Chloride Transport Characteristics of Concrete Exposed to Coastal Dredger Fill Silty Soil Environment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Concrete Mix Ratio
2.2. Preparation of Concrete Specimens
2.3. Test Environment and Methods
3. Results
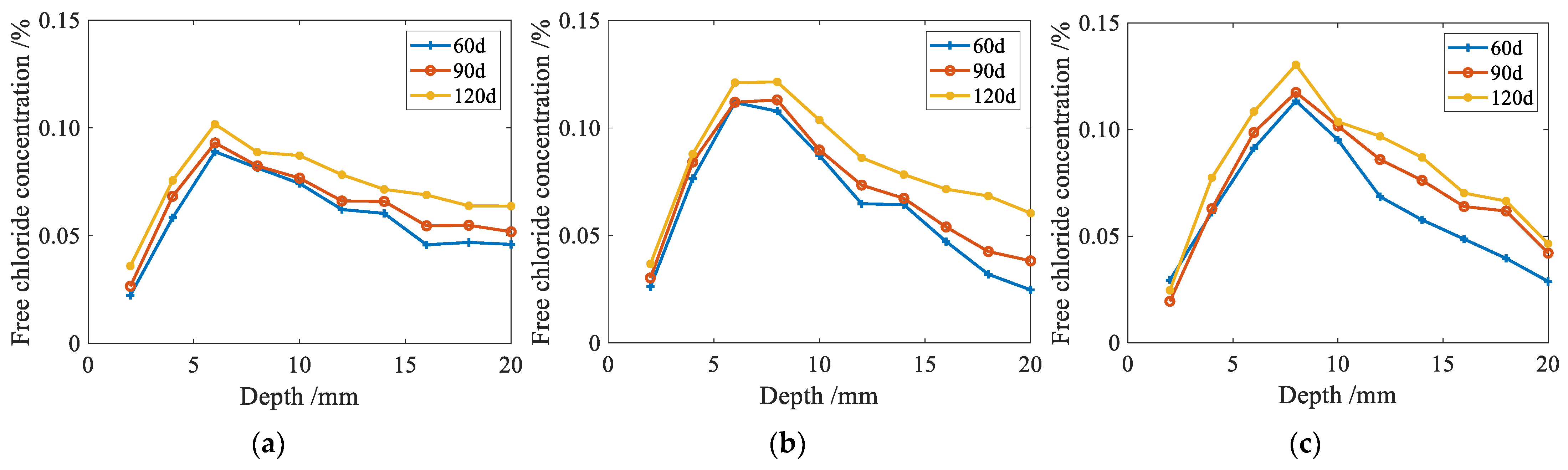
3.1. Chloride Profiles
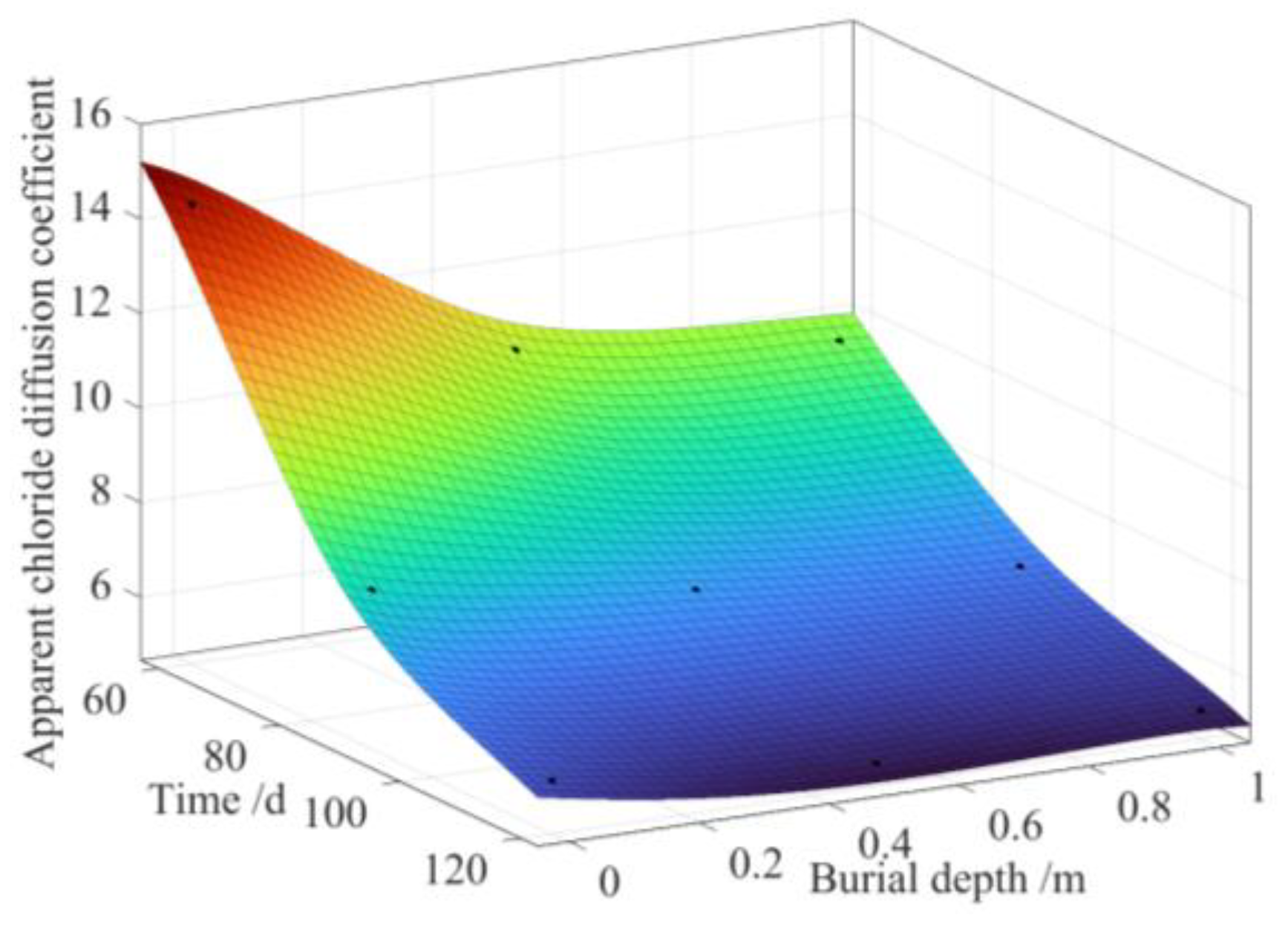
3.2. Time-Varying Chloride Transport
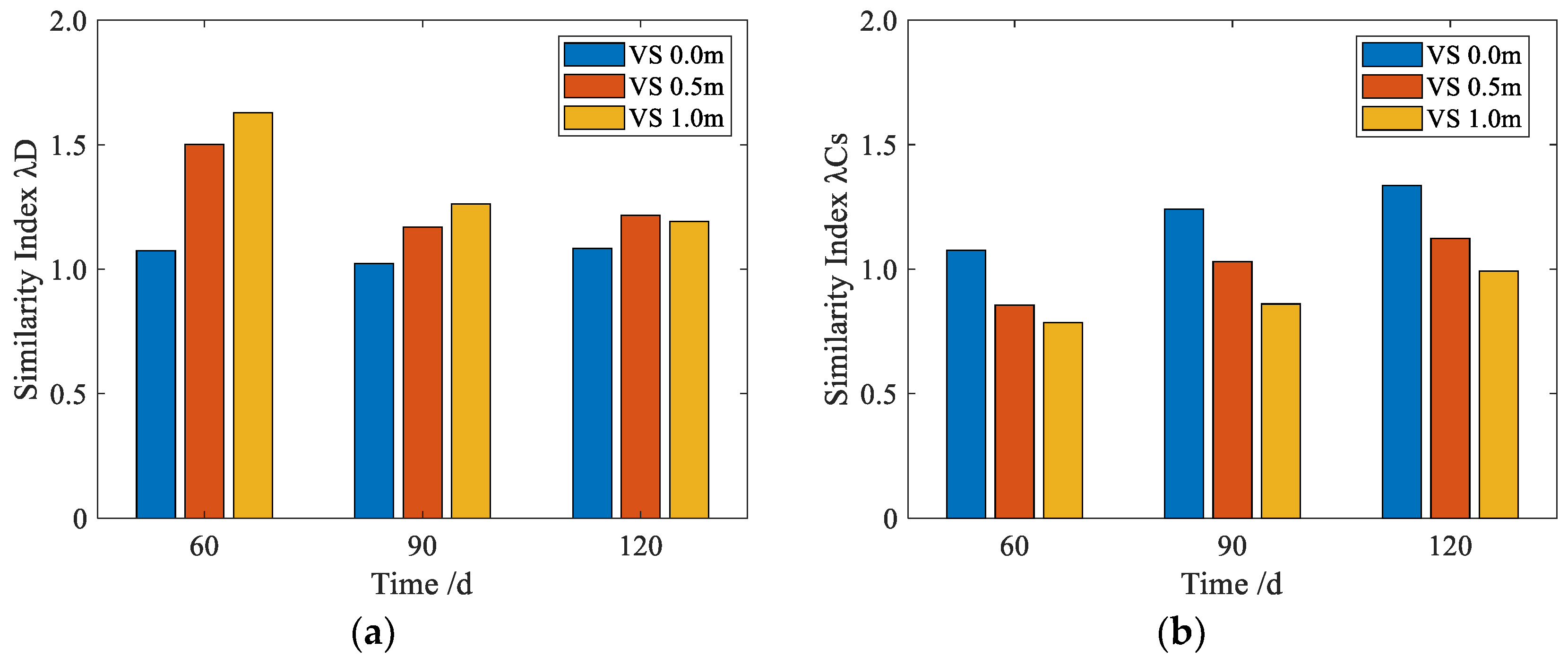
3.3. Similarity Analysis
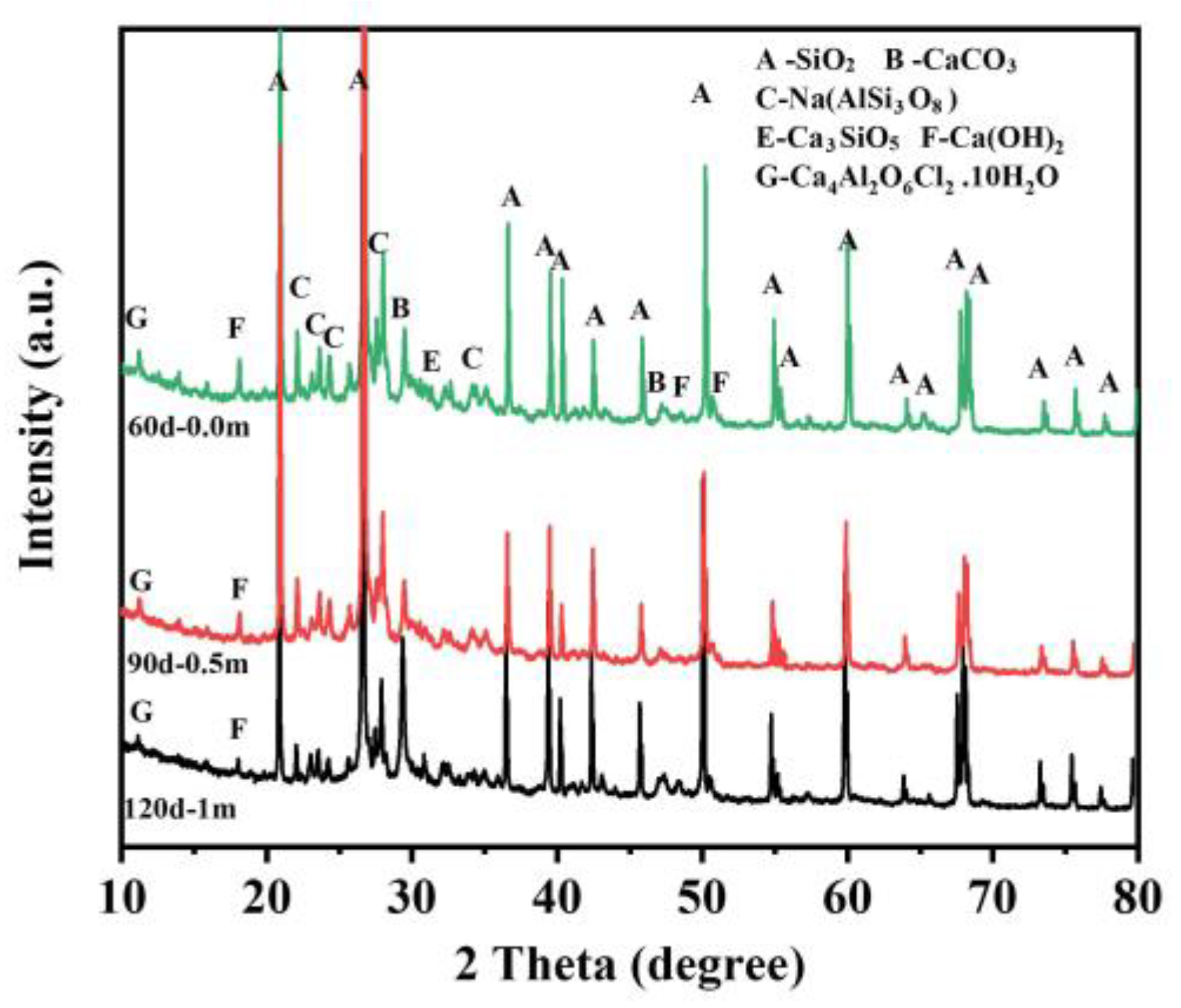
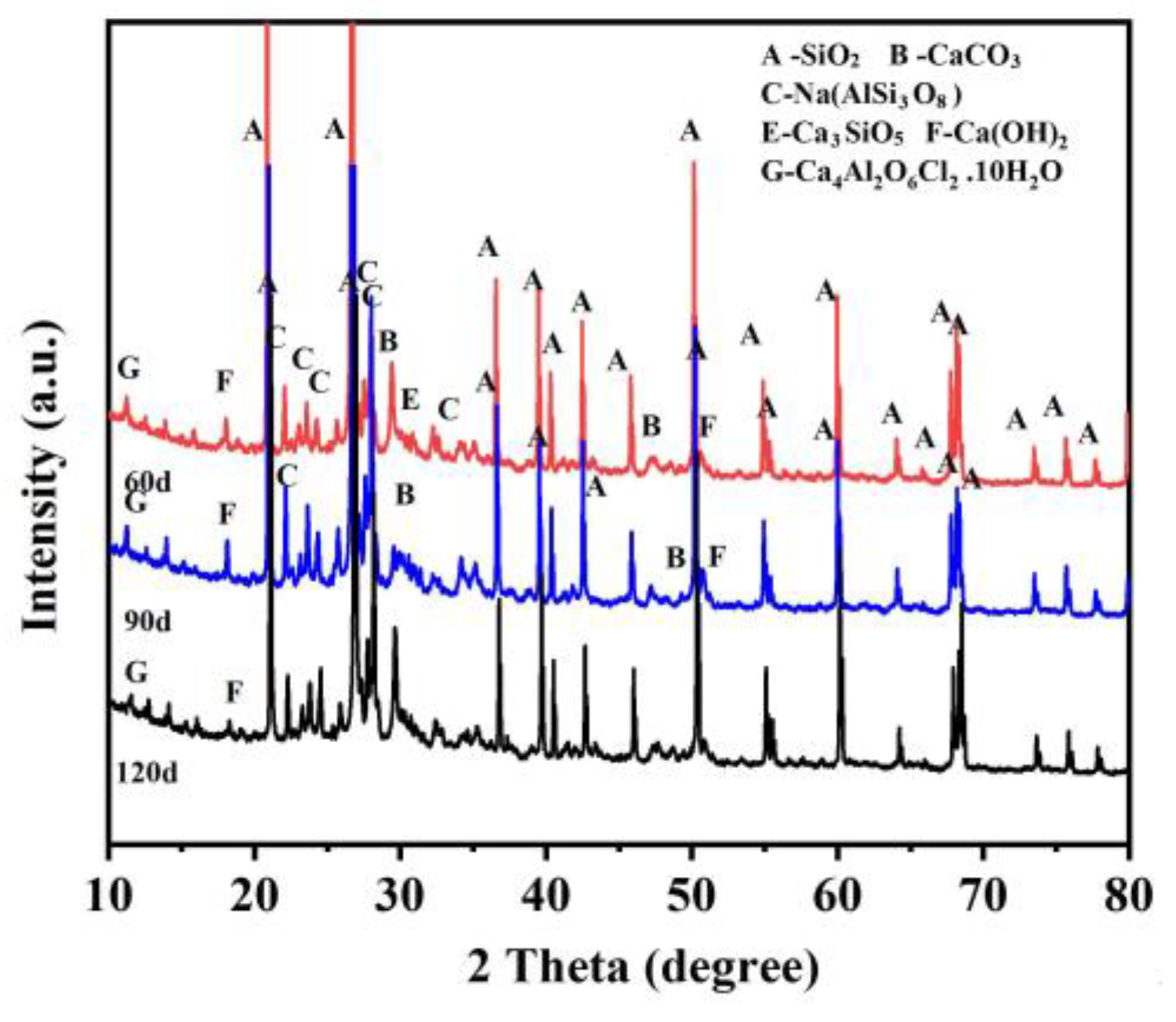
3.4. Concrete Corrosion Products Analysis
4. Conclusions
- (1)
- In the dredger fill silty soil environment, a direct correlation was observed between the increase in exposure time (ranging from 60 days to 120 days) and the corresponding augmentation in the free chloride content of the concrete specimens. Owing to the relatively diminished moisture content within the soil matrix, a distinct convection zone emerged adjacent to the concrete specimen surfaces at varying depths of interment and exposure durations. This convection zone manifested at an approximate depth of 6 to 8 mm. Furthermore, an elevation in burial depth corresponded to parallel increments in both the convection zone’s depth and chloride content.
- (2)
- In the dredger fill silty soil environment, the apparent chloride diffusion coefficient exhibited a diminishing trend as both the depth of interment and exposure time escalated. Divergent effects were noted concerning the impact of exposure time and burial depth on the apparent surface chloride concentration relative to the chloride diffusion coefficient evident within the concrete samples. Mathematical formulations were devised to represent the concrete’s apparent surface chloride concentrations and apparent chloride diffusion coefficients as dependent on the exposure time and burial depth parameters, yielding an exceptional coefficient of determination of up to 0.96.
- (3)
- The chloride corrosion similarity in the two distinct testing environments was significantly influenced by both the burial depth and exposure time of the concrete specimens. Notably, concrete samples interred at a depth of 0.0 m for a duration of 60 days exhibited satisfactory parallels with those placed within the simulated solution environment, as characterized by similarity indices (λD and λCs) of 1.07 and 1.11, respectively.
- (4)
- In contrast to the simulated solution environment, the space 5 mm from the surface of the concrete in the dredger fill silty soil environment usually remained in an unsaturated state, leading to unsatisfactory crystallization of Friedel’s salt. Therefore, the chloride corrosion potential of concrete may be more severe in the dredger fill silty soil environment.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qu, F.L.; Li, W.G.; Dong, W.K.; Tam, V.W.Y.; Yu, T. Durability deterioration of concrete under marine environment from material to structure: A critical review. J. Build. Eng. 2021, 35, 102074. [Google Scholar] [CrossRef]
- Hu, J.Y.; Zhang, S.S.; Chen, E.; Li, W. A review on corrosion detection and protection of existing reinforced concrete (RC) structures. Constr. Build. Mater. 2022, 325, 126718. [Google Scholar] [CrossRef]
- Li, K.F.; Zeng, J.J.; Tang, L.P.; Sorensen, H.E.; Borges, P.C.; Geiker, M.R.; Pedersen, M.T.; Zhang, P.; Surana, S.; Maddalena, R.; et al. Long-term field exposure of structural concretes in marine environment: State-of-the-art review by RILEM TC 289-DCM. Mater. Struct. 2022, 55, 205. [Google Scholar] [CrossRef]
- Wu, L.J.; Li, W.; Yu, X.N. Time-dependent chloride penetration in concrete in marine environments. Constr. Build. Mater. 2017, 152, 406–413. [Google Scholar] [CrossRef]
- Pack, S.W.; Jung, M.S.; Song, H.W.; Kim, S.H.; Ann, K.Y. Prediction of time dependent chloride transport in concrete structures exposed to a marine environment. Cem. Concr. Res. 2010, 40, 302–312. [Google Scholar] [CrossRef]
- Jin, Z.Q.; Zhao, X.; Zhao, T.J.; Li, J.Q. Chloride ions transportation behavior and binding capacity of concrete exposed to different marine corrosion zones. Constr. Build. Mater. 2018, 177, 170–183. [Google Scholar]
- Fjendbo, S.; Sorensen, H.E.; De Weerdt, K.; Jakobsen, U.H.; Geiker, M.R. Correlating the development of chloride profiles and microstructural changes in marine concrete up to ten years. Cem. Concr. Compos. 2022, 131, 104590. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Zhuang, H.X.; Shi, J.L.; Huang, J.; Zhang, J.Z. Time-dependent characteristic and similarity of chloride diffusivity in concrete. Mag. Concr. Res. 2018, 70, 129–137. [Google Scholar] [CrossRef]
- Wu, L.J.; Wang, W.Q.; Jiang, C.C. Study on the similarity of chloride penetration in concrete exposed to field and laboratory conditions. Mater. Struct. 2023, 56, 95. [Google Scholar] [CrossRef]
- Sun, L.; Wang, C.; Zhang, C.W.; Yang, Z.Y.; Li, C.; Qiao, P.Z. Experimental investigation on the bond performance of sea sand coral concrete with FRP bar reinforcement for marine environments. Adv. Struct. Eng. 2023, 26, 533–546. [Google Scholar] [CrossRef]
- Wu, L.J.; Xiang, Z.Y.; Jiang, H.; Liu, M.W.; Ju, X.L.; Zhang, W.X. A Review of Durability Issues of Reinforced Concrete Structures Due to Coastal Soda Residue Soil in China. J. Mar. Sci. Eng. 2022, 10, 1740. [Google Scholar] [CrossRef]
- Valipour, M.; Pargar, F.; Shekarchi, M.; Khani, S.; Moradian, M. In situ study of chloride ingress in concretes containing natural zeolite, metakaolin and silica fume exposed to various exposure conditions in a harsh marine environment. Constr. Build. Mater. 2013, 46, 63–70. [Google Scholar] [CrossRef]
- Sadati, S.; Arezoumandi, M.; Shekarchi, M. Long-term performance of concrete surface coatings in soil exposure of marine environments. Constr. Build. Mater. 2015, 94, 656–663. [Google Scholar] [CrossRef]
- Sadati, S.; Moradllo, M.K.; Shekarchi, M. Long-Term Performance of Silica Fume Concrete in Soil Exposure of Marine Environments. J. Mater. Civ. Eng. 2017, 29, 04017126. [Google Scholar] [CrossRef]
- Al-Tameemi, A.A.; Mahmood, M.S.; Albahadli, N.A.; Alkhudery, H.H.; Alturaihee, R.M. Impact of Soil-Borne Chloride on Rebars Corrosion in Reinforced Concrete Members. J. Eng. Sci. Technol. 2022, 17, 654–672. [Google Scholar]
- Yang, D.Q.; Yan, C.W.; Zhang, J.; Liu, S.G.; Li, J. Chloride threshold value and initial corrosion time of steel bars in concrete exposed to saline soil environments. Constr. Build. Mater. 2021, 267, 120979. [Google Scholar] [CrossRef]
- Zhang, F.; Wei, F.; Wu, X.J.; Hu, Z.P.; Li, X.G.; Gao, L.L. Study on Concrete Deterioration and Chloride Ion Diffusion Mechanism by Different Aqueous NaCl-MgSO4 Concentrations. Buildings 2022, 12, 1843. [Google Scholar] [CrossRef]
- Yang, D.Q.; Yan, C.W.; Liu, S.G.; Zhang, J.; Hu, Z.C. Stress-strain constitutive model of concrete corroded by saline soil under uniaxial compression. Constr. Build. Mater. 2019, 213, 665–674. [Google Scholar] [CrossRef]
- Yan, C.W.; Zhao, J.J.; Liu, S.G.; Zhang, J. Seismic Yield Strength of Reinforced Concrete Bridge Piers in a Saline Soil Environment. J. Perform. Constr. Facil. 2020, 34, 04019114. [Google Scholar] [CrossRef]
- Deng, Y.H.; Yan, C.W.; Li, J.; Liu, S.G.; Zhang, J.; Wang, J.J. Seismic deformation of reinforced concrete piers corroded by saline soil. Bull. Earthq. Eng. 2022, 20, 6763–6788. [Google Scholar] [CrossRef]
- Xu, G.; Li, Y.P.; Su, Y.B.; Xu, K. Chloride ion transport mechanism in concrete due to wetting and drying cycles. Struct. Concr. 2015, 16, 289–296. [Google Scholar]
- Yang, L.F.; Cai, R.; Yu, B. Investigation of computational model for surface chloride concentration of concrete in marine atmosphere zone. Ocean Eng. 2017, 138, 105–111. [Google Scholar] [CrossRef]
- Cai, R.; Hu, Y.S.; Yu, M.; Liao, W.Y.; Yang, L.F.; Kumar, A.; Ma, H.Y. Skin effect of chloride ingress in marine concrete: A review on the convection zone. Constr. Build. Mater. 2020, 262, 120566. [Google Scholar] [CrossRef]
- Zhang, Y.; Di Luzio, G.; Alnaggar, M. Coupled multi-physics simulation of chloride diffusion in saturated and unsaturated concrete. Constr. Build. Mater. 2021, 292, 123394. [Google Scholar] [CrossRef]
- He, H.; Shuang, E.; Wen, T.; Yao, J.; Wang, X.; He, C.; Yu, Y. Employing novel N-doped graphene quantum dots to improve chloride binding of cement. Constr. Build. Mater. 2023, 401, 132944. [Google Scholar] [CrossRef]
- Loser, R.; Lothenbach, B.; Leemann, A.; Tuchschmid, M. Chloride resistance of concrete and its binding capacity—Comparison between experimental results and thermodynamic modeling. Cem. Concr. Compos. 2010, 32, 34–42. [Google Scholar] [CrossRef]
- Yuan, Q.; Shi, C.J.; De Schutter, G.; Audenaert, K.; Deng, D.H. Chloride binding of cement-based materials subjected to external chloride environment—A review. Constr. Build. Mater. 2009, 23, 1–13. [Google Scholar] [CrossRef]
- Bullard, J.W.; Jennings, H.M.; Livingston, R.A. Mechanisms of cement hydration. Cem. Concr. Res. 2011, 41, 1208–1223. [Google Scholar] [CrossRef]
- Wang, H.L.; Dai, J.G.; Sun, X.Y.; Zhang, X.L. Time-Dependent and Stress-Dependent Chloride Diffusivity of Concrete Subjected to Sustained Compressive Loading. J. Mater. Civ. Eng. 2016, 28, 04016059. [Google Scholar] [CrossRef]
- Bao, J.W.; Wang, L.C. Combined effect of water and sustained compressive loading on chloride penetration into concrete. Constr. Build. Mater. 2017, 156, 708–718. [Google Scholar] [CrossRef]
- Zhang, L.H.; Jia, J.Q.; Meng, G.; Zhu, W.Q. Chloride diffusion in concrete subjected to compressive loading. Mag. Concr. Res. 2014, 66, 991–997. [Google Scholar] [CrossRef]
- Xu, J.; Li, F.M. Analytical Model for Load Dependence of Chloride Penetration into Concrete. J. Mater. Civ. Eng. 2017, 29, 04016279. [Google Scholar] [CrossRef]
- Yang, L.F.; Wang, L.; Yu, B. Time-varying behavior and its coupling effects with environmental conditions and cementitious material types on surface chloride concentration of marine concrete. Constr. Build. Mater. 2021, 303, 124578. [Google Scholar] [CrossRef]
- Zhou, S.J. Modeling Chloride Diffusion in Concrete with Linear Increase of Surface Chloride. ACI Mater. J. 2014, 111, 483–490. [Google Scholar]
- Zhou, S.J. Analytical Model for Square Root Increase of Surface Chloride Concentration and Decrease of Chloride Diffusivity. J. Mater. Civ. Eng. 2016, 28, 04015181. [Google Scholar] [CrossRef]
- Cai, R.; Yu, M.; Hu, Y.S.; Yang, L.F.; Ma, H.Y. Influence of data acquisition and processing on surface chloride concentration of marine concrete. Constr. Build. Mater. 2021, 273, 121705. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Lin, C.P.; Cui, Y.Q. Experiments of Chloride Ingression in Loaded Concrete Members under the Marine Environment. J. Mater. Civ. Eng. 2014, 26, 04014012. [Google Scholar] [CrossRef]
- Wu, J.; Li, H.M.; Wang, Z.; Liu, J.J. Transport model of chloride ions in concrete under loads and drying-wetting cycles. Constr. Build. Mater. 2016, 112, 733–738. [Google Scholar] [CrossRef]
- Song, H.W.; Lee, C.H.; Ann, K.Y. Factors influencing chloride transport in concrete structures exposed to marine environments. Cem. Concr. Compos. 2008, 30, 113–121. [Google Scholar] [CrossRef]
- Zhu, Q.; Jiang, L.H.; Chen, Y.; Xu, J.X.; Mo, L.L. Effect of chloride salt type on chloride binding behavior of concrete. Constr. Build. Mater. 2012, 37, 512–517. [Google Scholar] [CrossRef]
- Voinitchi, D.; Julien, S.; Lorente, S. The relation between electrokinetics and chloride transport through cement-based materials. Cem. Concr. Compos. 2008, 30, 157–166. [Google Scholar] [CrossRef]
- Long, W.J.; Zhang, X.H.; Feng, G.L.; Xie, J.; Xing, F.; Dong, B.Q.; Zhang, J.R.; Khayat, K.H. Investigation on chloride binding capacity and stability of Friedel’s salt in graphene oxide reinforced cement paste. Cem. Concr. Compos. 2022, 132, 104603. [Google Scholar] [CrossRef]
- Machner, A.; Hemstad, P.; De Weerdt, K. Towards the Understanding of the pH Dependency of the Chloride Binding of Portland Cement Pastes. Nord. Concr. Res. 2018, 58, 143–162. [Google Scholar] [CrossRef]





| Cement/kg | Water/kg | Fine Aggregates/kg | Coarse Aggregates/kg | w/c |
|---|---|---|---|---|
| 475 | 190 | 547 | 1164 | 0.4 |
| Components | K | Na | Ca | Mg | Cl− | SO₄2− | HCO3− |
|---|---|---|---|---|---|---|---|
| Molarity (mg/L) | 352 | 4750 | 705 | 315 | 3976 | 573 | 2501 |
| Percentage concentration (%) | 0.0352 | 0.4750 | 0.0705 | 0.0315 | 0.3976 | 0.0573 | 0.2501 |
| Components | KCl | NaCl | MgCl2 | CaCl2 | MgSO4 | NaHCO3 |
|---|---|---|---|---|---|---|
| Molarity (mg/L) | 672 | 3175 | 613 | 1958 | 733 | 3573 |
| Percentage concentration (%) | 0.0626 | 0.2961 | 0.0571 | 0.1826 | 0.0683 | 0.3332 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Jiang, C.; Wang, W.; Gao, X.; Xia, Y. Chloride Transport Characteristics of Concrete Exposed to Coastal Dredger Fill Silty Soil Environment. Buildings 2023, 13, 2398. https://doi.org/10.3390/buildings13092398
Wu L, Jiang C, Wang W, Gao X, Xia Y. Chloride Transport Characteristics of Concrete Exposed to Coastal Dredger Fill Silty Soil Environment. Buildings. 2023; 13(9):2398. https://doi.org/10.3390/buildings13092398
Chicago/Turabian StyleWu, Lingjie, Chenchi Jiang, Weiqiang Wang, Xiang Gao, and Yufeng Xia. 2023. "Chloride Transport Characteristics of Concrete Exposed to Coastal Dredger Fill Silty Soil Environment" Buildings 13, no. 9: 2398. https://doi.org/10.3390/buildings13092398





