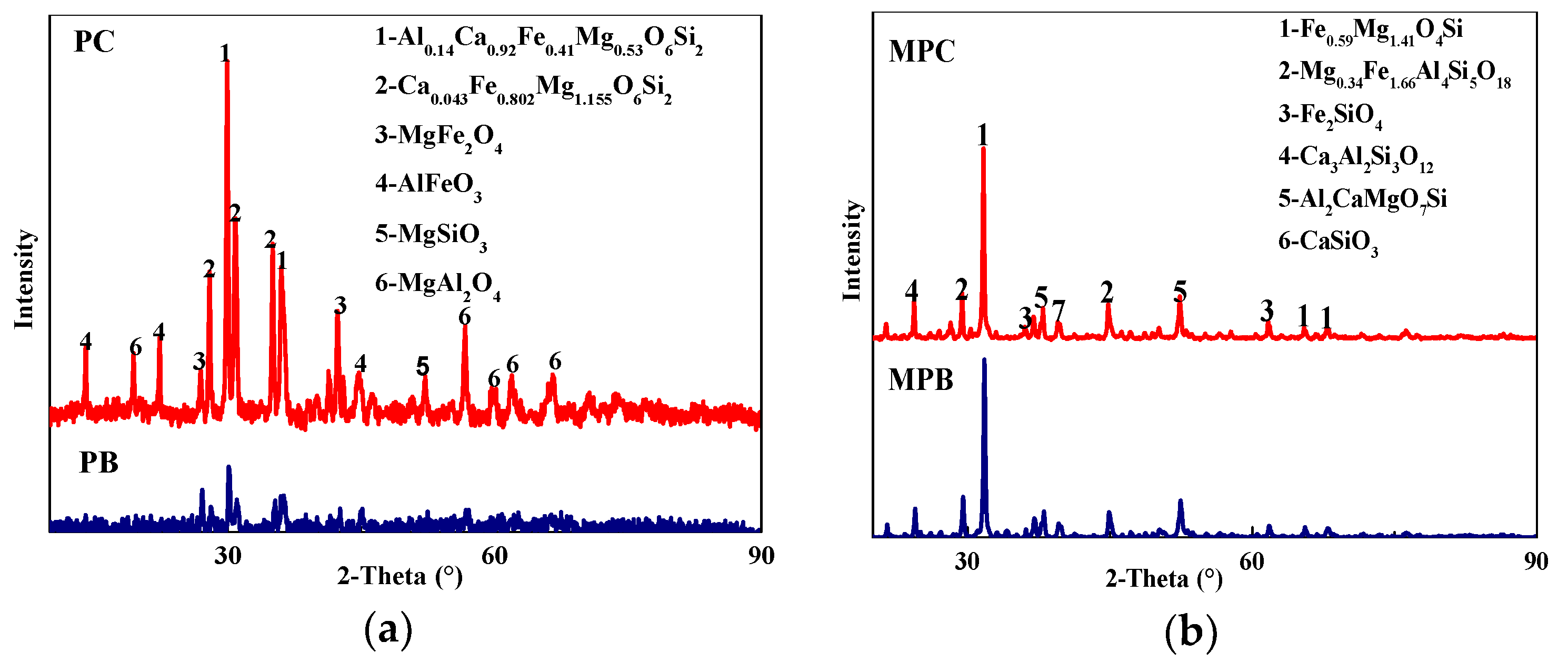
| Samples | TFe | FeO | SiO2 | CaO | MgO | Al2O3 | R (w(CaO)/w(SiO2)) |
|---|---|---|---|---|---|---|---|
| S | 57.74 | 8.55 | 5.02 | 9.65 | 1.79 | 1.94 | 1.92 |
| PA | 65.72 | 1.58 | 2.19 | 2.21 | 0.63 | 0.74 | 1.01 |
| PB | 65.73 | 0.83 | 4.63 | 0.66 | 0.34 | 0.34 | 0.14 |
| PC | 65.10 | 0.65 | 5.18 | 1.18 | 0.48 | 0.28 | 0.23 |
| PD | 63.81 | 0.00 | 8.13 | 0.31 | 0.49 | 0.43 | 0.04 |
| Sample | Pellets in Mixed Burden (%) | Sinters in Mixed Burden (%) |
|---|---|---|
| PB | 100 | 0 |
| PC | 100 | 0 |
| MPB | 37 | 63 |
| MPC | 37 | 63 |
| Sample | FeO | TFe | CaO | SiO2 | Al2O3 | MgO | MFe |
|---|---|---|---|---|---|---|---|
| PB | 57.54 | 75.88 | 1.00 | 6.33 | 0.27 | 0.50 | 23.24 |
| PC | 60.34 | 77.80 | 0.74 | 5.64 | 0.37 | 0.57 | 22.93 |
| MPB | 49.00 | 69.75 | 7.43 | 6.92 | 2.05 | 1.34 | 29.19 |
| MPC | 51.28 | 76.55 | 7.19 | 5.46 | 1.95 | 1.42 | 28.45 |
| Sample | TFe | FeO | MgO | SiO2 | CaO | Al2O3 |
|---|---|---|---|---|---|---|
| PB | 16.23 | 18.83 | 4.11 | 56.88 | 10.06 | 7.85 |
| PC | 22.33 | 20.11 | 3.36 | 55.36 | 8.01 | 4.24 |
| MPB | 13.66 | 17.67 | 8.23 | 29.08 | 32.38 | 11.04 |
| MPC | 11.40 | 14.09 | 6.58 | 32.15 | 33.02 | 14.03 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Jiang, X.; Shen, F.; Zheng, H.; Gao, Q.; Zhang, X. Influence of SiO2 on the Compressive Strength and Reduction-Melting of Pellets. Metals 2019, 9, 852. https://doi.org/10.3390/met9080852
Guo H, Jiang X, Shen F, Zheng H, Gao Q, Zhang X. Influence of SiO2 on the Compressive Strength and Reduction-Melting of Pellets. Metals. 2019; 9(8):852. https://doi.org/10.3390/met9080852
Chicago/Turabian StyleGuo, He, Xin Jiang, Fengman Shen, Haiyan Zheng, Qiangjian Gao, and Xin Zhang. 2019. "Influence of SiO2 on the Compressive Strength and Reduction-Melting of Pellets" Metals 9, no. 8: 852. https://doi.org/10.3390/met9080852





