Performance Evaluation of a Multifunctional Road Marking Coating for Tunnels Based on Nano SiO2 and TiO2 Modifications
Abstract
:1. Introduction
2. Preparation of Materials and Test Methods
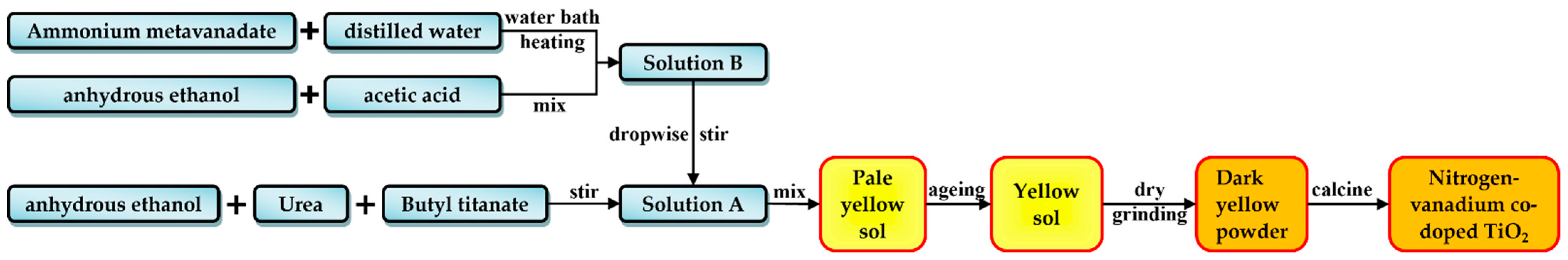
2.1. Preparation of Materials
2.2. Test Methods
2.2.1. Flame Retardant Tests
- (1)
- TG-DTG tests
- (2)
- DSC tests:
- (3)
- FTIR tests
- (4)
- SEM tests
2.2.2. Exhaust Degradation Tests
2.2.3. Self-Cleaning Tests
- (1)
- Self-cleaning performance tests
- (2)
- Water droplet contact angle tests
3. Results and Discussion
3.1. Evaluation of Thermal Stability
3.1.1. TG-DTG Results Analysis
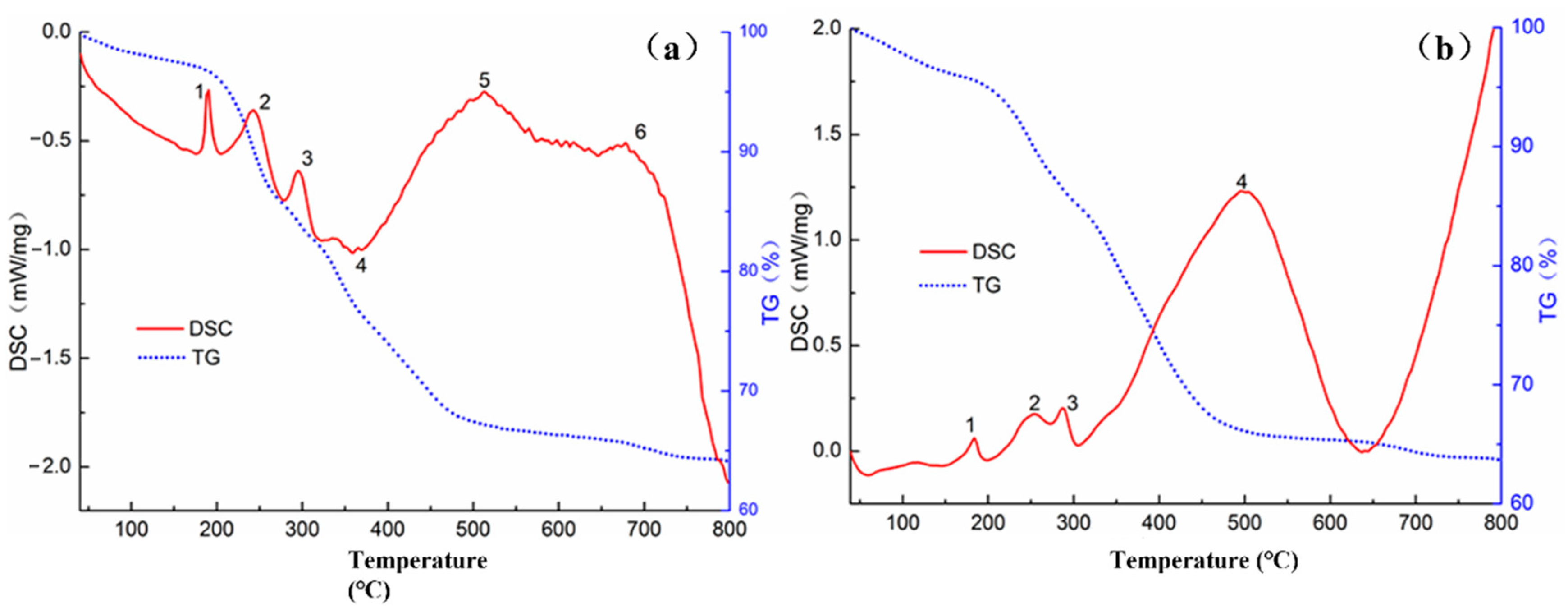
3.1.2. DSC Results Analysis
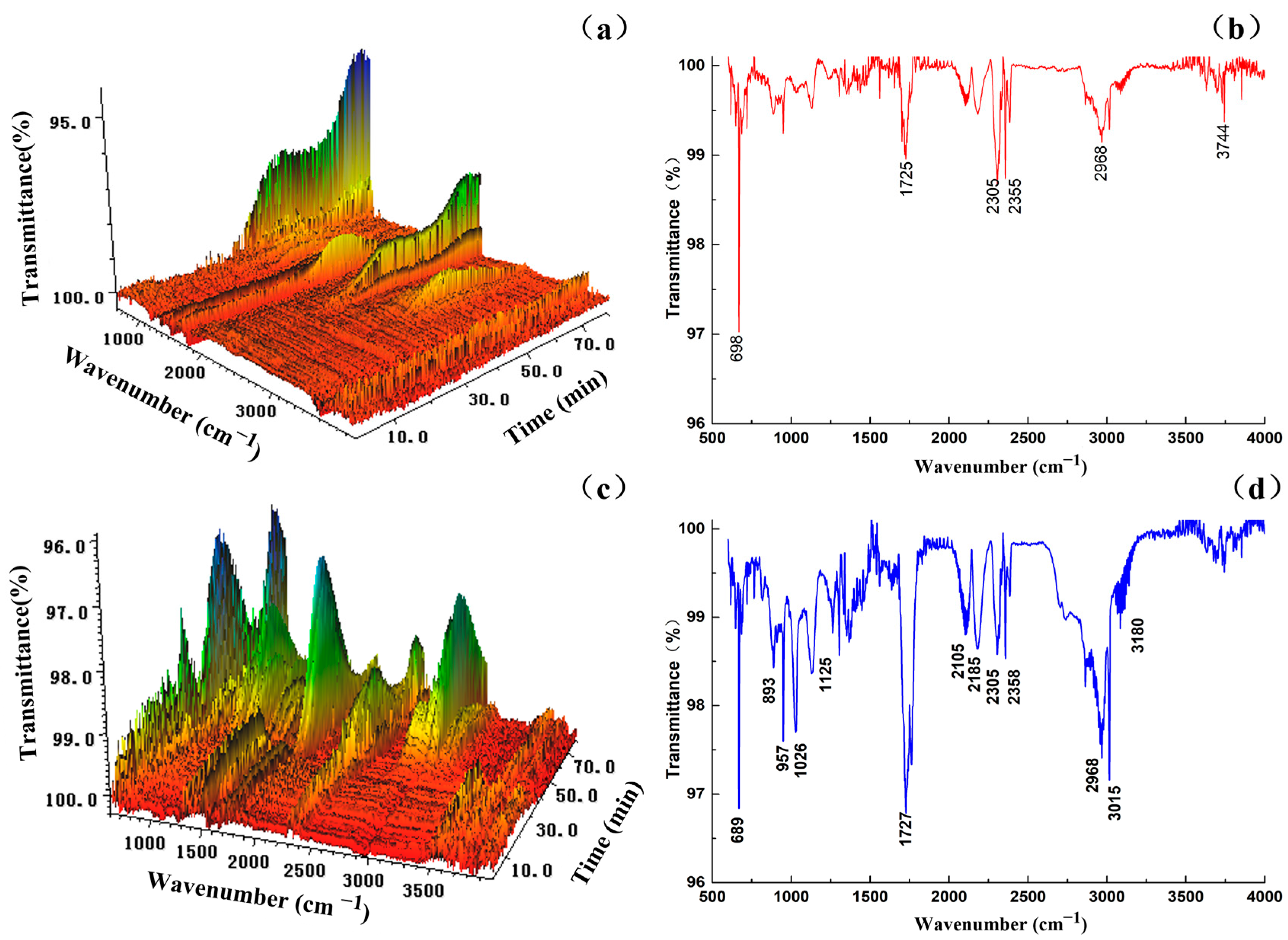
3.1.3. Analysis of Combustion Volatiles FTIR Results
3.1.4. Micro-Morphological Analysis of the Charcoal Layer after Combustion
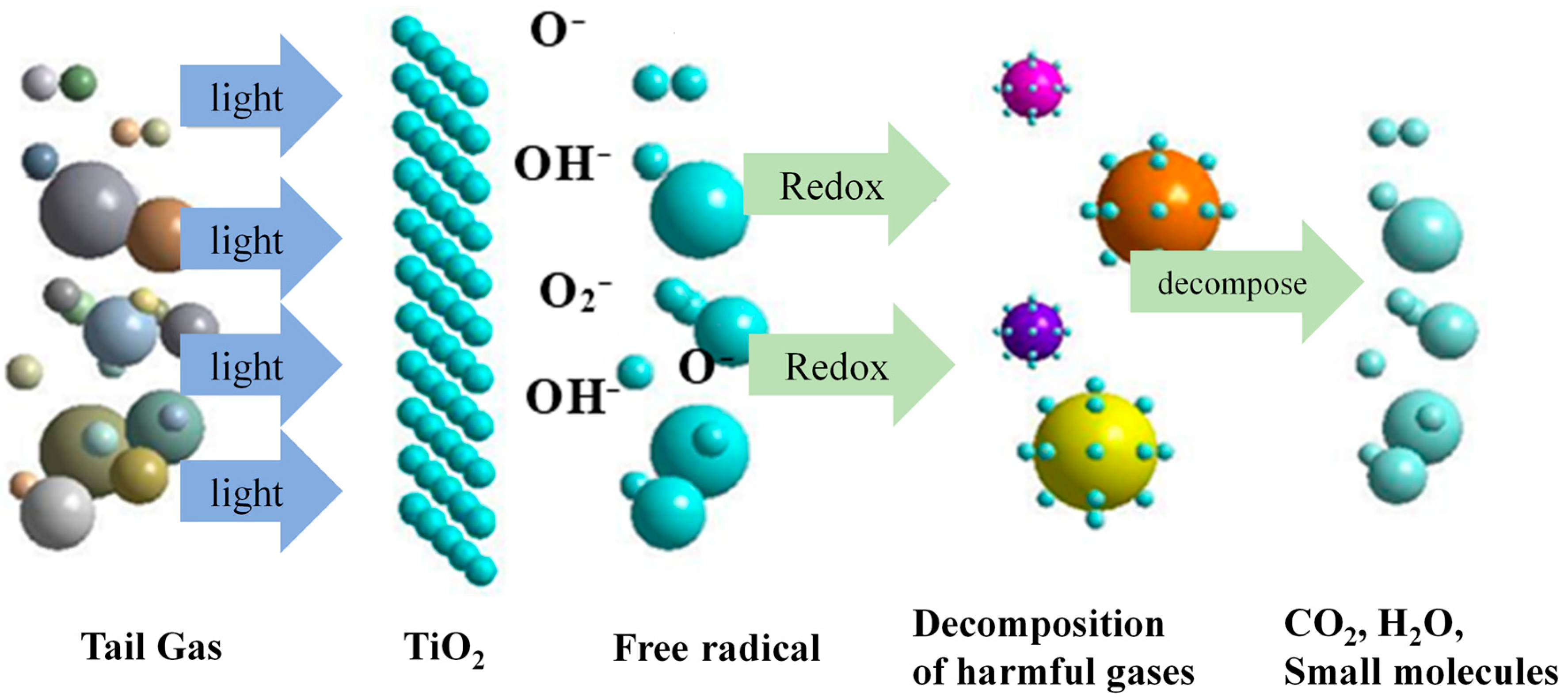
4. Assessment of Tail Gas Degradation
Self-Cleaning Performance Evaluation
5. Conclusions
- The multifunctional road marking coating in the tunnel developed in this study effectively reduces the thermal conductivity of the carbon layer during the combustion process through the physical changes in the flame retardant system. The heat resistance is formed by nano SiO2 with high fracture bonding energy and has good high-temperature stability by forming titanium pyrophosphate with a ceramic-like structure with nano TiO2, which is conducive to the enhancement of high-temperature stability whilst not producing hazardous volatile gases.
- N–V co-doping reduces the forbidden band energy gap of TiO2, broadens the absorption range of visible light by nano TiO2, and improves the catalytic efficiency of visible light. The degradation rates of multifunctional road marking coating in tunnels for the four harmful components NOx, HC, CO, and CO2 in automobile exhaust fumes are 0.0044 min−1, 0.0014 min−1, 0.00044 min−1, and 0.00051 min−1, respectively. The degradation efficiencies of NOx, HC, CO, and CO2 reached 23.4%, 8.3%, 2.5%, and 2.9%, respectively, with better absorption of automobile exhaust.
- The solid–liquid phase separation in the multifunctional road marking coating in the tunnel causes the nano SiO2 and nano TiO2 particles to form a pile up on the surface of the paint, which in turn constructs a microstructure similar to the “micro–nanometer micro-convex” on the surface of the lotus leaf. The contact angle of water droplets on the coating surface reaches 134.2°, which has better hydrophobic self-cleaning performance.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, Y.; Wang, B.; Jiang, J.; Li, H.; Chen, W.; Ye, J. Study on evaluation model of residual bearing capacity of shield tunnel lining structures after fire. China J. Highw. Transp. 2023, 1–18. Available online: http://kns.cnki.net/kcms/detail/61.1313.U.20231110.0911.004.html (accessed on 1 February 2024).
- Chen, H.; Liu, T.; You, X.; Yuan, D.; Ping, Y.; Zhang, Q. Experimental investigation on fire damage to staggered segmental lining of shield tunnel. Tunn. Undergr. Space Technol. 2023, 141, 105359. [Google Scholar] [CrossRef]
- Li, Q.; Yao, J.; Liang, T. Experimental study on the effect of fireproof coating and cooling methods on the mechanical properties of concrete exposed to high temperature. Constr. Build. Mater. 2023, 376, 131045. [Google Scholar] [CrossRef]
- Nakamura, T.; Nomura, Y.; Tanaka, A. Study on Spraying Method Using Fireproof Coating Spraying Robot. AIJ J. Technol. Des. 2023, 29, 639–644. [Google Scholar] [CrossRef]
- Moosaei, H.R.; Zareei, A.R.; Salemi, N. Elevated Temperature Performance of Concrete Reinforced with Steel, Glass, and Polypropylene Fibers and Fire-proofed with Coating. Int. J. Eng. Trans. A Basics 2022, 35, 917–930. [Google Scholar] [CrossRef]
- Li, Q.; Li, H.; Liang, T. Effect of non-intumescent fireproof coating on the durability of concrete exposed to elevated temperature. Constr. Build. Mater. 2023, 404, 133211. [Google Scholar] [CrossRef]
- Wang, Z.F.; Cheng, W.C.; Wang, Y.Q. Investigation into geohazards during urbanization process of Xi’an, China. Nat. Hazards 2018, 92, 1937–1953. [Google Scholar] [CrossRef]
- Qiao, R.; Shao, Z.; Wei, W.; Zhang, Y.Y. Theoretical Investigation into the Thermo-Mechanical Behaviours of Tunnel Lining during RABT Fire Development. Arab. J. Sci. Eng. 2019, 44, 4807–4818. [Google Scholar] [CrossRef]
- Zhang, Y.; Weng, X.; Song, Z.; Sun, Y. Modeling of Loess Soaking Induced Impacts on a Metro Tunnel Using a Water Soaking System in Centrifuge. Geofluids 2019, 2019, 5487952. [Google Scholar] [CrossRef]
- Qiu, J.; Yang, T.; Wang, X.; Wang, L.; Zhang, G. Review of the flame retardancy on highway tunnel asphalt pavement. Constr. Build. Mater. 2019, 195, 468–482. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhong, H.; Fang, Y.; Zhang, W.; Liu, K.; Fang, J. Rockburst Prediction Model Based on Entropy Weight Integrated with Grey Relational BP Neural Network. Adv. Civil. Eng. 2019, 2019, 3453614. [Google Scholar] [CrossRef]
- Kim, J.H.J.; Lim, Y.M.; Won, J.P.; Park, H.G. Fire resistant behavior of newly developed bottom-ash-based cementitious coating applied concrete tunnel lining under RABT fire loading. Constr. Build. Mater. 2010, 24, 1984–1994. [Google Scholar] [CrossRef]
- Alhawat, H.; Hamid, R.; Baharom, S.; Azmi, M.R.; Kaish, A.B.M.A. Thermal behaviour of unloaded concrete tunnel lining through an innovative large-scale tunnel fire experimental testing setup. Constr. Build. Mater. 2021, 283, 122718. [Google Scholar] [CrossRef]
- Radzi, N.A.M.; Hamid, R.; Mutalib, A.A. A Review of Methods, Issues and Challenges of Small-scale Fire Testing of Tunnel Lining Concrete. J. Appl. Sci. 2016, 16, 293–301. [Google Scholar] [CrossRef]
- Ibrahim, R.K.; Hamid, R.; Taha, M.R. Fire resistance of high-volume fly ash mortars with nanosilica addition. Constr. Build. Mater. 2012, 36, 779–786. [Google Scholar] [CrossRef]
- Abhilash, P.P.; Nayak, D.K.; Sangoju, B.; Kumar, R.; Kumar, V. Effect of nano silica in concrete; a review. Constr. Build. Mater. 2021, 278, 122347. [Google Scholar] [CrossRef]
- Pereyra, A.M.; Canosa, G.; Giudice, C.A. Nanostructured protective coating systems, fireproof and environmentally friendly, suitable for the protection of metallic substrates. Ind. Eng. Chem. Res. 2010, 49, 2740–2746. [Google Scholar] [CrossRef]
- Zhang, H.J.; Wang, Y.C.; Li, F.; Zhao, J.P. In-situ polymerized zinc phytate chelated Si-C-P geopolymer hybrid coating constructed by incorporating chitosan oligosaccharide and DOPO for flame-retardant plywood. Constr. Build. Mater. 2023, 397, 132416. [Google Scholar] [CrossRef]
- Ng, Y.H.; Suri, A.; Dasari, A.; Tan, K.H. Thermal decomposition and fire response of non-halogenated polymer-based thermal coatings for concrete structures. Surf. Coat. Technol. 2017, 320, 396–403. [Google Scholar] [CrossRef]
- Abniki, M.; Shirkavand Hadavand, B.; Najafi, F.; Ghasedi, I. Synthesis of the effective flame retardant via modification of epoxy resin with phenylboronic acid. J. Macromol. Sci. Part. A 2022, 59, 411–420. [Google Scholar] [CrossRef]
- Wu, J.; Luo, Y.; Qin, Z. Composite-modified nano TiO2 for the degradation of automobile exhaust in tunnels. Constr. Build. Mater. 2023, 408, 133805. [Google Scholar] [CrossRef]
- Oliveira, H.G.; Silva, E.D.; Longo, C. Electrochemical and photocatalytic properties of TiO2/WO3 photoelectrodes. In Solar Hydrogen and Nanotechnology V; SPIE: San Diego, CA, USA, 2010; Volume 7770, pp. 11–19. [Google Scholar] [CrossRef]
- Luu, C.L.; Nguyen, Q.T.; Ho, S.T. Synthesis and characterization of Fe-doped TiO2 photocatalyst by the sol-gel method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2010, 1, 015008. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Peng, T.; You, H.; Zeng, L.; Qin, Y. Preparation and Visible Photocatalytic Properties of N-Doped TiO2/Muscovite Nanocomposites. Clays Clay Miner. 2021, 69, 254–262. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, K.; Chong, D.; Niu, D.; Lin, P.; Liu, X.; Niu, Y.; Jing, R. Evaluation of photocatalytic micro-surfacing mixture: Road performance, vehicle exhaust gas degradation capacity, and environmental impacts. Constr. Build. Mater. 2022, 345, 128367. [Google Scholar] [CrossRef]
- Liu, G.; Xia, H.; Niu, Y.; Zhao, X.; Zhang, G.; Song, L.; Chen, H. Photocatalytic performance of doped TiO2/AC coating and its UV stability research. Prog. Org. Coat. 2020, 148, 105882. [Google Scholar] [CrossRef]
- Xia, H.; Liu, G.; Zhang, R.; Song, L.; Chen, H. The photocatalytic degradation of vehicle exhausts by an Fe/N/Co-TiO2 waterborne coating under visible light. Materials 2019, 12, 3378. [Google Scholar] [CrossRef]
- Liu, W.; Wang, S.; Zhang, J.; Fan, J. Photocatalytic degradation of vehicle exhausts on asphalt pavement by TiO2/rubber composite structure. Constr. Build. Mater. 2015, 81, 224–232. [Google Scholar] [CrossRef]
- Lu, C.; Zheng, M.; Liu, J.; Zhu, R.; Su, Y. Characterization of self-cleaning pavement coatings with catalytic-hydrophobic synergistic effects. Constr. Build. Mater. 2023, 397, 132246. [Google Scholar] [CrossRef]
- Jiang, B.; Zu, X.; Tang, F.; Wu, Z.; Lu, J.; Wei, Q.; Zhang, X. Surface modification on nanoscale titanium dioxide by radiation: Preparation and characterization. J. Appl. Polym. Sci. 2006, 100, 3510–3518. [Google Scholar] [CrossRef]
- Qi, Y.; Chen, S.; Sun, H.; Zhang, J. A multifunctional cool material with contamination-resistant and anti-icing characters based on the application of fluorinated TiO2. Sol. Energy Mater. Sol. Cells 2019, 201, 110091. [Google Scholar] [CrossRef]
- Qi, Y.; Chen, S.; Zhang, J. Fluorine modification on titanium dioxide particles: Improving the anti-icing performance through a very hydrophobic surface. Appl. Surf. Sci. 2019, 476, 161–173. [Google Scholar] [CrossRef]
- Qi, Y.; Xiang, B.; Zhang, J. Effect of titanium dioxide (TiO2) with different crystal forms and surface modifications on cooling property and surface wettability of cool roofing materials. Sol. Energy Mater. Sol. Cells 2017, 172, 34–43. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, H.; Zhu, H.; Guo, Y. Ultra-hydrophobic modification of TiO2 nanoparticles via thermal decomposition of polytetrafluoroethylene. Powder Technol. 2014, 253, 548–552. [Google Scholar] [CrossRef]
- Xi, Y.; Qi, Y.; Mao, Z.; Yang, Z.; Zhang, J. Surface hydrophobic modification of TiO2 and its application to preparing PMMA/TiO2 composite cool material with improved hydrophobicity and anti-icing property. Constr. Build. Mater. 2021, 266, 120916. [Google Scholar] [CrossRef]
- GB/T31815-2015; Self-Cleaning Coatings for Exterior Surfaces of Buildings. Code of China: Beijing, China, 2015.








| Ingredient | Content (%) | Provenience | Ingredient | Content (%) | Provenience |
|---|---|---|---|---|---|
| Polyacrylic Resin Component A | 19.73 | Xuchuan Chemical (Suzhou) Co., Ltd., Suzhou, China | Ammonium Polyphosphate | 3.99 | Sinopharm Chemical Reagent Co., Ltd., Shanghai, China |
| Curing agent component B | 15.51 | Xuchuan Chemical (Suzhou) Co., Ltd., Suzhou, China | Pentaerythritol | 3.46 | Sinopharm Chemical Reagent Co., Ltd., Shanghai, China |
| Aluminum Hydroxide | 7.24 | Zibo Aotai new material technology CO., Ltd., Zibo, China | Melamine | 3.24 | Sinopharm Chemical Reagent Co., Ltd., Shanghai, China |
| Expanded Vermiculite | 2.16 | Nantong Xingchen Synthetic Material, Co., Ltd., Nantong, China | Reagent | 7.82 | Jiangsu Baoying Asphalt Technology Co., Ltd., Baoying, China |
| Modified nano TiO2 | 10.46 | Jiangsu Chuangwei Transportation Technology Development Co., Ltd., Nanjing, China | Silicone Water Repellent | 13.94 | Nantong Xingchen Synthetic Material, Co., Ltd., Nantong, China |
| Glass Beads | 3.48 | Jiangsu Chuangwei Transportation Technology Development Co., Ltd., Nanjing, China | Nano SiO2 | 8.97 | Nantong Xingchen Synthetic Material, Co., Ltd., Nantong, China |
| Materials | Characteristic Peak | Starting Temperature (°C) | Peak Temperature (°C) | Termination Temperature (°C) |
|---|---|---|---|---|
| (a) Road marking coating base material | 1 | 173 | 187 | 208 |
| 2 | 206 | 240 | 278 | |
| 3 | 278 | 297 | 316 | |
| 4 | 339 | 361 | 420 | |
| 5 | 420 | 510 | 572 | |
| 6 | 641 | 676 | 716 | |
| (b) Multifunctional marking paint | 1 | 143 | 180 | 202 |
| 2 | 202 | 260 | 275 | |
| 3 | 275 | 305 | 310 | |
| 4 | 310 | 635 | 640 |
| Bond-Type | Type of Vibration | Frequency (cm−1) |
|---|---|---|
| -NH- | Stretching vibration | 3500~3300 |
| O=C=O | Asymmetric stretching vibration | 2305, 2358 |
| -C-H- | Symmetric stretching vibration | 3300~3000 |
| -C-H | Out-of-plane bending vibration | 689 |
| -O- | In-plane bending vibration | 1125 |
| -OH | Stretching vibration | 4000~3500 |
| -NH | Out-of-plane bending vibration | 893, 957 |
| -NH | Deformation vibration | 1026 |
| C=O | Stretching vibration | 2185 |
| C=O | Conjugate | 1740~1700 |
| C-O-C | Stretching vibration | 2105 |
| -CH2- | Stretching vibration | 2968 |
| Exhaust Gas Composition | NOx | HC | CO | CO2 |
|---|---|---|---|---|
| Photodegradation efficiency (%) | 23.4% | 8.3% | 2.5% | 2.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quan, X.; Yang, L.; Li, H.; Chen, Y.; Shi, S. Performance Evaluation of a Multifunctional Road Marking Coating for Tunnels Based on Nano SiO2 and TiO2 Modifications. Buildings 2024, 14, 459. https://doi.org/10.3390/buildings14020459
Quan X, Yang L, Li H, Chen Y, Shi S. Performance Evaluation of a Multifunctional Road Marking Coating for Tunnels Based on Nano SiO2 and TiO2 Modifications. Buildings. 2024; 14(2):459. https://doi.org/10.3390/buildings14020459
Chicago/Turabian StyleQuan, Xiujie, Liang Yang, Hui Li, Yan Chen, and Shuang Shi. 2024. "Performance Evaluation of a Multifunctional Road Marking Coating for Tunnels Based on Nano SiO2 and TiO2 Modifications" Buildings 14, no. 2: 459. https://doi.org/10.3390/buildings14020459





