Spatial Distribution of Wettability in Aluminum Surfaces Treated with an Atmospheric-Pressure Remote-Plasma
Abstract
:1. Introduction
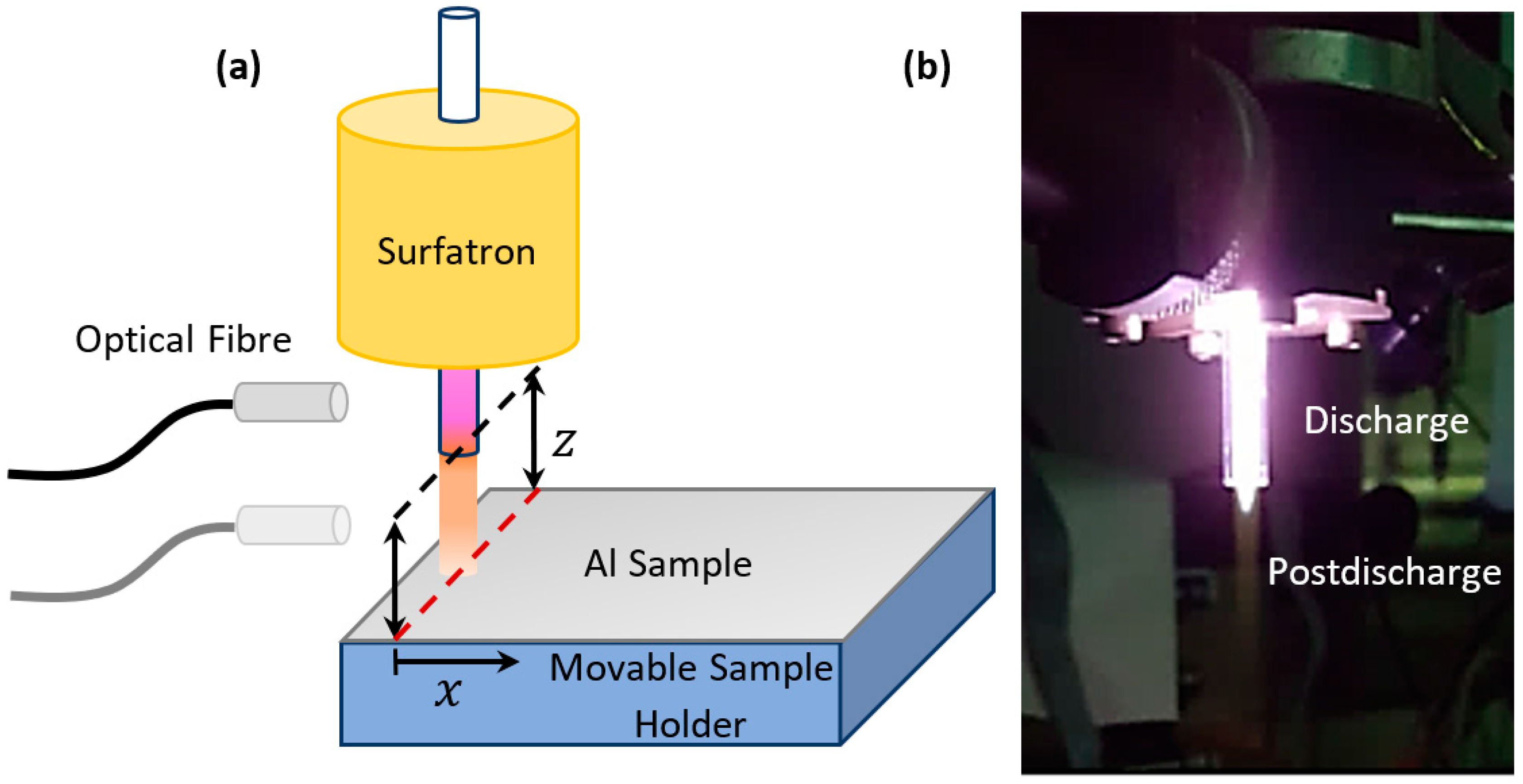
2. Experimental and Methods
3. Results and Discussion
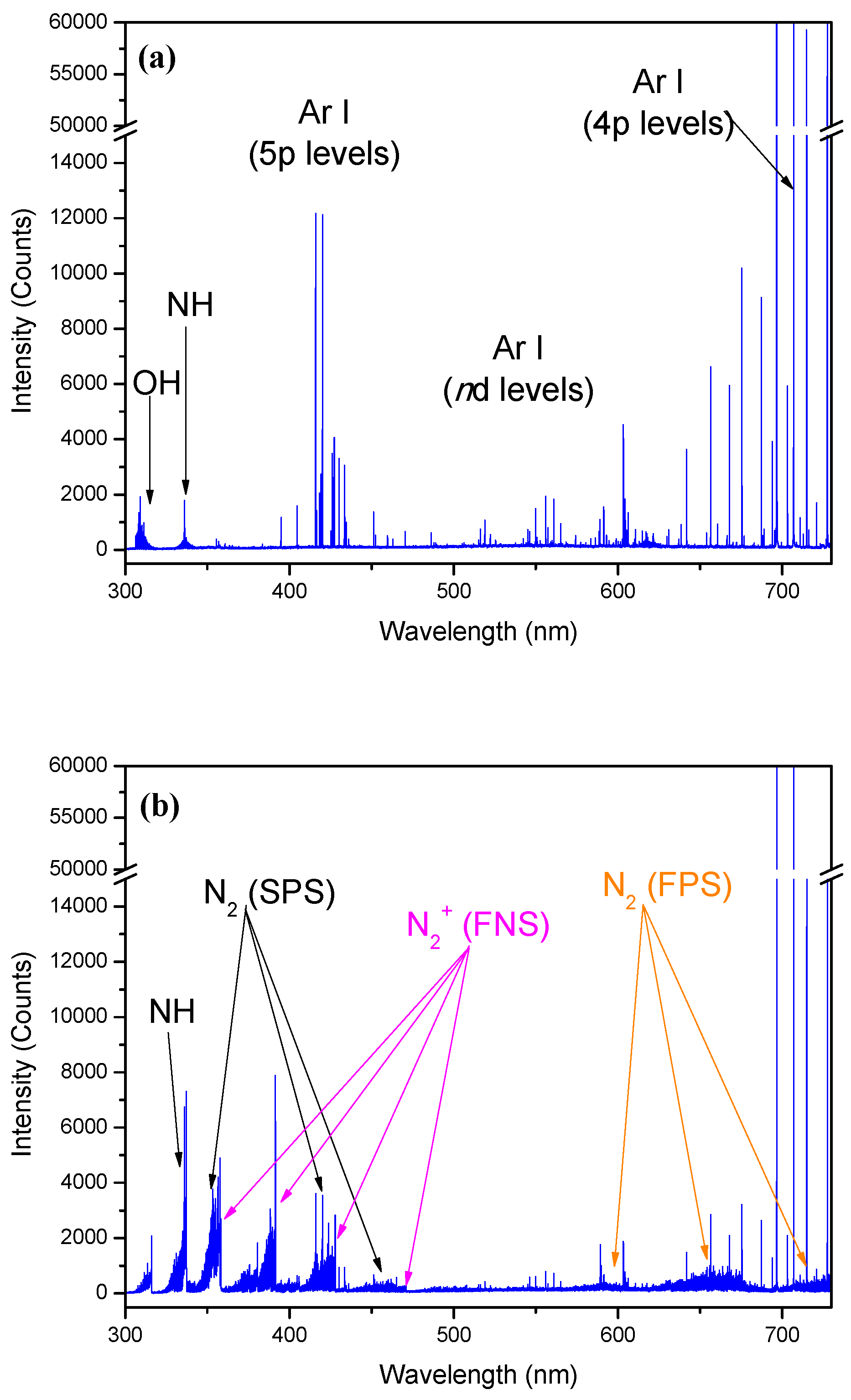
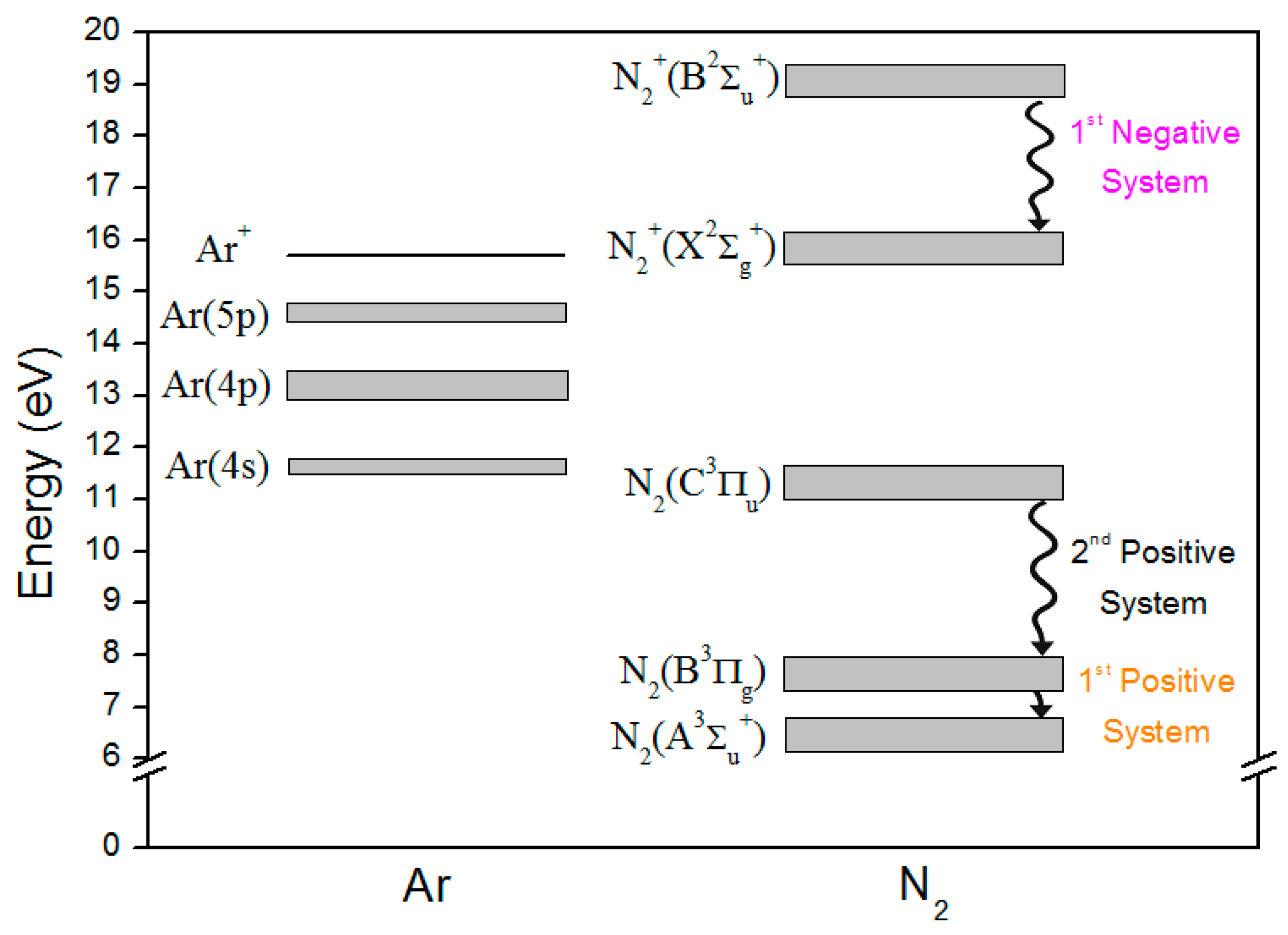
3.1. Active Species in the Discharge
3.2. Active Species in the Postdischarge
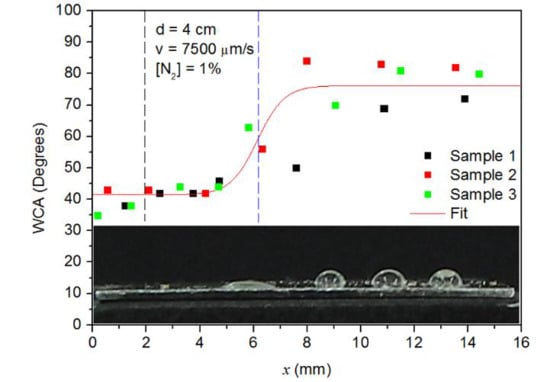
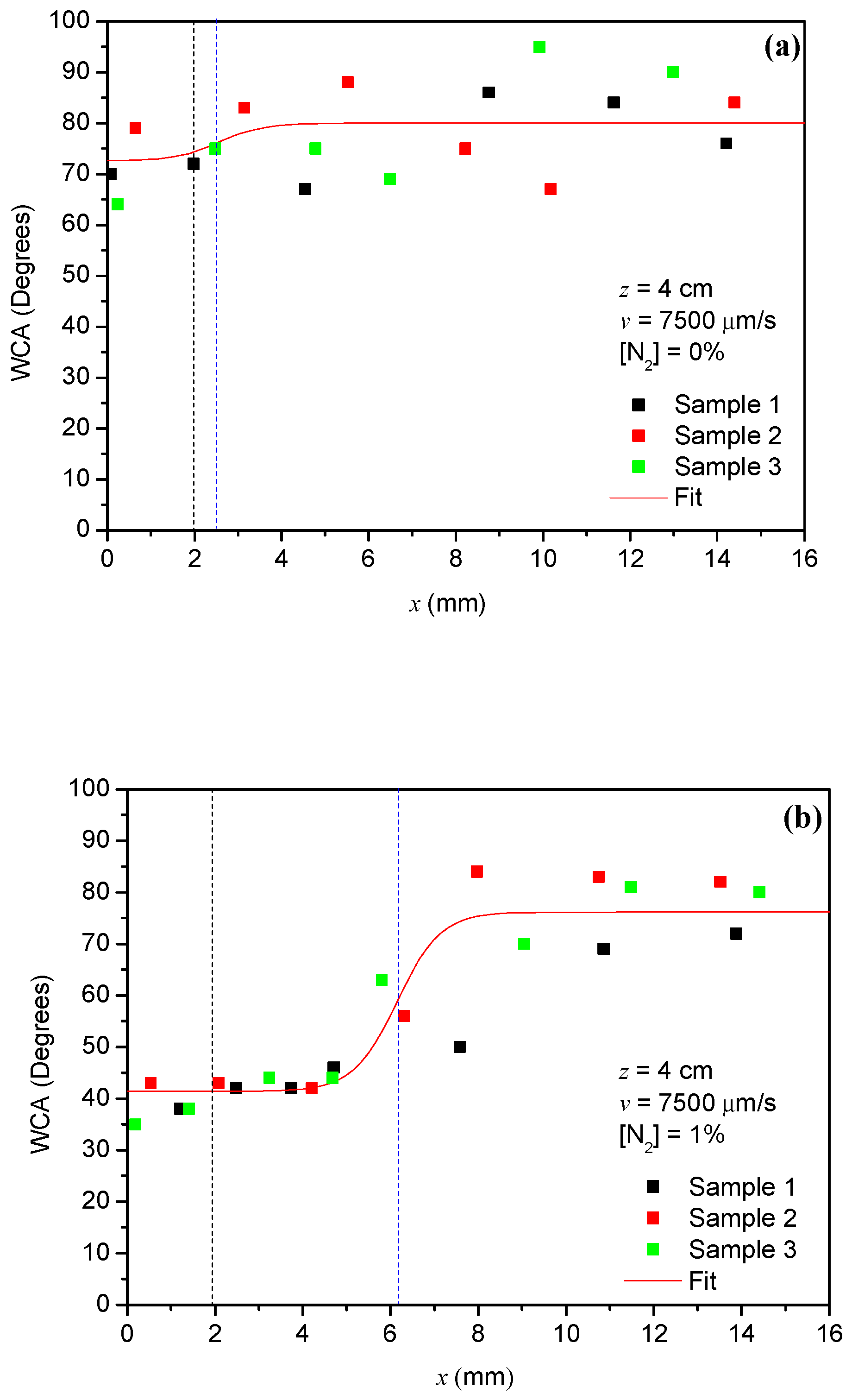
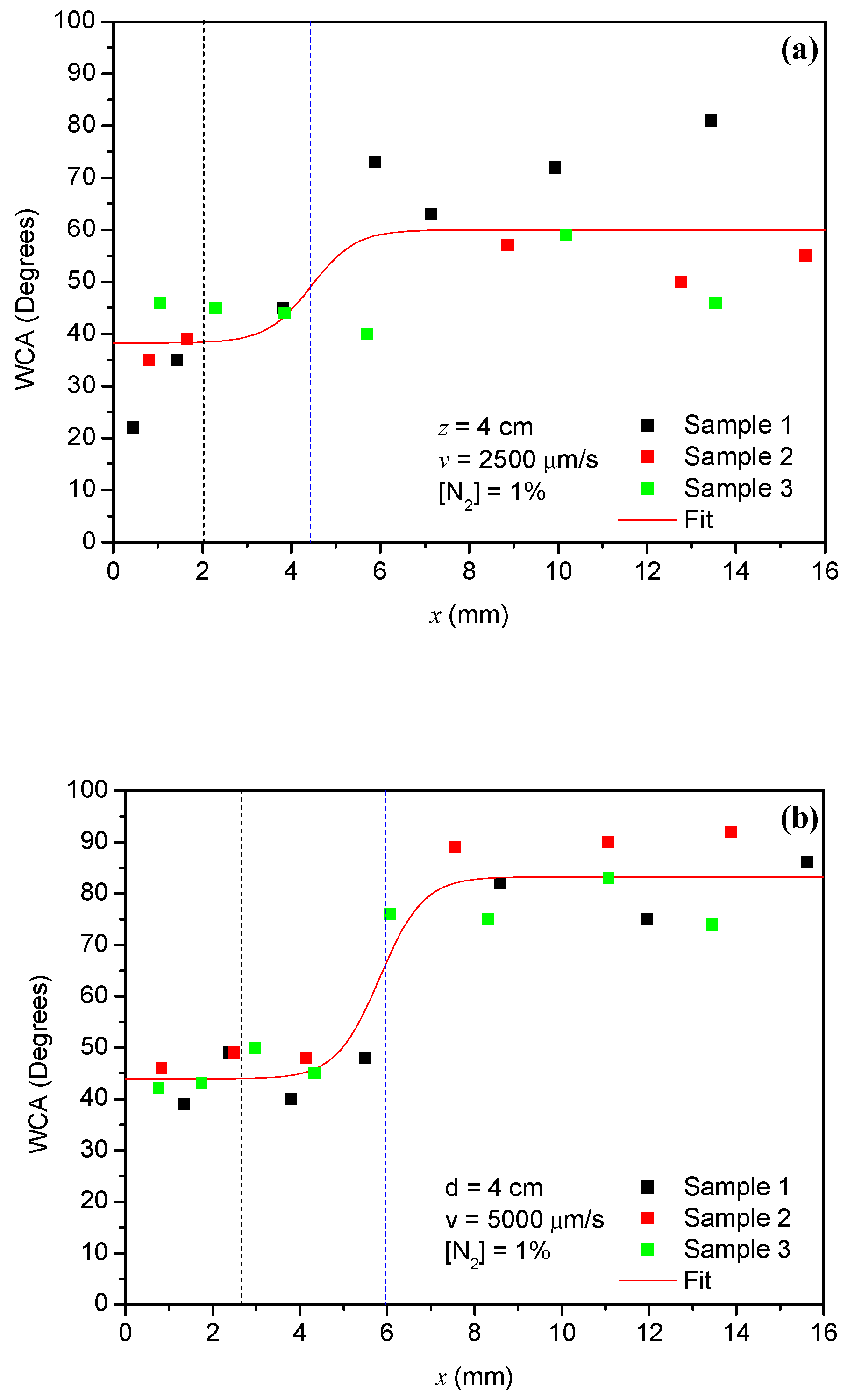
3.3. Spatial Distribution of the Plasma Action on the Surface of Aluminum Samples
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Richard Prabakar, S.J.; Hwang, Y.-H.; Bae, E.G.; Lee, D.K.; Pyo, M. Graphene oxide as a corrosion inhibitor for the aluminum current collector in lithium ion batteries. Carbon 2013, 52, 128–136. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Li, S.; Wang, Y.; Hana, Z.; Rena, L. Fabrication of a superhydrophobic graphene surface with excellent mechanical abrasion and corrosion resistance on an aluminum alloy substrate. RSC Adv. 2014, 4, 45389. [Google Scholar] [CrossRef]
- Polini, W.; Sorrentino, L. Improving the wettability of 2024 aluminum alloy by means of cold plasma treatment. Appl. Surf. Sci. 2003, 214, 1–4. [Google Scholar] [CrossRef]
- Morent, R.; de Geyter, N.; Verschuren, J.; de Clerck, K.; Kiekens, P.; Leis, C. Non-thermal plasma treatment of textiles. Surf. Coat. Technol. 2008, 202, 3427–3449. [Google Scholar] [CrossRef]
- Xu, X. Dielectric barrier-discharge-properties and applications. Thin Solid Films 2001, 390, 237–242. [Google Scholar] [CrossRef]
- Prysiazhnyi, V.; Matoušek, J.; Černák, M. Steel surface treatment and following aging effect after coplanar barrier discharge plasma in air, nitrogen and oxygen. Chem. Listy 2012, 106, 1475–1481. [Google Scholar]
- Prysiazhnyi, V. Atmospheric pressure plasma treatment and following aging effect of chromium surfaces. J. Surf. Eng. Mater. Adv. Technol. 2013, 3, 138–145. [Google Scholar] [CrossRef]
- Bónová, I.; Zahoranová, A.; Kováčik, D.; Zahoran, M.; Mičušík, M.; Černák, M. Atmospheric pressure plasma treatment of flat aluminum surface. App. Surf. Sci. 2015, 331, 79–86. [Google Scholar] [CrossRef]
- Prysiazhnyi, V.; Zaporojchenko, V.; Kersten, H.; Černák, M. Influence of humidity on atmospheric pressure air plasma treatment of aluminum surface. Appl. Surf. Sci. 2012, 258, 5467–5471. [Google Scholar] [CrossRef]
- Prysiazhnyi, V.; Vasina, P.; Panyala, N.R.; Havel, J.; Černák, M. Air DCSBD plasma treatment of Al surface at atmospheric pressure. Surf. Coat. Technol. 2012, 206, 3011–3016. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yoshizaki, A.; Kuroki, T.; Okubo, M. Aluminum surface treatment using three different plasma-assisted dry chemical processes. IEEE Trans. Ind. Appl. 2004, 40, 1220–1225. [Google Scholar] [CrossRef]
- Hnilica, J.; Kudrle, V.; Potočnaková, L. Surface treatment by atmospheric-pressure surfatron jet. IEEE Trans. Plasma Sci. 2012, 40, 2925–2930. [Google Scholar] [CrossRef]
- Shin, D.H.; Bang, C.U.; Kim, J.H.; Hong, Y.C.; Uhm, H.S.; Park, D.K.; Him, J.K. Treatment of metal surface by atmospheric microwave plasma jet. IEEE Trans. Plasma Sci. 2006, 34, 1241–1246. [Google Scholar] [CrossRef]
- Muñoz, J.; Bravo, J.A.; Calzada, M.D. Aluminum metal surface cleaning and activation by atmospheric-pressure remote plasma. Appl. Surf. Sci. 2017, 407, 72. [Google Scholar] [CrossRef]
- Moisan, M.; Zakrzewski, Z.; Pantel, R. The theory and characteristics of an efficient surface wave launcher (surfatron) producing long plasma columns. J. Phy. D: Appl. Phys. 1979, 12, 219–237. [Google Scholar] [CrossRef]
- Kabouzi, Y.; Calzada, M.D.; Moisan, M.; Tran, K.C.; Trassy, C. Radial contraction of microwave sustained plasma columns at atmospheric pressure. J. Appl. Phys. 2002, 91, 1008–1019. [Google Scholar] [CrossRef]
- Bravo, J.A.; Muñoz, J.; Sáez, M.; Calzada, M.D. Atmospheric pressure Ar-N2 surface-wave discharge morphology. IEEE Trans. Plasma Sci. 2011, 39, 2114–2115. [Google Scholar] [CrossRef]
- Callede, G.; Deschamps, J.; Godert, J.L.; Ricard, A. Active nitrogen atom in an atmospheric pressure flowing Ar-N2 microwave discharge. J. Phys. D: Appl. Phys. 1991, 24, 909–914. [Google Scholar] [CrossRef]
- Ricard, A.; Tétreault, J.; Hubert, J. Nitrogen atom recombination in high Ar-N2 flowing post-discharges. J. Phy. B: At. Mol. Opt. Phy. 1991, 24, 1115–1123. [Google Scholar] [CrossRef]
- Bravo, J.A.; Rincón, R.; Muñoz, J.; Sanchez, A.; Calzada, M.D. Spectroscopic characterization of argon-nitrogen Surface-wave discharges in dielectric tubes at atmospheric pressure. Plasma Chem. Plasma Process. 2015, 35, 993–1014. [Google Scholar] [CrossRef]
- Rincón, R.; Yubero, C.; Calzada, M.D.; Moyano, L.; Zea, L. Plasma technology as a new food preservation technique. In Microbial Food Safety and Preservation Techniques; Ravishankar Rai, V., Bai, J.A., Eds.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Yuan, Y. Contact Angle and Wetting Properties. In Surface Science Techniques; Bracco, G., Holst, B., Eds.; Springer: Berlin, Germany, 2013. [Google Scholar]
- Calzada, M.D.; Garcia, M.C.; Luque, J.M.; Santiago, I. Influence of the thermodynamic equilibrium state in the excitation of samples by a plasma at atmospheric pressure. J. Appl. Phy. 2002, 92, 2269. [Google Scholar] [CrossRef]
- Kudela, J.; Odrobina, I.; Kando, M. High-speed camera study of the surface wave discharge propagation in xenon. Jpn. J. Appl. Phy. 1998, 37, 4169. [Google Scholar] [CrossRef]
- Calzada, M.D.; Moisan, M.; Gamero, A.; Sola, A. Experimental investigation and characterization of the departure from local thermodynamic equilibrium along a surface-wave-sustained discharge at atmospheric pressure. J. Appl. Phy. 1996, 80, 46. [Google Scholar] [CrossRef]
- Jonkers, J.; de Regt, J.M.; van der Mullen, J.A.M.; Vos, H.P.C.; de Groote, F.P.; Timmermans, E.A.H. On the electron temperatures and densities produced by the “torche à injection axiale”. Spectrochim. Acta Part B 1996, 51, 1385. [Google Scholar] [CrossRef]
- Muñoz, J.; Calzada, M.D. Experimental study on equilibrium deviations in atmospheric pressure argon/helium surface wave discharges. Spectrochim. Acta Part B 2010, 65, 1014–1021. [Google Scholar] [CrossRef]
- Muñoz, J.; Rincón, R.; Melero, C.; Dimitrijević, M.S.; González, C.; Calzada, M.D. Validation of the van der Waals broadening method for the determination of gas temperature in microwave discharges sustained in argon–neon mixtures. J. Quant. Spectrosc. Radiat. Transfer 2018, 206, 135–141. [Google Scholar] [CrossRef]
- Ricard, A. Reactive Plasmas; Société Française du Vide: Paris, France, 1996. [Google Scholar]
- Henriques, J.; Tatarova, E.; Guerra, V.; Ferreira, C.M. Wave driven N2-Ar discharge. I. Self-consistent theoretical model. J. Appl. Phys. 2001, 91, 5632–5639. [Google Scholar] [CrossRef]
- Guerra, V.; Tatarova, E.; Dias, F.M.; Ferreira, C.M. On the self-consistent modeling of a traveling wave sustained nitrogen discharge. J. Appl. Phys. 2002, 91, 2648–2661. [Google Scholar] [CrossRef] [Green Version]
- Rincón, R.; Muñoz, J.; Sáez, M.; Calzada, M.D. Spectroscopic characterization of atmospheric pressure argon plasmas sustained with the Torche à Injection Axiale sur Guide d’Ondes. Spectrochim. Acta, Part B 2013, 81, 26–35. [Google Scholar] [CrossRef]
- Szili, E.J.; Al-Bataineh, S.A.; Bryant, P.M.; Short, R.D.; Bradley, J.W.; Steele, D.A. Controlling the spatial distribution of polymer surface treatments using atmospheric-pressure microplasma jets. Plasma Processes Polym. 2011, 8, 38–50. [Google Scholar] [CrossRef]
- Mézerette, D.; Belmonte, T.; Hugon, R.; Henrion, G.; Czerwiec, T.; Michel, H. Study of the surface mechanisms in an Ar-N2 post-discharge cleaning process. Surf. Coat. Technol. 2003, 169–170, 181–185. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz, J.; Rincón, R.; Calzada, M.D. Spatial Distribution of Wettability in Aluminum Surfaces Treated with an Atmospheric-Pressure Remote-Plasma. Metals 2019, 9, 937. https://doi.org/10.3390/met9090937
Muñoz J, Rincón R, Calzada MD. Spatial Distribution of Wettability in Aluminum Surfaces Treated with an Atmospheric-Pressure Remote-Plasma. Metals. 2019; 9(9):937. https://doi.org/10.3390/met9090937
Chicago/Turabian StyleMuñoz, José, Rocío Rincón, and María Dolores Calzada. 2019. "Spatial Distribution of Wettability in Aluminum Surfaces Treated with an Atmospheric-Pressure Remote-Plasma" Metals 9, no. 9: 937. https://doi.org/10.3390/met9090937