Comparison of Mechanical and Antibacterial Properties of TiO2/Ag Ceramics and Ti6Al4V-TiO2/Ag Composite Materials Using Combined SLM-SPS Techniques
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Specimens Preparation
2.3. Mechanical and Microstructural Characterization
2.4. Antibacterial Assay
3. Results
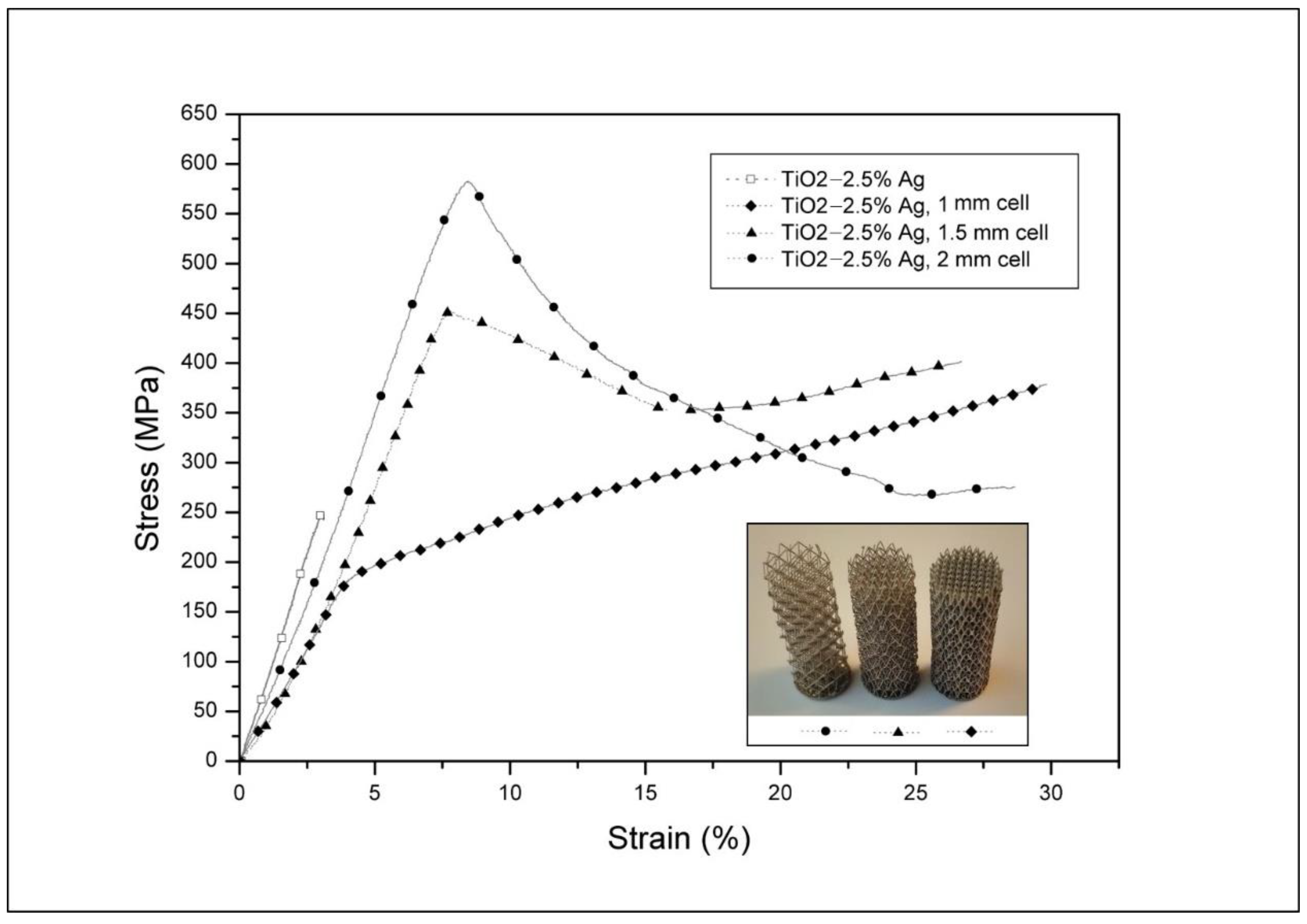
3.1. Structural Study and Mechanical Properties of the Composite Materials and Ceramics
3.2. Antibacterial Activity of the Surfaces
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- He, C.; Yu, Y.; Hu, X.; Larbot, A. Influence of silver doping on the photocatalytic activity of titania film. Appl. Surf. Sci. 2002, 200, 239–247. [Google Scholar] [CrossRef]
- Sung-Suh, H.M.; Choi, J.R.; Hah, H.J.; Koo, S.M.; Bae, Y.C. Comparison of Ag deposition effects on the photocatalytic activity of nanoparticulate TiO2 under visible and UV light irradiation. J. Photochem. Photobiol. Chem. 2004, 163, 37–44. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, H.; Huo, K.; Cui, L.; Zhang, W.; Ni, H.; Zhang, Y.; Wu, Z.; Chu, P.K. Antibacterial nano-structured titania coating incorporated with silver nanoparticles. Biomaterials 2011, 32, 5706–5716. [Google Scholar] [CrossRef] [PubMed]
- Oja Acik, I.; Junolainen, A.; Mikli, V.; Danilson, M.; Krunks, M. Growth of ultra-thin TiO2 films by spray pyrolysis on different substrates. Appl. Surf. Sci. 2009, 256, 1391–1394. [Google Scholar] [CrossRef]
- Kim, H.C.; Park, H.K.; Shon, I.J.; Ko, I.Y. Fabrication of ultra-fine TiO2 Ceramics by a high-frequency induction heated sintering method. J. Ceram. Process. Res. 2006, 7, 327. [Google Scholar]
- Matsuno, H.; Yokoyama, A.; Watari, F.; Uo, M.; Kawasaki, T. Biocompatibility and osteogenesis of refractory metal implants, titanium, hafnium, niobium, tantalum and rhenium. Biomaterials 2001, 22, 1253–1262. [Google Scholar] [CrossRef]
- Asa’ad, F.; Pagni, G.; Pilipchuk, S.P.; Giannì, A.B.; Giannobile, W.V.; Rasperini, G. 3D-Printed Scaffolds and Biomaterials: Review of Alveolar Bone Augmentation and Periodontal Regeneration Applications. Int. J. Dent. 2016, 2016, 1239842. [Google Scholar] [CrossRef]
- Vandenbroucke, B.; Kruth, J.P. Selective laser melting of biocompatible metals for rapid manufacturing of medical parts. Rapid Prototyp. J. 2007, 13, 196–203. [Google Scholar] [CrossRef]
- Könönen, M.; Kivilahti, J. Concise review biomaterials & bioengineering: Fusing of dental ceramics to titanium. J. Dent. Res. 2001, 80, 848–854. [Google Scholar]
- Zhang, C.; Chaudhary, U.; Lahiri, D.; Godavarty, A.; Agarwal, A. Photocatalytic activity of spark plasma sintered TiO2-graphene nanoplatelet composite. Scr. Mater. 2013, 68, 719–722. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, J.H.; Hong, S.H.; Kim, D.Y. Preparation of nanostructured TiO2 ceramics by spark plasma sintering. Mater. Res. Bull. 2003, 38, 925–930. [Google Scholar] [CrossRef]
- Noh, J.H.; Jung, H.S.; Lee, J.K.; Kim, J.R.; Hong, K.S. Microwave dielectric properties of nanocrystalline TiO2 prepared using spark plasma sintering. J. Eur. Ceram. Soc. 2007, 2, 2937–2940. [Google Scholar]
- Bernasconi, R.; Carrara, E.; Hoop, M.; Mushtaq, F.; Chen, X.; Nelson, B.J.; Pané, S.; Credi, C.; Levi, M.; Magagnin, L. Magnetically navigable 3D printed multifunctional microdevices for environmental applications. Addit. Manuf. 2019, 28, 127–135. [Google Scholar] [CrossRef]
- Visnapuu, M.; Rosenberg, M.; Truska, E.; Nommiste, E.; Sutka, A.; Kahru, A.; Rahn, M.; Vija, H.; Orupold, K.; Kisand, V.; et al. UVA-induced antimicrobial activity of ZnO/Ag nanocomposite covered surfaces. Colloids Surf. B Biointerfaces 2018, 169, 222–232. [Google Scholar] [PubMed]
- Rahmani, R.; Antonov, M.; Kamboj, N. Modelling of impact-abrasive wear of ceramic, metallic, and composite materials. Proc. Est. Acad. Sci. 2019, 68, 191–197. [Google Scholar]
- Rahmani, R.; Antonov, M.; Kollo, L. Wear Resistance of (Diamond-Ni)-Ti6Al4V Gradient Materials Prepared by Combined Selective Laser Melting and Spark Plasma Sintering Techniques. Adv. Tribol. 2019, 2019, 5415897. [Google Scholar] [CrossRef]
- Rahmani, R.; Antonov, M.; Kollo, L. Selective Laser Melting of Diamond-Containing or Postnitrided Materials Intended for Impact-Abrasive Conditions: Experimental and Analytical Study. Adv. Mater. Sci. Eng. 2019, 2019, 4210762. [Google Scholar] [CrossRef]
- Velasco, S.C.; Cavaleiro, A.; Carvalho, S. Functional properties of ceramic-Ag nanocomposite coatings produced by magnetron sputtering. Prog. Mater. Sci. 2016, 84, 158–191. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, J.H.; Tseng, C.C.; Chang, Y.K.; Chang, S.Y.; Wu, W. Antibacterial behavior of TaN-Ag nanocomposite thin films with and without annealing. Surf. Coat. Technol. 2008, 202, 5586–5589. [Google Scholar]
- Wickens, D.J.; West, G.; Kelly, P.J.; Verran, J.; Lynch, S.; Whitehead, K.A. Antimicrobial activity of nanocomposite zirconium nitride/silver coatings to combat external bone fixation pin infections. Int. J. Artif. Organs. 2012, 35, 817–825. [Google Scholar]
- Hsieh, J.H.; Chang, C.C.; Li, C.; Liu, S.J.; Chang, Y.K. Effects of Ag contents on antibacterial behaviors of TaON-Ag nanocomposite thin films. Surf. Coat. Technol. 2010, 205, 337–340. [Google Scholar]
- Huang, H.L.; Chang, Y.Y.; Lai, M.C.; Lin, C.R.; Lai, C.H.; Shieh, T.M. Antibacterial TaN-Ag coatings on titanium dental implants. Surf. Coat. Technol. 2010, 205, 1636–1641. [Google Scholar] [CrossRef]
- Jamuna-Thevi, K.; Bakar, S.A.; Ibrahim, S.; Shahab, N.; Toff, M.R.M. Quantification of silver ion release, in vitro cytotoxicity and antibacterial properties of nanostuctured Ag doped TiO2 coatings on stainless steel deposited by RF magnetron sputtering. Vacuum 2011, 86, 235–241. [Google Scholar] [CrossRef]
- Song, D.H.; Uhm, S.H.; Kim, S.E.; Kwon, J.S.; Han, J.G.; Kim, K.N. Synthesis of titanium oxide thin films containing antibacterial silver nanoparticles by a reactive magnetron co-sputtering system for application in biomedical implants. Mater. Res. Bull. 2012, 47, 2994–2998. [Google Scholar]
- Ferraris, M.; Miola, S.; Ferraris, S.; Gautier, G.; Maina, G. Chemical, mechanical, and antibacterial properties of silver nanocluster-silica composite coatings obtained by sputtering. Adv. Eng. Mater. 2010, 12, 276–282. [Google Scholar]










© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahmani, R.; Rosenberg, M.; Ivask, A.; Kollo, L. Comparison of Mechanical and Antibacterial Properties of TiO2/Ag Ceramics and Ti6Al4V-TiO2/Ag Composite Materials Using Combined SLM-SPS Techniques. Metals 2019, 9, 874. https://doi.org/10.3390/met9080874
Rahmani R, Rosenberg M, Ivask A, Kollo L. Comparison of Mechanical and Antibacterial Properties of TiO2/Ag Ceramics and Ti6Al4V-TiO2/Ag Composite Materials Using Combined SLM-SPS Techniques. Metals. 2019; 9(8):874. https://doi.org/10.3390/met9080874
Chicago/Turabian StyleRahmani, Ramin, Merilin Rosenberg, Angela Ivask, and Lauri Kollo. 2019. "Comparison of Mechanical and Antibacterial Properties of TiO2/Ag Ceramics and Ti6Al4V-TiO2/Ag Composite Materials Using Combined SLM-SPS Techniques" Metals 9, no. 8: 874. https://doi.org/10.3390/met9080874





