The Properties of Magnesium Silicate Hydrate Prepared from the Magnesium Silicate Minerals in the Earth’s Crust
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
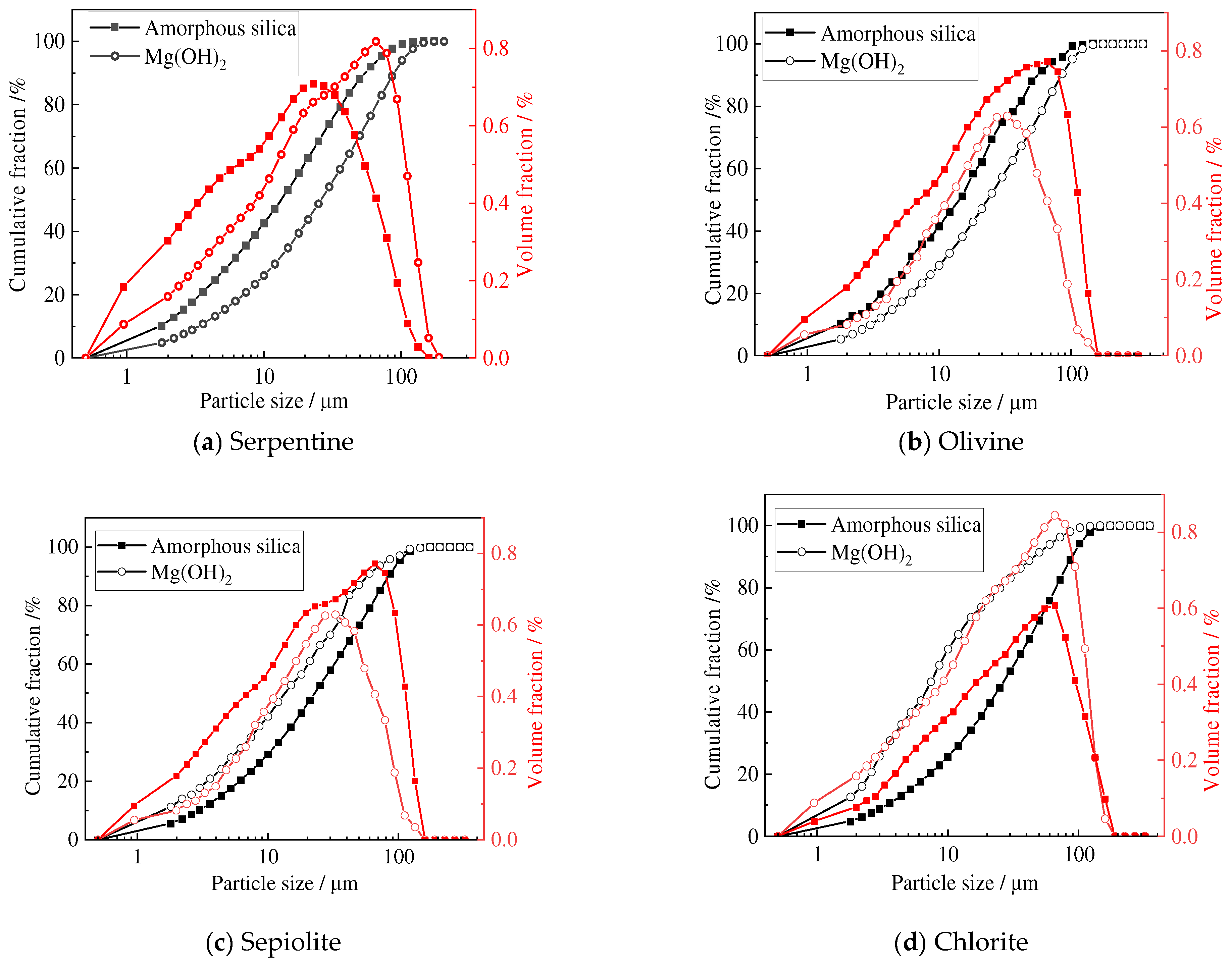
3.1. Composition and Particle Size Distribution of the Residue and Mg(OH)2
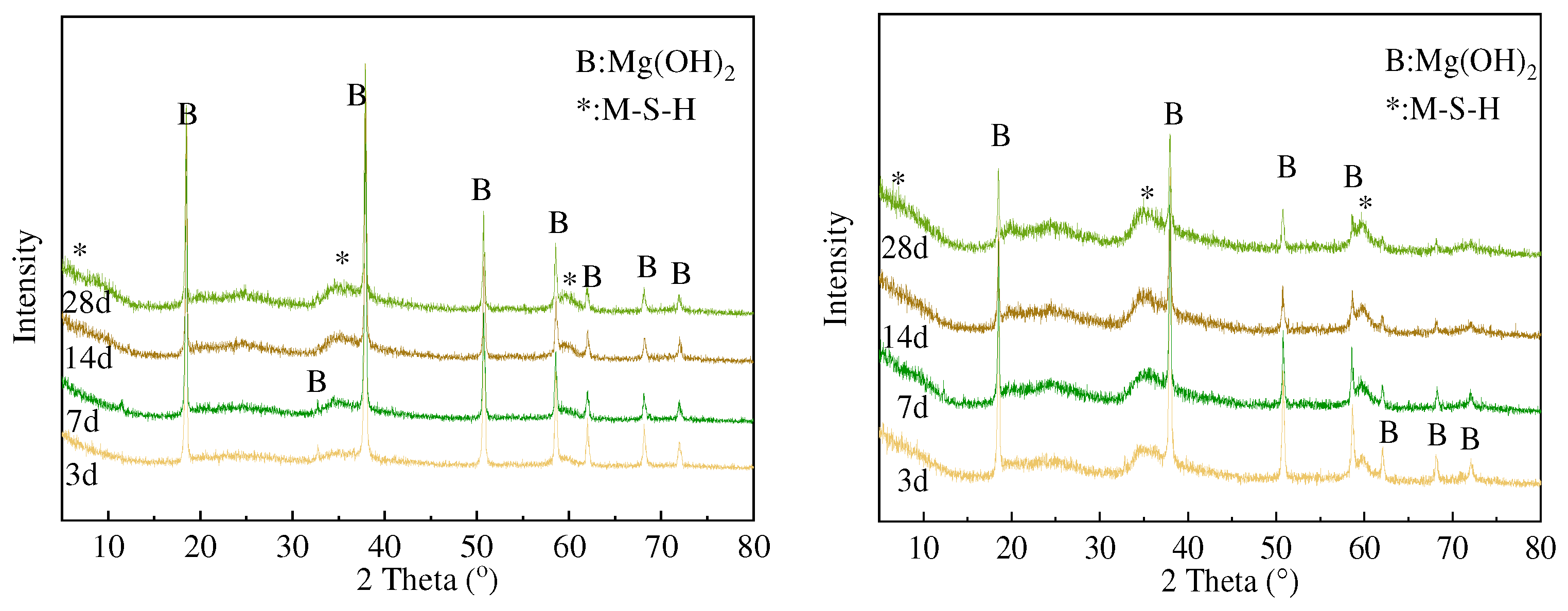
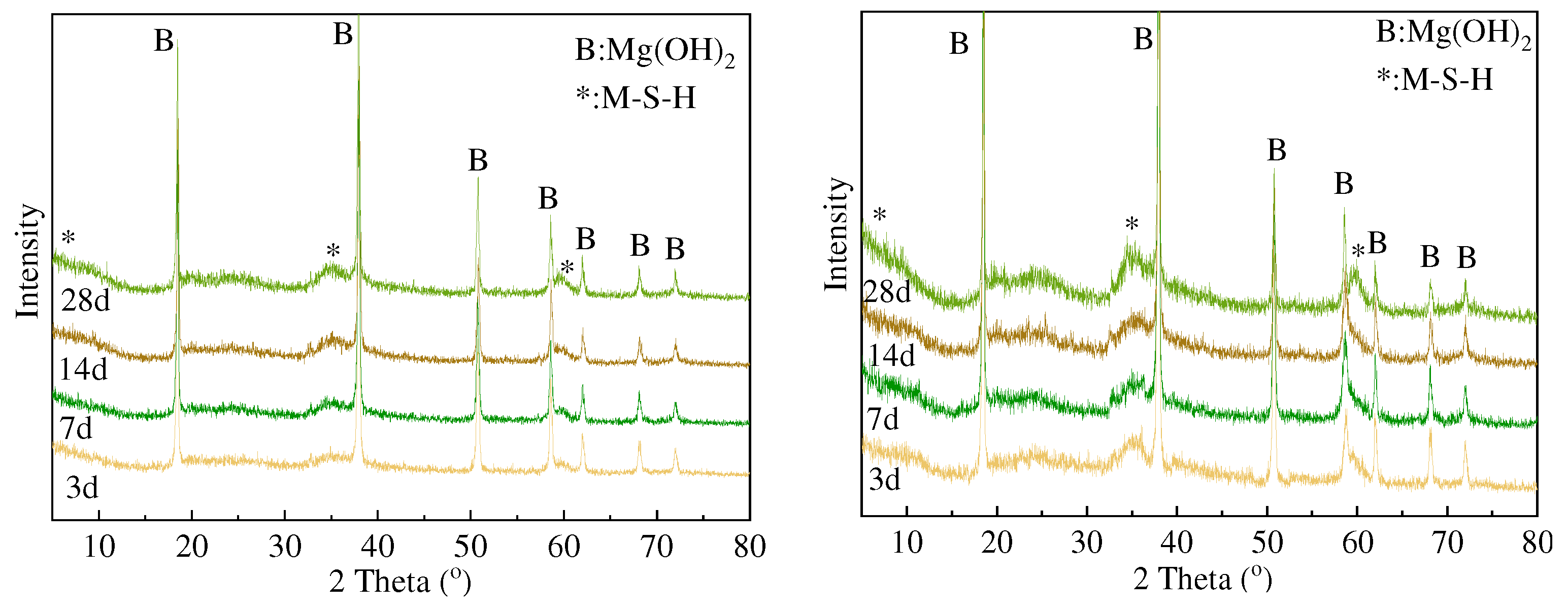
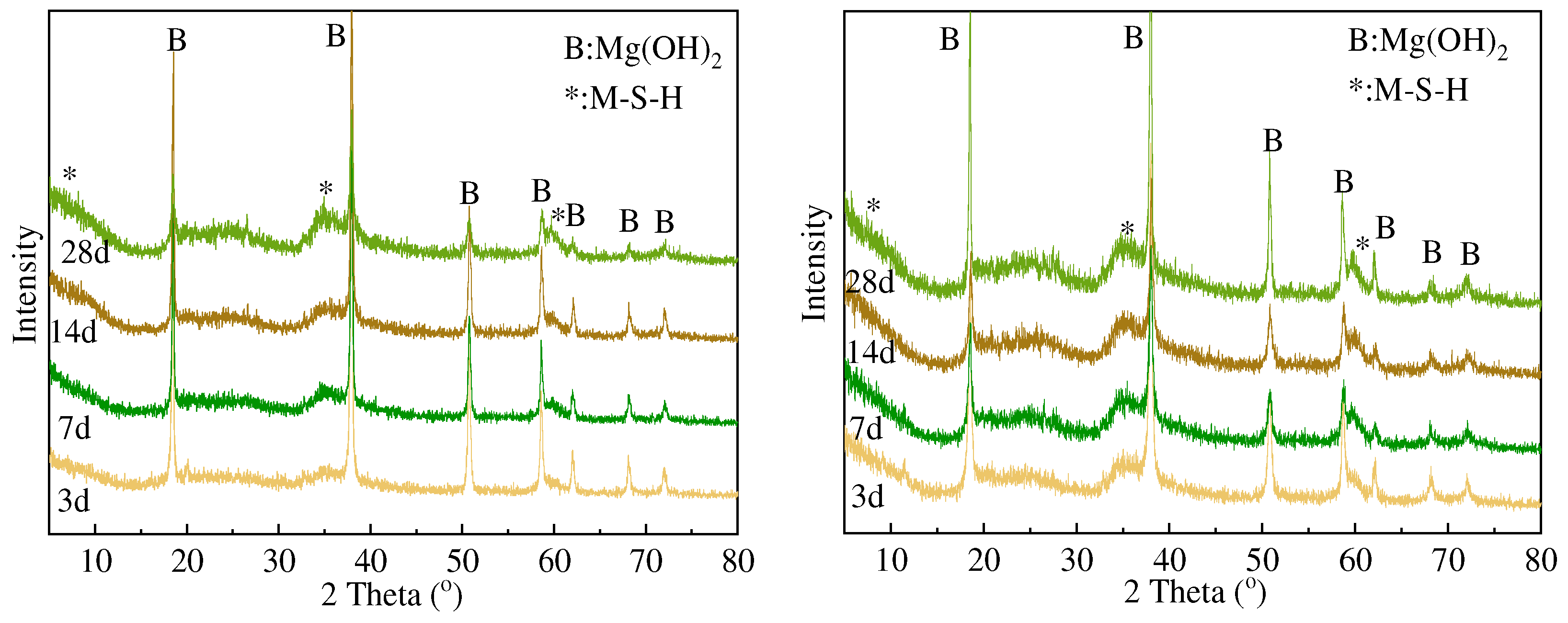
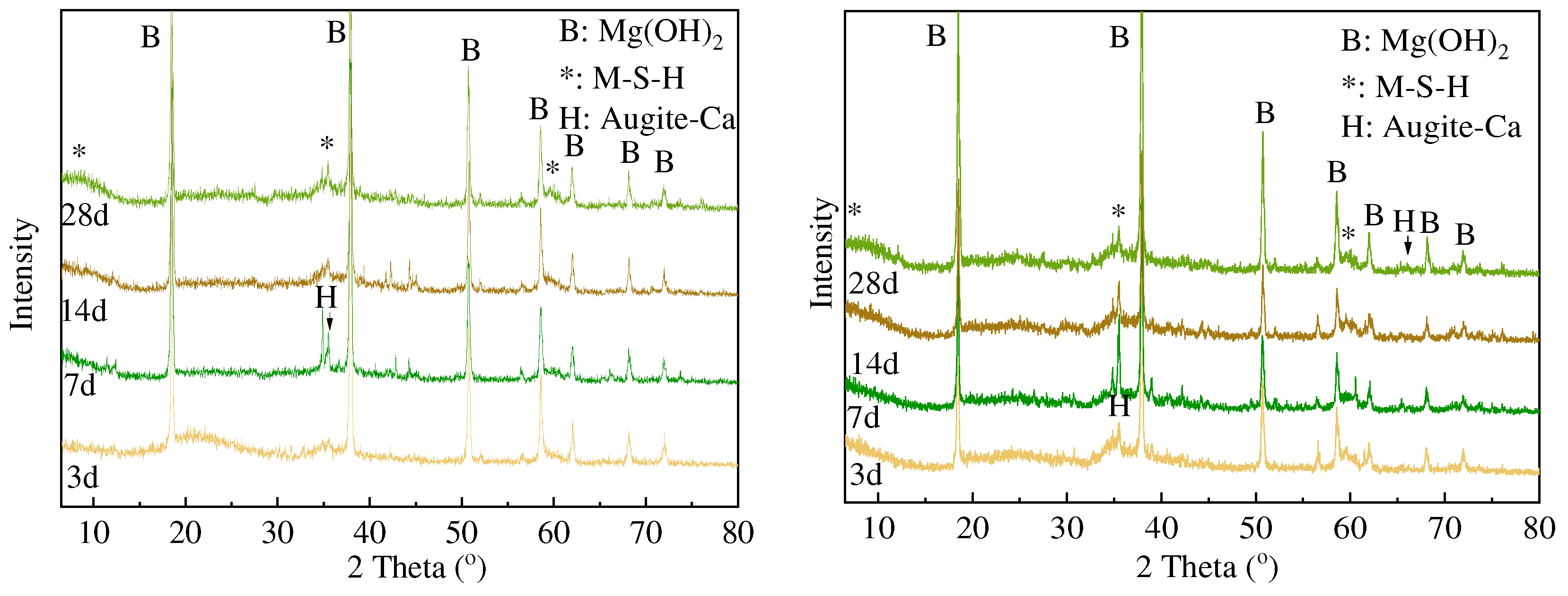
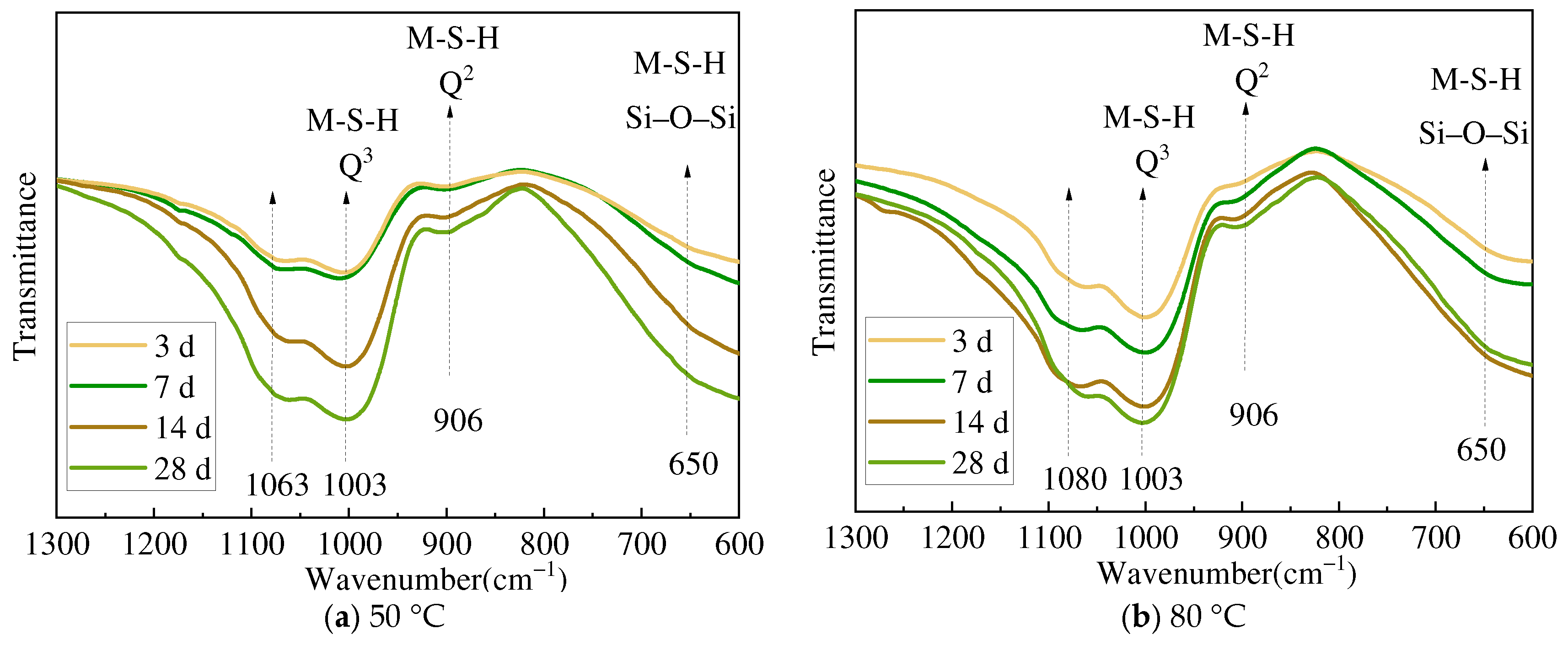
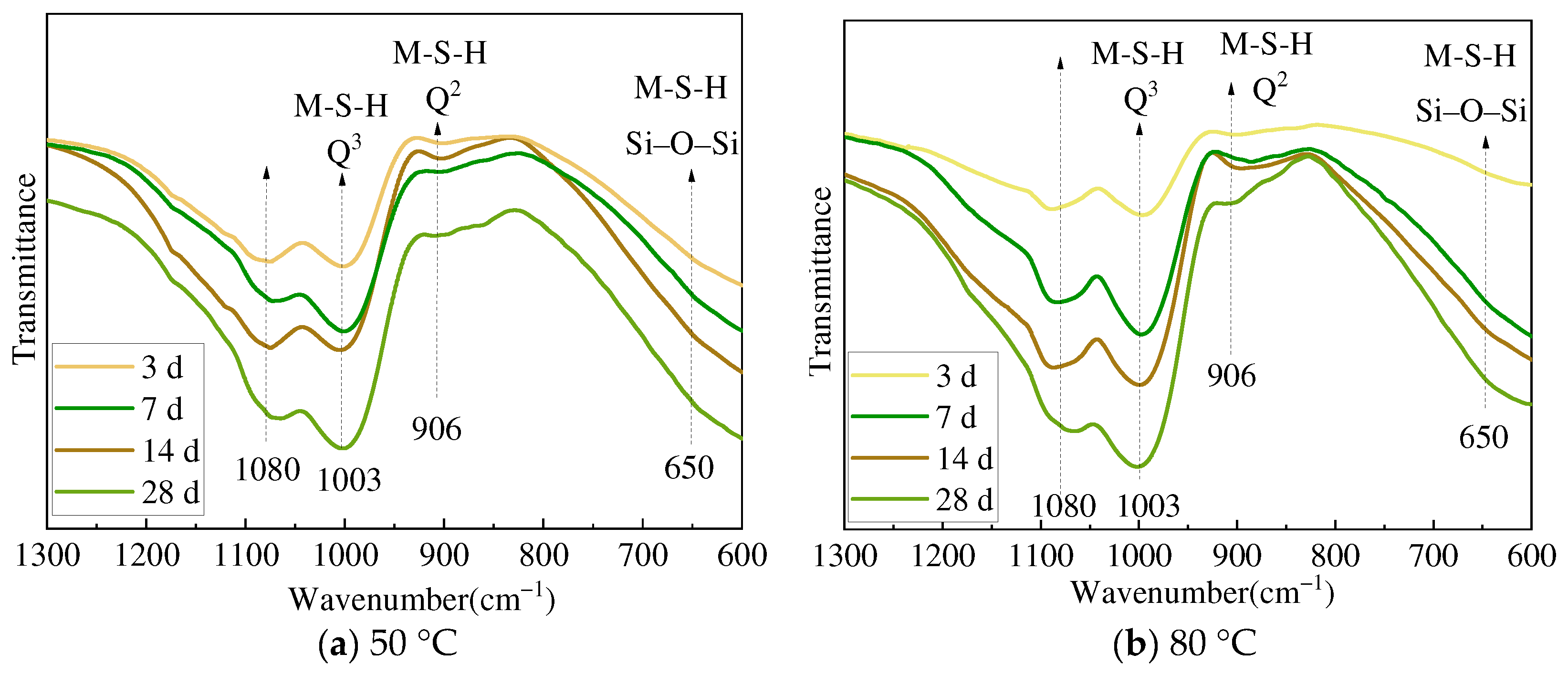
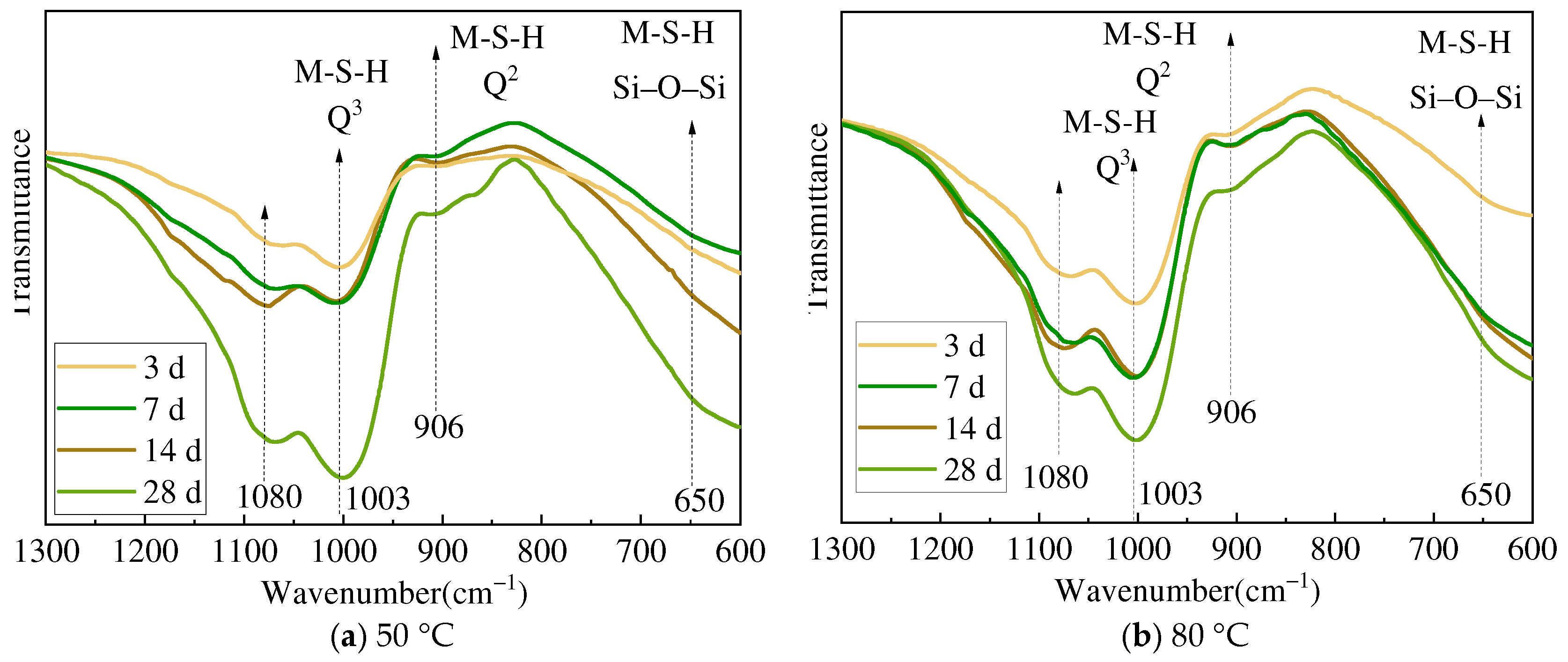
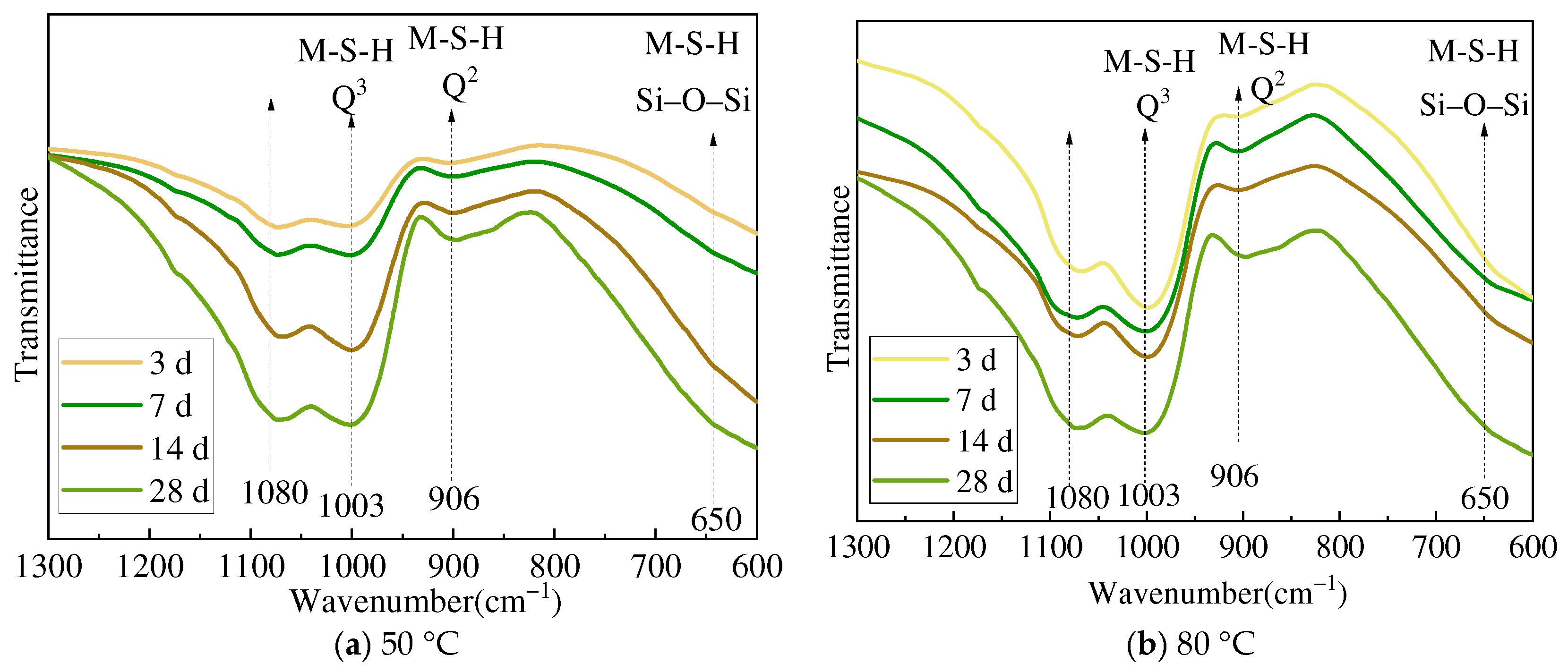
3.2. XRD and FTIR Analysis
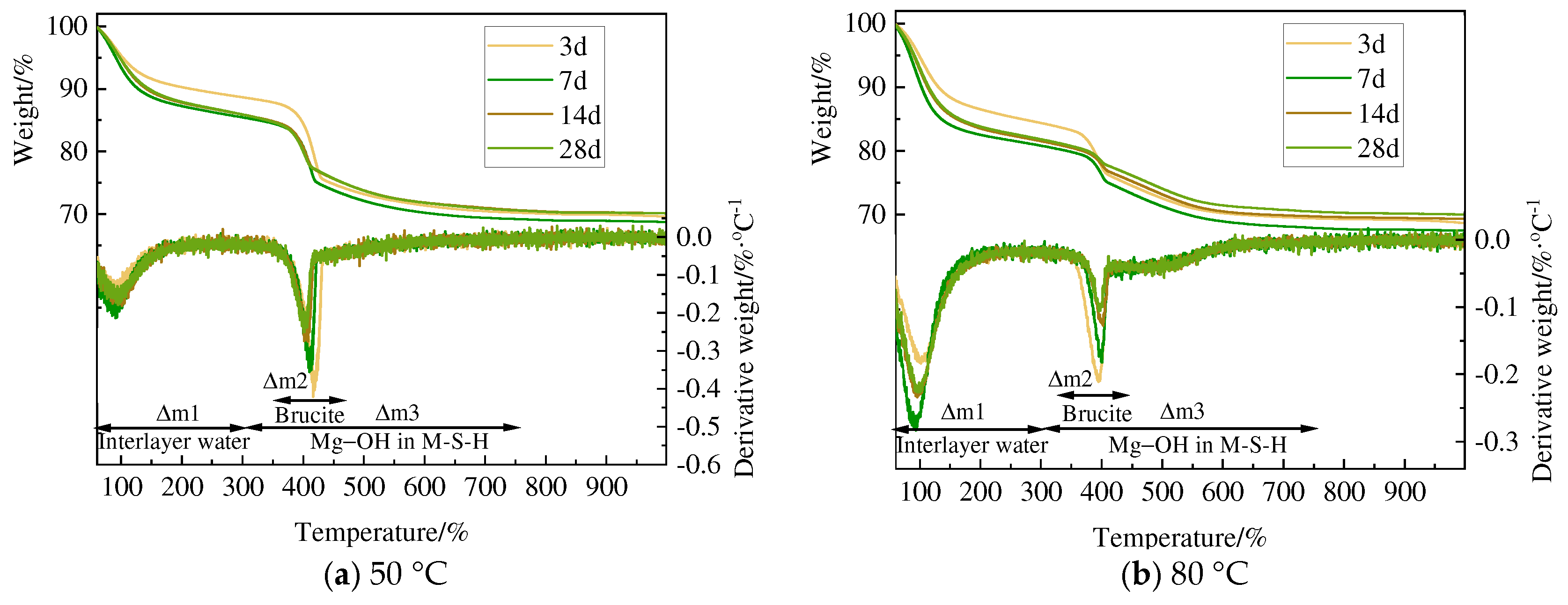
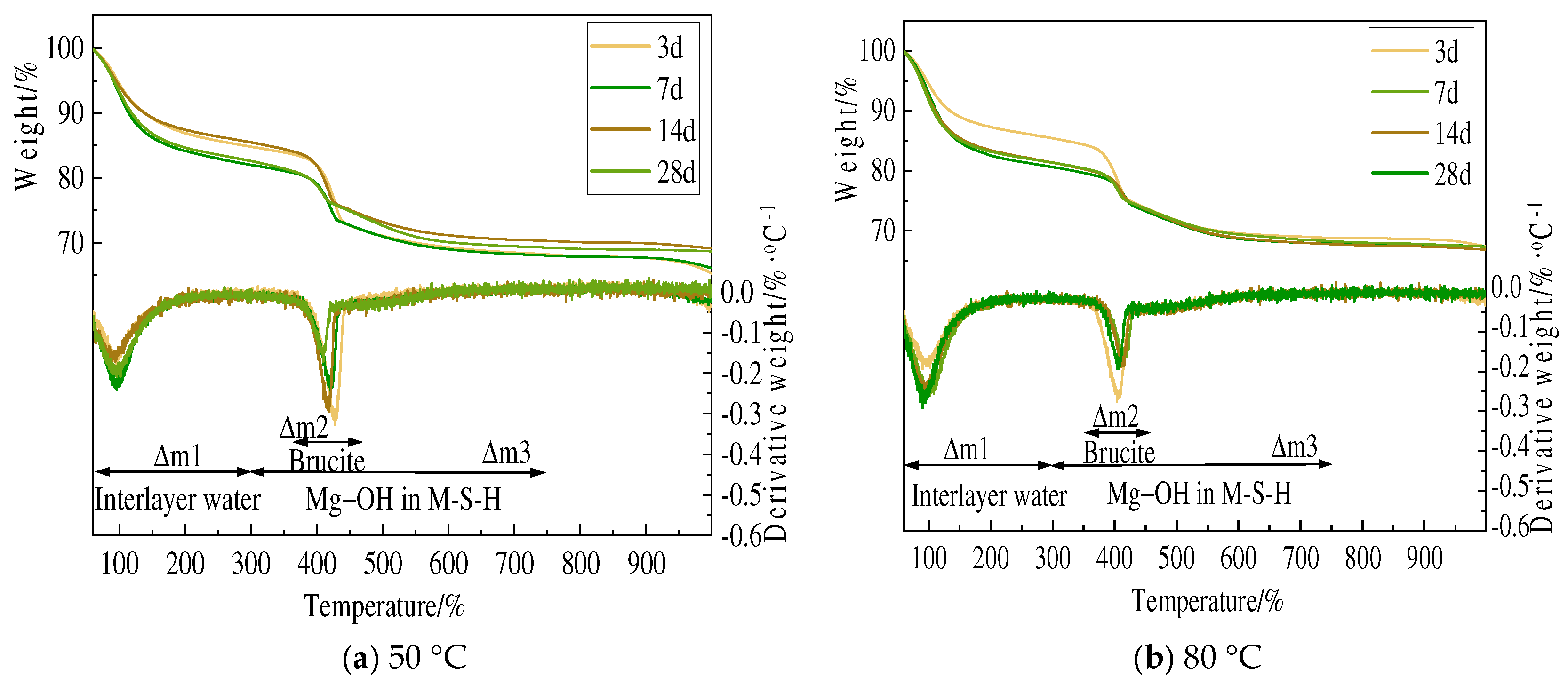
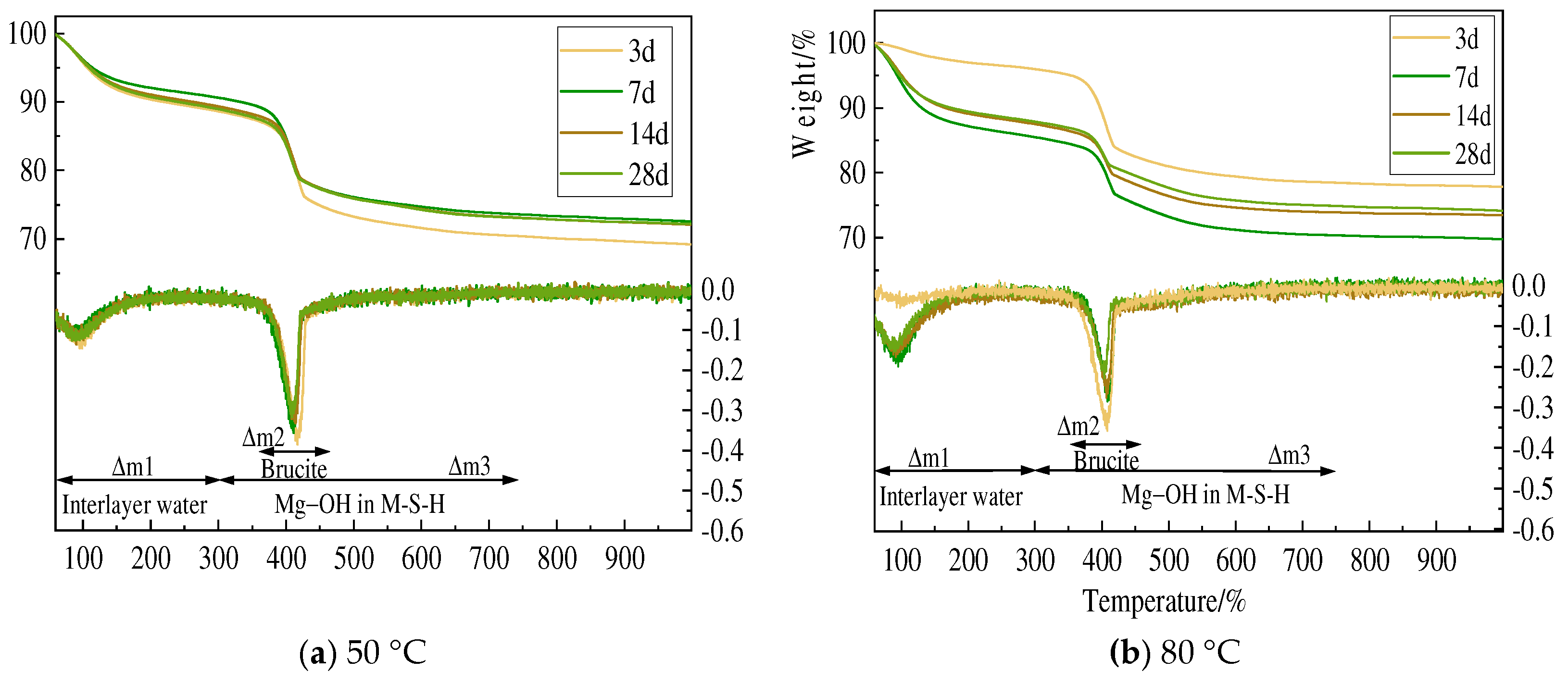
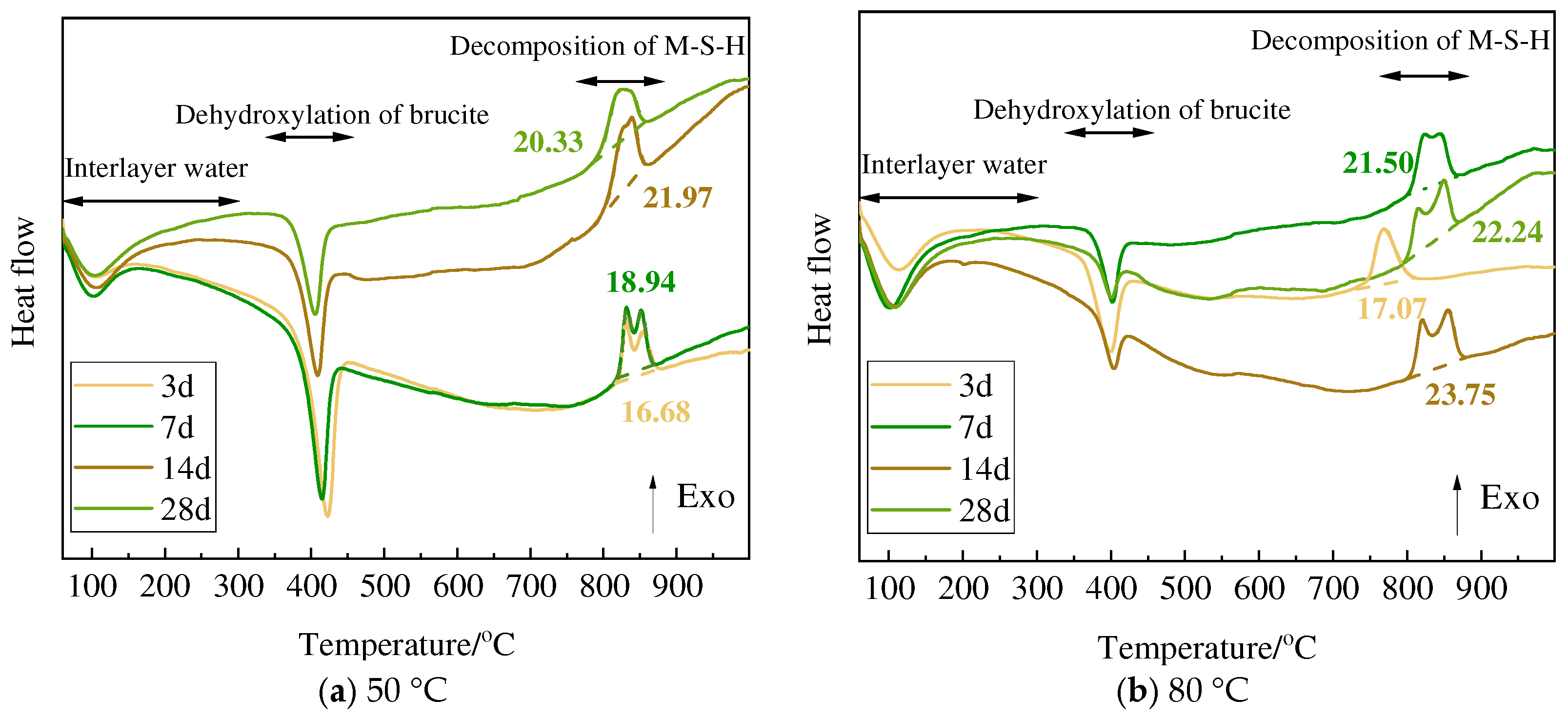
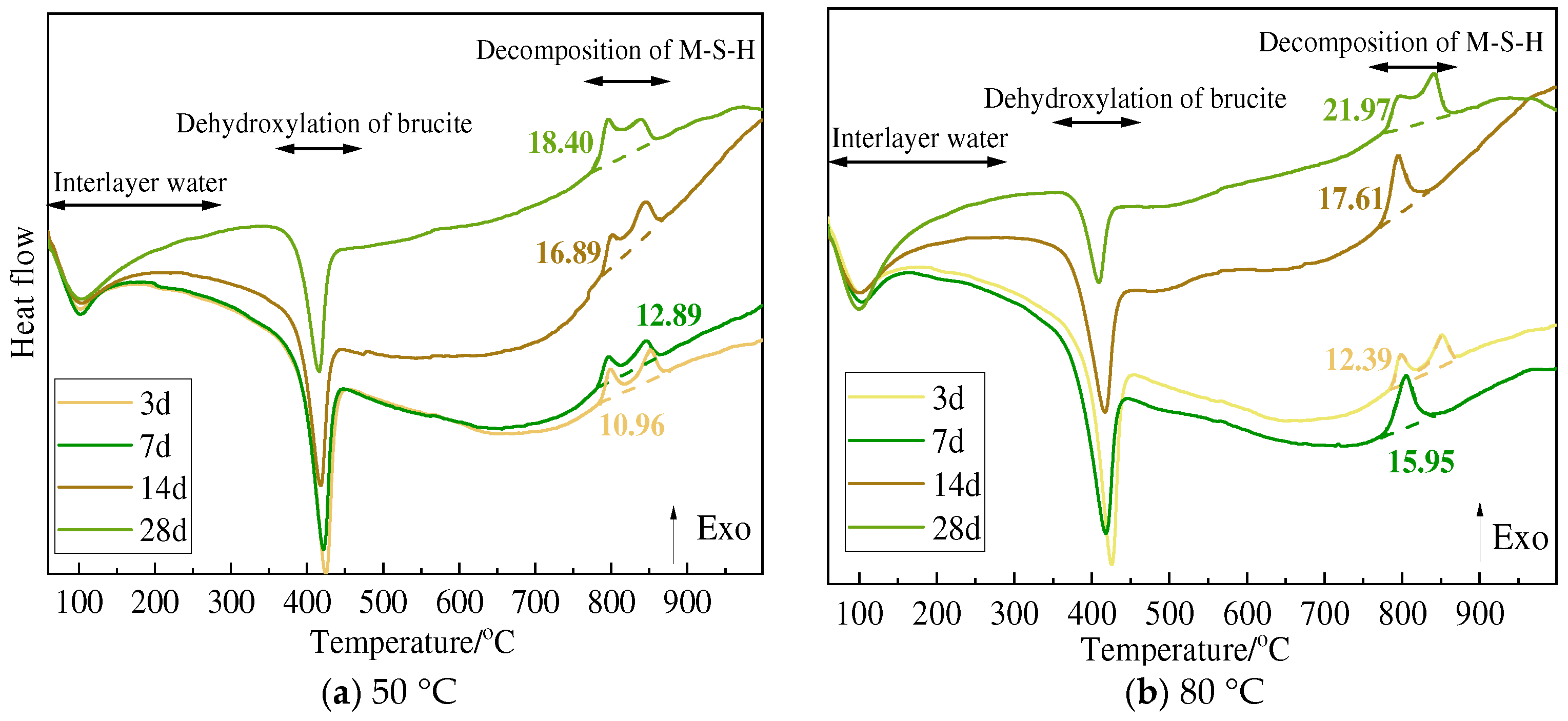
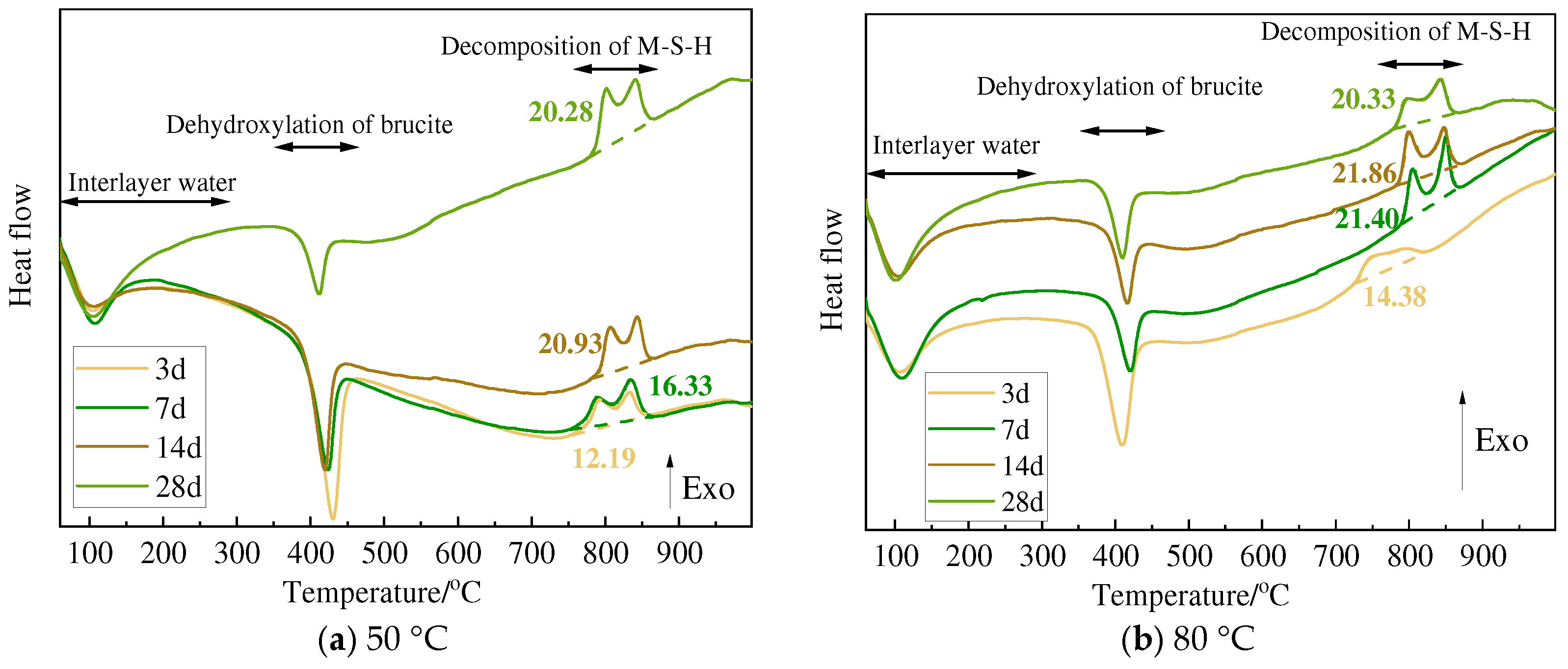
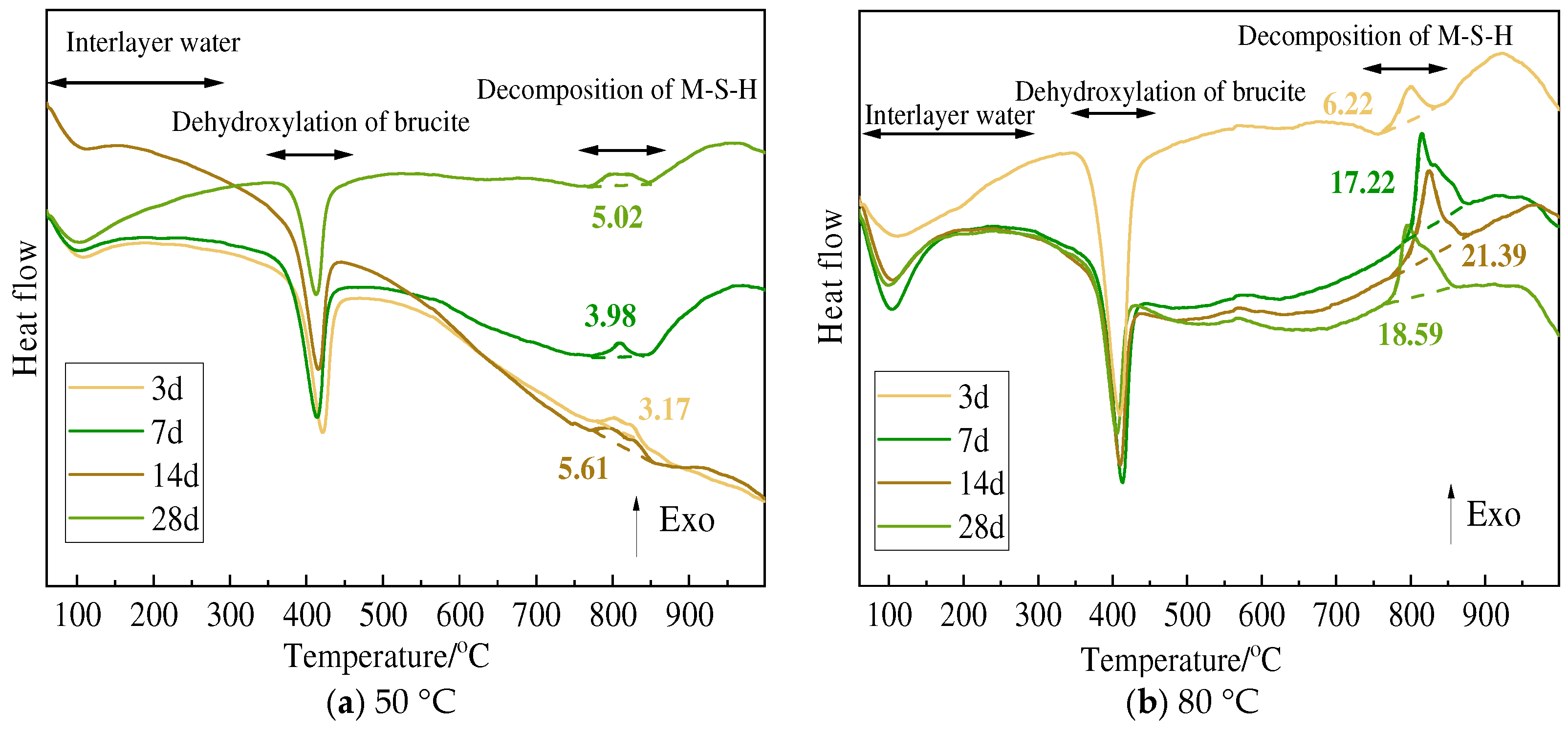
3.3. TG and DSC Analysis
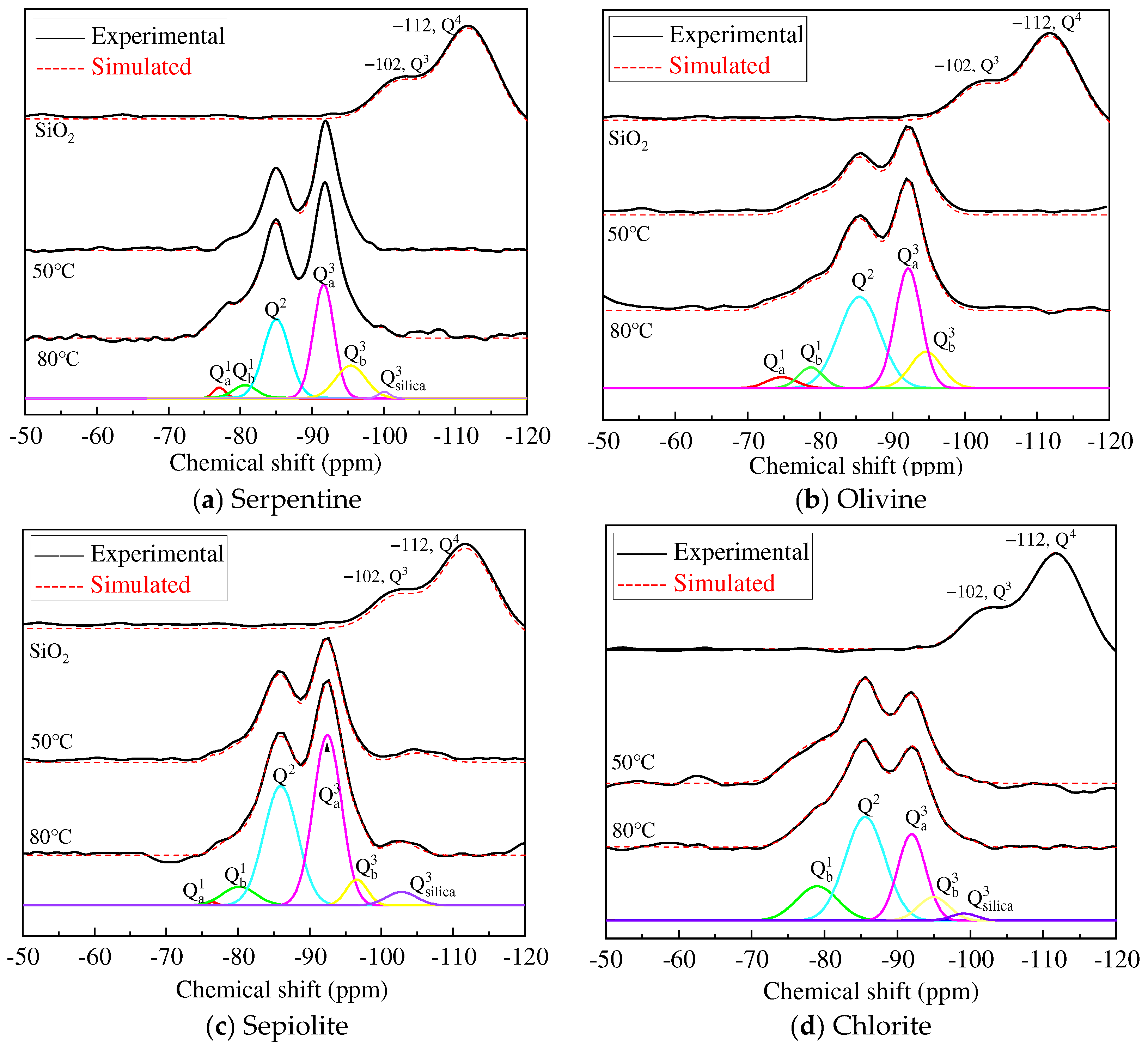
3.4. NMR Analysis
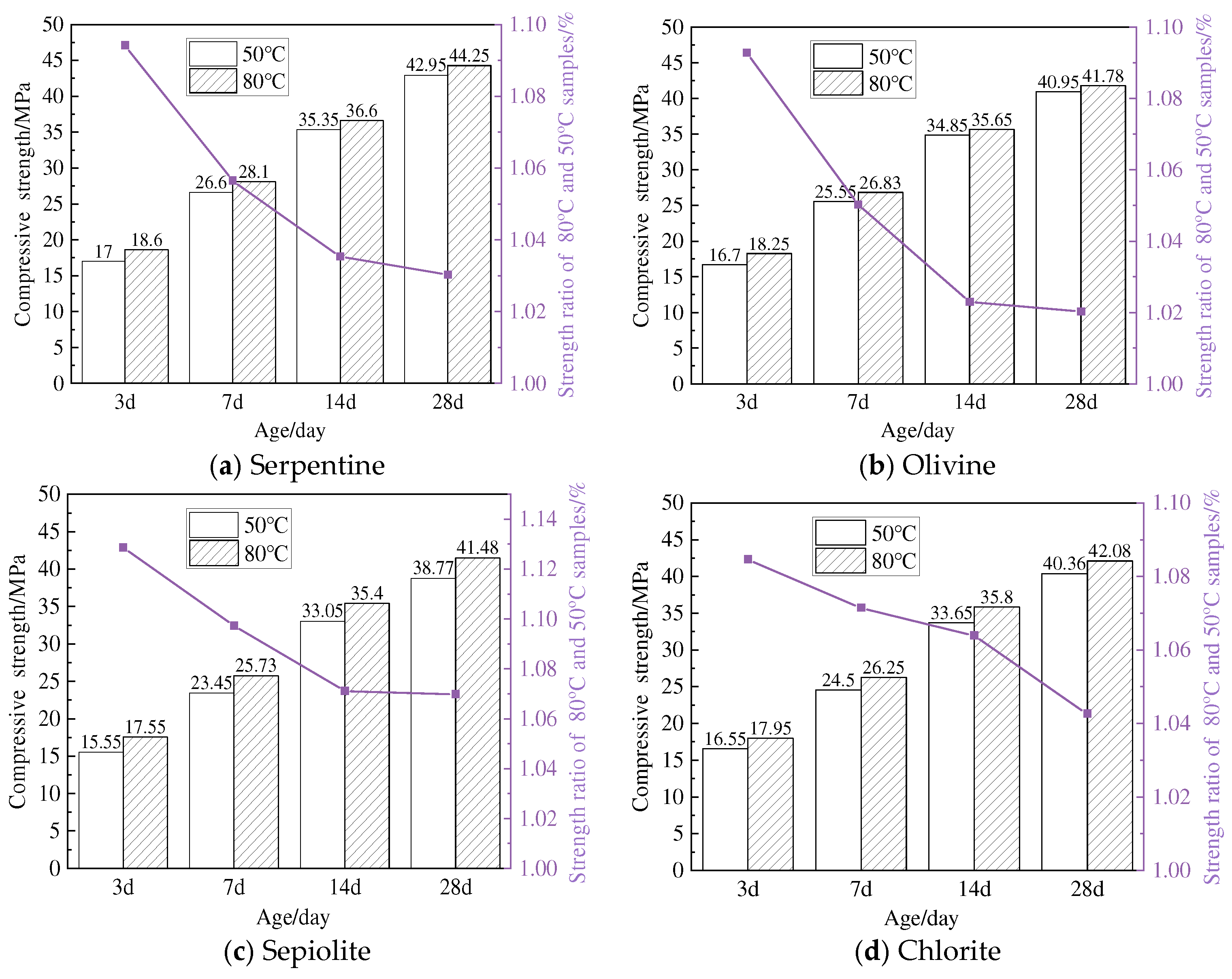
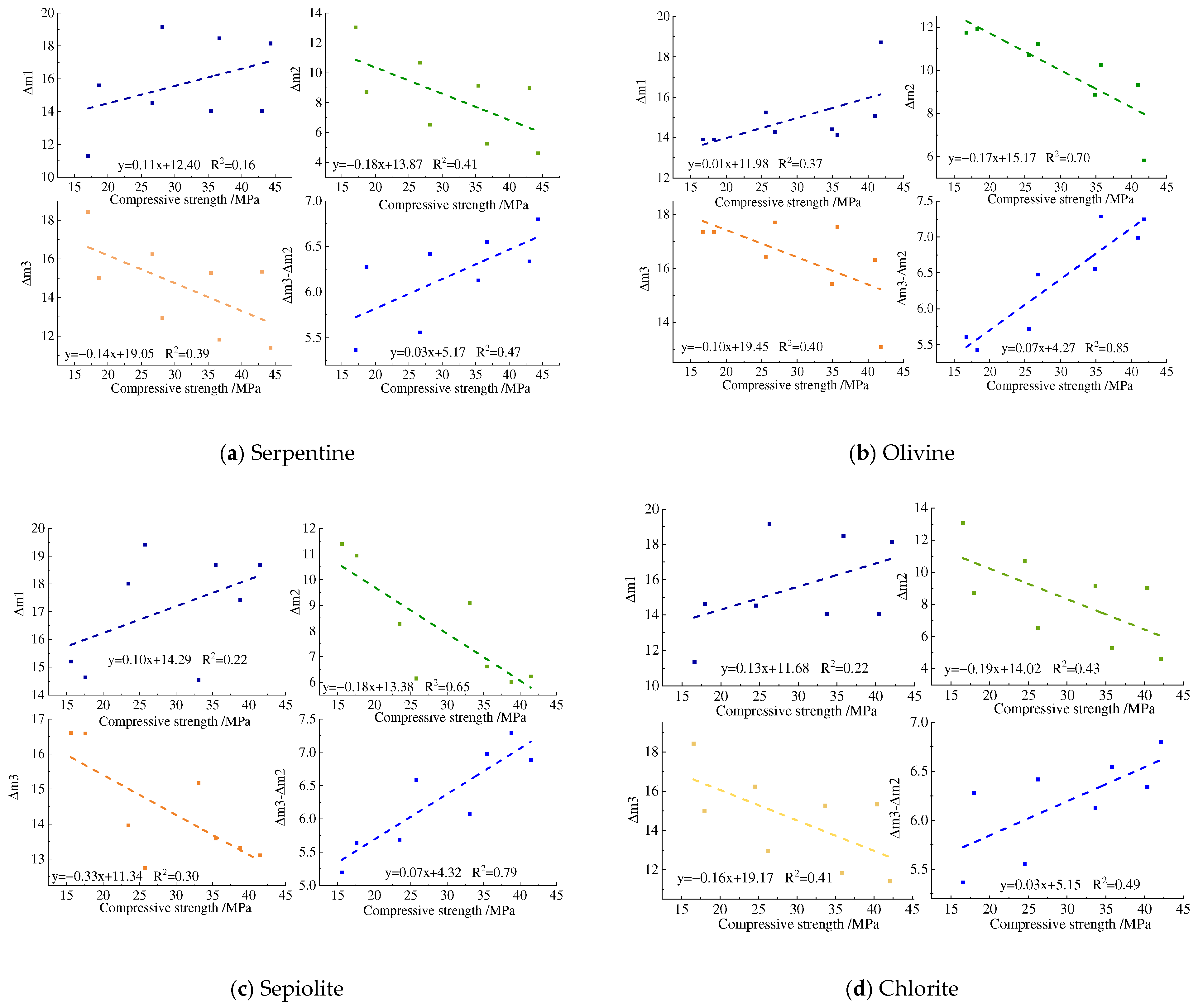
3.5. Compressive Strength Analysis
4. Conclusions
- (1)
- The amorphous silica obtained from the acid leaching of serpentine, olivine, sepiolite, and chlorite reach a purity of over 90%. The silica derived from serpentine, olivine, and sepiolite has an average particle size of about 20 μm, while the silica from acid-leached chlorite has an average particle size of about 65 μm.
- (2)
- Amorphous silica and magnesium oxide prepared using magnesium hydroxide could form magnesium silicate hydrate within 3 days of hydration. Although there are certain numerical differences between the minerals used as raw materials, in general, the hydration reaction accelerates by 10–20% with increased curing time and temperature, and the degree of polymerization of the silicate tetrahedra of newly formed M-S-H increases.
- (3)
- The M-S-H prepared using four types of magnesium silicate minerals forms a silicate tetrahedral structure predominantly characterized by the chrysotile-like T:O structure. The resulting magnesium silicate hydrate cementitious materials could achieve a compressive strength of 40 MPa at 28 days, and the compressive strength shows a good correlation with the reduction in magnesium hydroxide content and the increase in magnesium silicate hydrate.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gartner, E.; Sui, T. Alternative cement clinkers. Cem. Concr. Res. 2018, 114, 27–39. [Google Scholar] [CrossRef]
- Simoni, M.; Woo, C.L.; Zhao, H.; Iuga, D.; Svora, P.; Hanein, T.; Kinoshita, H.; Walkley, B. Reaction mechanisms, kinetics, and nanostructural evolution of magnesium silicate hydrate (M-S-H) gels. Cem. Concr. Res. 2023, 174, 107295. [Google Scholar] [CrossRef]
- Bernard, E.; Lothenbach, B.; Le Goff, F.; Pochard, I.; Dauzères, A. Effect of magnesium on calcium silicate hydrate (C-S-H). Cem. Concr. Res. 2017, 97, 61–72. [Google Scholar] [CrossRef]
- Bernard, E.; Lothenbach, B.; Rentsch, D.; Pochard, I.; Dauzères, A. Formation of magnesium silicate hydrates (M-S-H). Phys. Chem. Earth 2017, 99, 142–157. [Google Scholar] [CrossRef]
- Nied, D.; Enemark-Rasmussen, K.; L‘Hopital, E.; Skibsted, J.; Lothenbach, B. Properties of magnesium silicate hydrates (MSH). Cem. Concr. Res. 2016, 79, 323–332. [Google Scholar] [CrossRef]
- Zhang, T.; Cheeseman, C.; Vandeperre, L. Development of low pH cement systems forming magnesium silicate hydrate (MSH). Cem. Concr. Res. 2011, 41, 439–442. [Google Scholar] [CrossRef]
- Sonat, C.; Unluer, C. Development of magnesium-silicate-hydrate (M-S-H) cement with rice husk ash. J. Clean. Prod. 2019, 211, 787–803. [Google Scholar] [CrossRef]
- Du, Y. Preparation of Magnesium-Silicate-Hydrate Cement by Replacing Silica Fume with Pulverized Fuel Ash. Ph.D. Thesis, Dalian University of Technology, Dalian, China, 2016. [Google Scholar]
- Chen, S.; Wang, L. Mechanical properties and reaction products of reactive MgO modified circulating fluidized bed combustion slag-silica fume composites. Acta Mater. Compos. Sin. 2018, 35, 1288–1297. [Google Scholar]
- Abbdel-Gawwad, H.A.; El-Aleem, S.A.; Amer, A.A.; El-Didamony, H.; Arif, M.A. Combined impact of silicate-amorphicity and MgO-reactivity on the performance of Mg-silicate cement. Construct Build. Mater. 2018, 189, 78–85. [Google Scholar] [CrossRef]
- Du, Y.C.; Wang, X.K.; Wu, J.S.; Wang, J.S.; Li, Y.; Dai, H.X. Mg3Si4O10(OH)2 and MgFe2O4 in situ grown on diatomite: Highly efficient adsorbents for the removal of Cr(VI). Micropor. Mesopor. Mat. 2018, 271, 83–91. [Google Scholar] [CrossRef]
- Temuujin, J.; Okada, K.; Mackenzie, K.J.D. Formation of layered magnesium silicate during the aging of magnesium hydroxide-silica mixtures. J. Am. Ceram. Soc. 2010, 81, 754–759. [Google Scholar] [CrossRef]
- Wedepohl, K.H. The composition of the continental crust. Miner. Mag. 1994, 58, 1217–1232. [Google Scholar] [CrossRef]
- Ruiter, D.; Austrheim, H. Formation of magnesium silicate hydrate cement in nature. J. Geol. Soc. 2018, 175, 308–320. [Google Scholar] [CrossRef]
- Lu, Z.; Wan, P.; Su, G.; Li, H. A Study of the cementitious behaviour of dehydrating serpentine in hydrothermal condition. J. Chin. Ceram. Soc. 1997, 25, 384–388. (In Chinese) [Google Scholar]
- Bernard, E.; Lothenbach, B.; Cau-Dit-Coumes, C.; Pochard, I.; Rentsch, D. Aluminum incorporation into magnesium silicate hydrate (M-S-H). Cem. Concr. Res. 2020, 128, 105931. [Google Scholar] [CrossRef]
- GB/T 17671-1999; Method of Testing Cements—Determination of Strength. State Administration of Quality and Technical Supervision of China: Beijing, China, 1999.
- Li, Z.; Yu, Q.; Chen, X.; Liu, H.; Zhang, J.; Zhang, J.; Yang, Y.; Wei, J. The role of MgO in the thermal behavior of MgO–silica fume pastes. J. Therm. Anal. Calorim. 2017, 127, 1897–1909. [Google Scholar] [CrossRef]
- Brew, D.R.M.; Glasser, F.P. Synthesis and characterisation of magnesium silicate hydrate gels. Cem. Concr. Res. 2005, 35, 85–98. [Google Scholar] [CrossRef]
- Lothenbach, B.; Nied, D.; L‘Hôpital, E.; Achiedo, G.; Dauzères, A. Magnesium and calcium silicate hydrates. Cem. Concr. Res. 2015, 77, 60–68. [Google Scholar] [CrossRef]
- Zhang, T.; Zou, J.; Wang, B.; Wu, Z.; Jia, Y.; Cheeseman, C.R. Characterization of magnesium silicate hydrate (MSH) gel formed by reacting MgO and Silicafume. Materials 2018, 11, 909. [Google Scholar] [CrossRef]
- Zhang, T.; Li, T.; Zou, J.; Li, Y.; Zhi, S.; Jia, Y.; Cheeseman, C.R. Immobilization of radionuclide 133Cs by magnesium silicate hydrate cement. Materials 2019, 13, 146. [Google Scholar] [CrossRef]
- Zhang, S.; Liao, N.; Li, Y.; Chatterjee, A.; Zhang, Y.; Sang, S. M-S-H formation in MgO-SiO2 slurries via wet milling for magnesia based castables. Ceram. Int. 2021, 47, 10880–10886. [Google Scholar] [CrossRef]
- Chabrol, K.; Gressier, M.; Pebere, N.; Menu, M.-J.; Martin, F.; Bonino, J.-P.; Marichal, C.; Brendle, J. Functionalization of synthetic talc-like phyllosilicates by alkoxyorganosilane grafting. J. Mater. Chem. 2010, 20, 9695–9706. [Google Scholar] [CrossRef]
- Park, D.G.; Duchamp, J.C.; Duncan, T.M.; Burlitch, J.M. Preparation of forsterite by pyrolysis of a xerogel: The effect of water. Chem. Mater. 1994, 6, 1990–1995. [Google Scholar] [CrossRef]
- Walling, S.A.; Kinoshita, H.; Bernal, S.A.; Collier, N.C.; Provis, J.L. Structure and properties of binder gels formed in the system Mg(OH)2-SiO2-H2O for immobilisation of Magnox sludge. Dalton Trans. 2015, 44, 8126–8137. [Google Scholar] [CrossRef]
- Roosz, C.; Grangeon, S.; Blanc, P.; Montouillout, V.; Lothenbach, B.; Henocq, P.; Giffaut, E.; Vieillard, P.; Gaboreau, S. Crystal structure of magnesium silicate hydrates (M-S-H): The relation with 2:1 Mg-Si phyllosilicates. Cem. Concr. Res. 2015, 73, 228–237. [Google Scholar] [CrossRef]
- Martini, F.; Tonelli, M.; Geppi, M.; Ridi, F.; Borsacchi, S.; Calucci, L. Hydration of MgO/SiO2 and Portland cement mixtures: A structural investigation of the hydrated phases by means of X-ray diffraction and solid state NMR spectroscopy. Cem. Concr. Res. 2017, 102, 60–67. [Google Scholar] [CrossRef]
- Tonelli, M.; Martini, F.; Calucci, L.; Fratini, E.; Geppi, M.; Ridi, F.; Borsacchi, S.; Baglioni, P. Structural characterization of magnesium silicate hydrate: Towards the design of eco-sustainable cements. Dalton Trans. 2016, 45, 3294–3304. [Google Scholar] [CrossRef]
- Ma, Y.; Li, W.; Jin, M.; Liu, J.; Zhang, J.; Huang, J.; Lu, C.; Zeng, H.; Wang, J.; Zhao, H.; et al. Influences of leaching on the composition, structure and morphology of calcium silicate hydrate (C–S–H) with different Ca/Si ratios. J. Build. Eng. 2022, 58, 105017. [Google Scholar] [CrossRef]
- Masse, S.; Zanni, H.; Lecourtier, J.; Roussel, J.; Rivereau, A. 29Si solid state NMR study of tricalcium silicate and cement hydration at high temperature. Cem. Concr. Res. 1993, 23, 1169–1177. [Google Scholar] [CrossRef]
- Bernard, E.; Lothenbach, B.; Chlique, C.; Wyrzykowski, M.; Dauzères, A.; Pochard, I.; Cau-Dit-Coumes, C. Characterization of magnesium silicate hydrate (M-S-H). Cem. Concr. Res. 2019, 116, 309–330. [Google Scholar] [CrossRef]
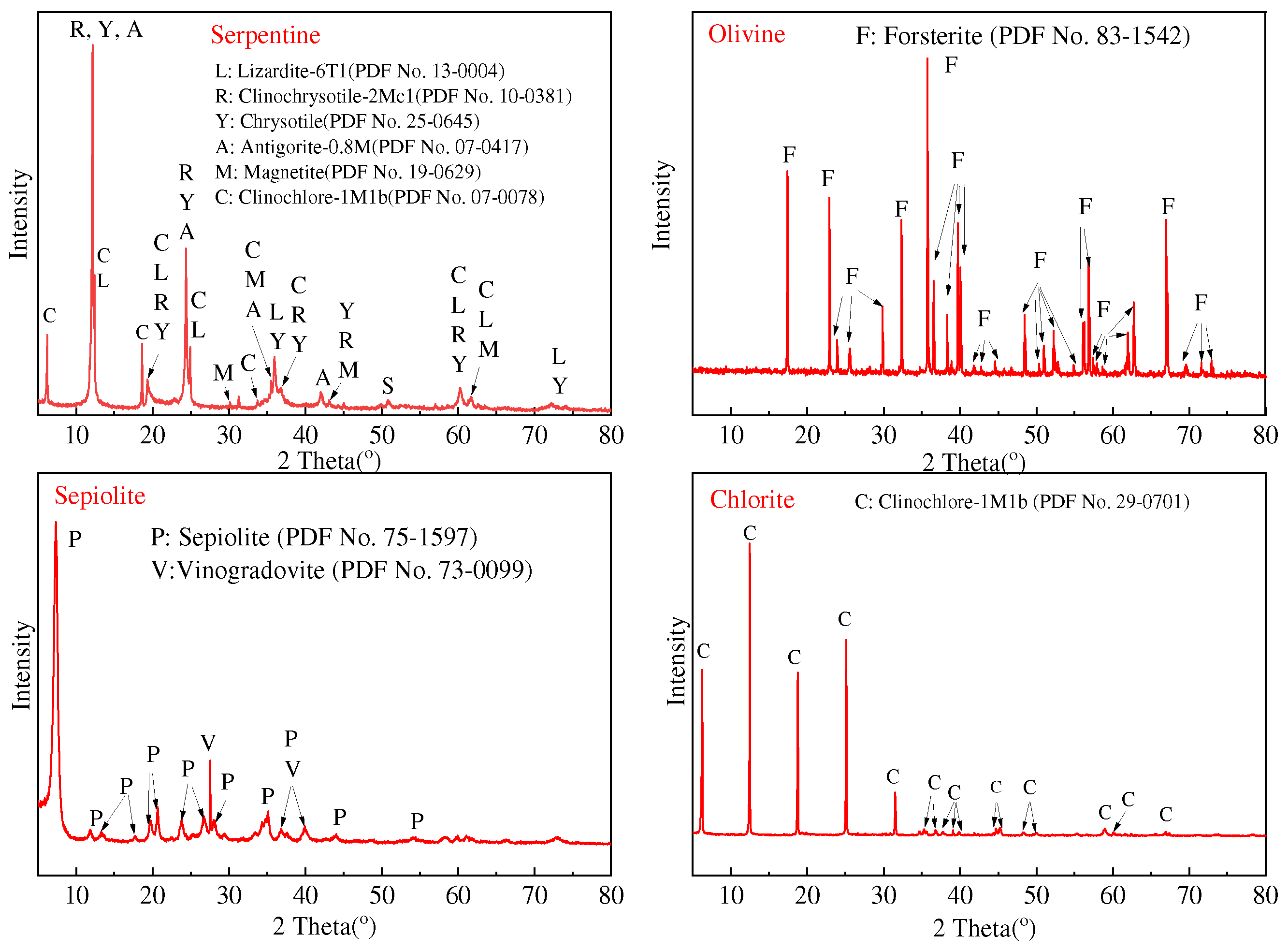
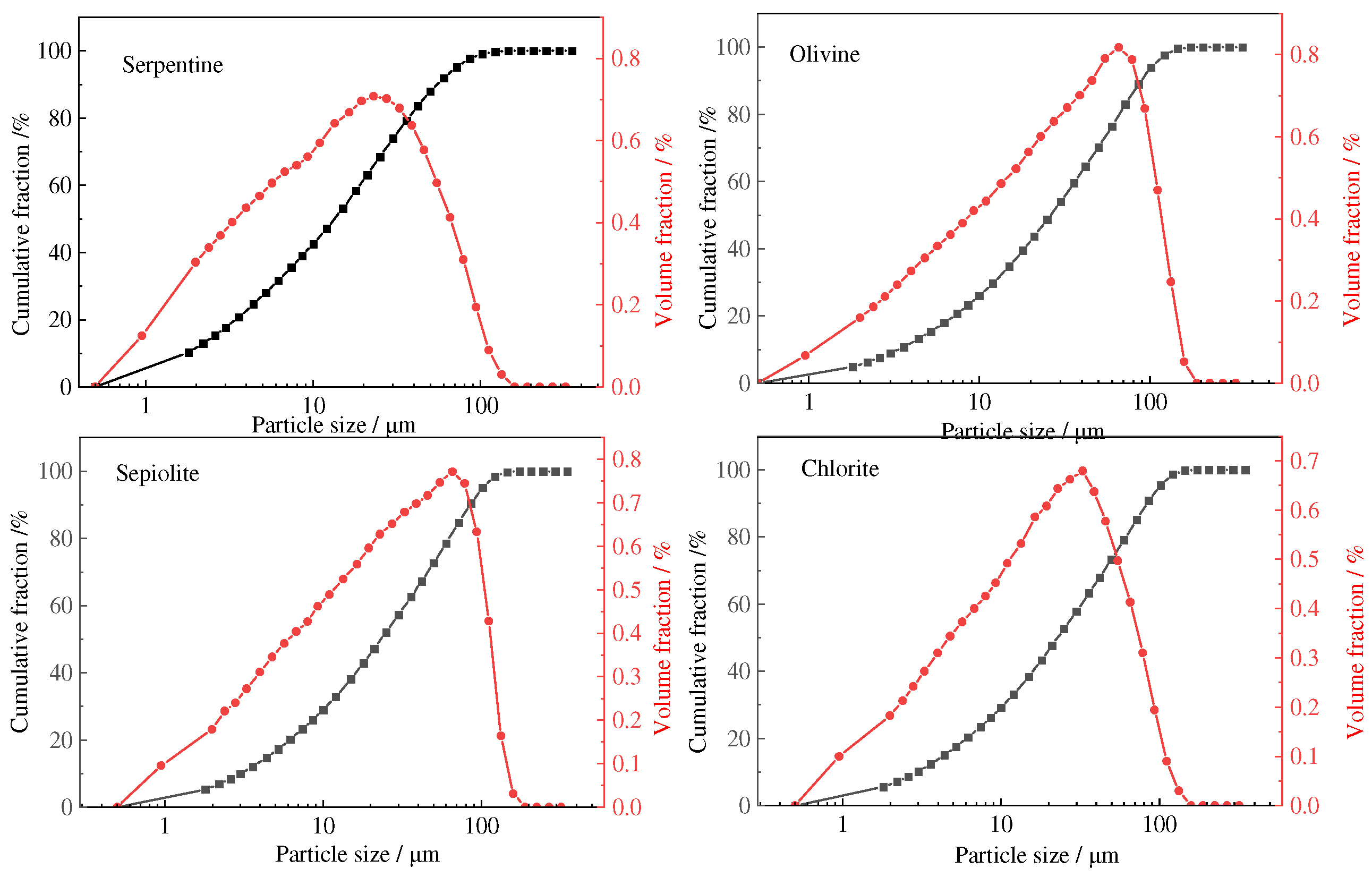
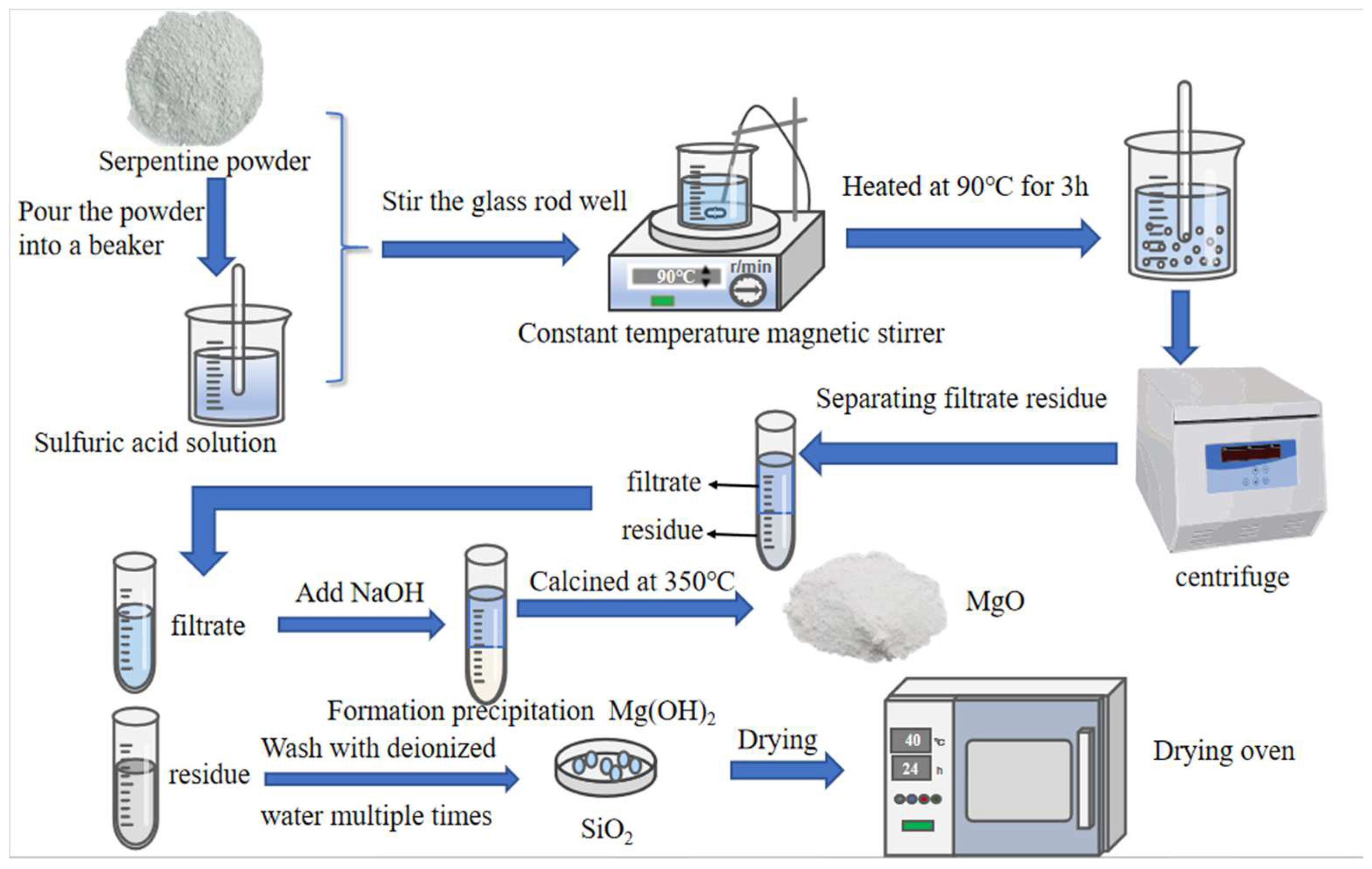
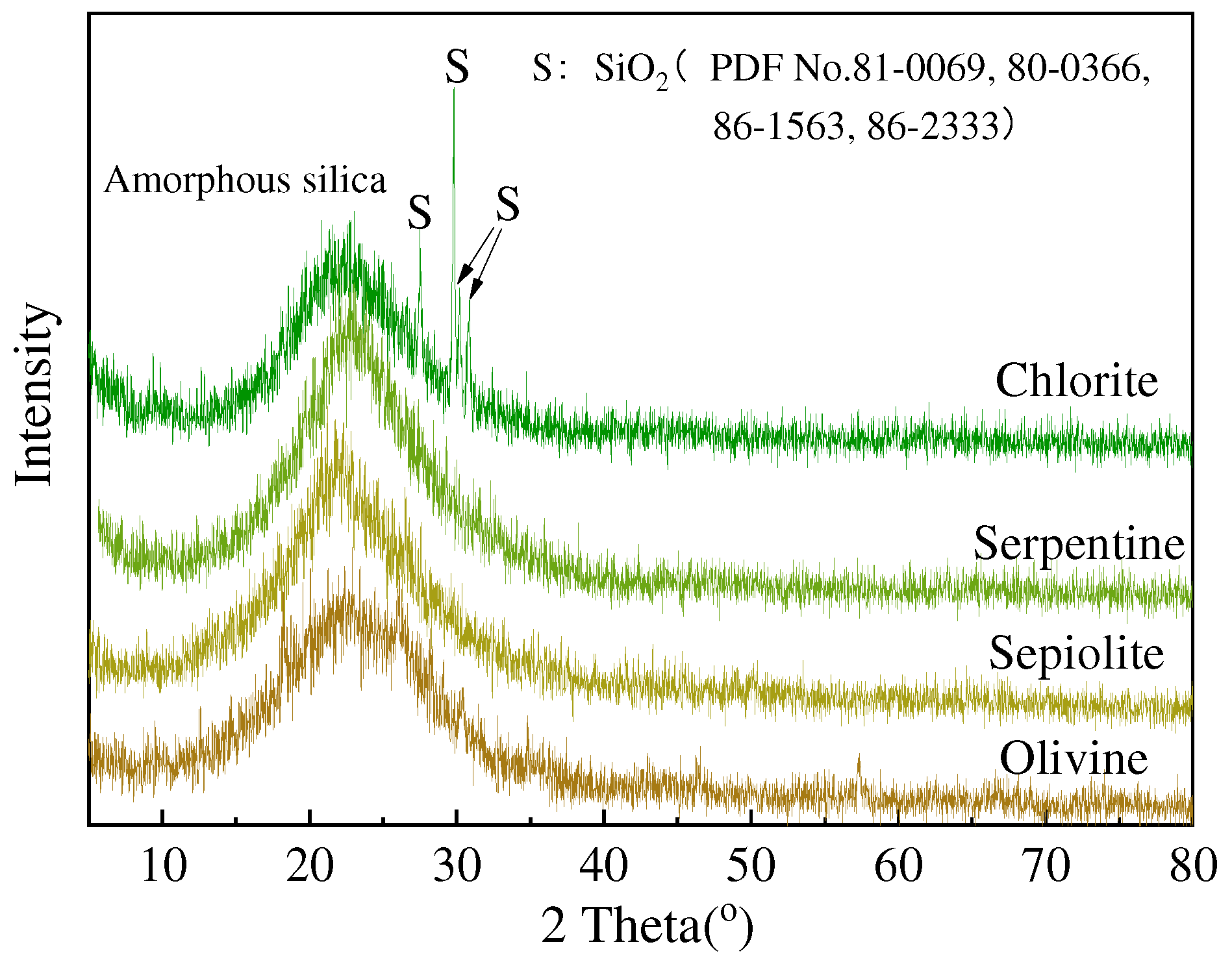


| Materials | MgO | SiO2 | Al2O3 | Fe2O3 | CaO | K2O | Na2O | TiO2 |
|---|---|---|---|---|---|---|---|---|
| Serpentine Olivine | 43.48 47.89 | 45.95 41.77 | 0.62 0.23 | 9.11 9.97 | 0.37 0.08 | 0.02 0.01 | 0.11 0.04 | 0.02 0.01 |
| Sepiolite | 28.47 | 67.45 | 2.03 | 0.49 | 0.44 | 0.65 | 0.28 | 0.09 |
| Chlorite | 34.71 | 42.12 | 12.70 | 6.96 | 3.12 | 0.12 | 0.05 | 0.22 |
| Materials | SiO2 | MgO | SO3 | Al2O3 | Fe2O3 | CaO | K2O | Na2O | TiO2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Residue | Serpentine Olivine | 94.68 95.74 | 0.78 1.35 | 3.97 1.33 | 0.09 0.25 | 0.34 0.92 | 0.06 0.16 | 0.01 0.02 | 0.05 0.08 | 0.02 0.15 |
| Sepiolite | 95.69 | 1.72 | 1.45 | 0.65 | 0.20 | 0.15 | / | / | 0.14 | |
| Chlorite | 90.87 | 5.44 | 0.88 | / | 2.61 | / | / | / | 0.20 | |
| Mg(OH)2 | Serpentine Olivine | 0.52 2.03 | 98.22 96.82 | 0.52 0.32 | 0.06 0.21 | 0.64 0.19 | / 0.12 | / 0.01 | 0.04 0.30 | / 0.01 |
| Sepiolite | 0.38 | 98.69 | 0.05 | 0.60 | / | 0.08 | / | 0.20 | / | |
| Chlorite | 1.94 | 97.12 | 0.24 | 0.33 | 0.24 | / | 0.02 | 0.11 | / |
| Curing Tem. | Mass Loss | Minerals | 3 d | 7 d | 14 d | 28 d |
|---|---|---|---|---|---|---|
| 50 °C | Δm1 | Serpentine | 11.34 | 14.55 | 14.07 | 14.07 |
| Olivine | 13.92 | 15.25 | 14.43 | 15.09 | ||
| Sepiolite | 15.21 | 18.02 | 14.56 | 17.43 | ||
| Chlorite | 11.32 | 9.34 | 10.61 | 11.00 | ||
| Δm2 | Serpentine | 13.07 | 10.70 | 9.16 | 9.02 | |
| Olivine | 11.75 | 10.72 | 8.87 | 9.33 | ||
| Sepiolite | 11.41 | 8.28 | 9.10 | 6.02 | ||
| Chlorite | 12.58 | 11.56 | 10.14 | 10.14 | ||
| Δm3 | Serpentine | 18.44 | 16.25 | 15.28 | 15.35 | |
| Olivine | 17.36 | 16.44 | 15.43 | 16.32 | ||
| Sepiolite | 16.61 | 13.97 | 15.18 | 13.32 | ||
| Chlorite | 18.28 | 17.98 | 16.28 | 15.94 | ||
| Δm3 − Δm2 | Serpentine | 5.37 | 5.56 | 6.13 | 6.34 | |
| Olivine | 5.61 | 5.72 | 6.56 | 6.99 | ||
| Sepiolite | 5.20 | 5.69 | 6.08 | 7.30 | ||
| Chlorite | 5.70 | 6.42 | 6.14 | 5.80 | ||
| 80 °C | Δm1 | Serpentine | 14.63 | 19.18 | 18.49 | 18.17 |
| Olivine | 13.92 | 14.30 | 14.15 | 18.73 | ||
| Sepiolite | 14.64 | 19.43 | 18.70 | 18.70 | ||
| Chlorite | 14.48 | 12.46 | 12.09 | 13.99 | ||
| Δm2 | Serpentine | 8.74 | 6.54 | 5.29 | 4.62 | |
| Olivine | 11.93 | 11.23 | 10.25 | 5.83 | ||
| Sepiolite | 10.96 | 6.16 | 6.62 | 6.23 | ||
| Chlorite | 8.60 | 7.60 | 6.60 | 5.89 | ||
| Δm3 | Serpentine | 15.02 | 12.96 | 11.84 | 11.42 | |
| Olivine | 17.36 | 17.71 | 17.54 | 13.08 | ||
| Sepiolite | 16.60 | 12.75 | 13.60 | 13.12 | ||
| Chlorite | 15.13 | 13.60 | 13.00 | 12.50 | ||
| Δm3 − Δm2 | Serpentine | 6.28 | 6.42 | 6.57 | 6.80 | |
| Olivine | 5.43 | 6.48 | 7.29 | 7.25 | ||
| Sepiolite | 5.64 | 6.59 | 6.98 | 6.89 | ||
| Chlorite | 6.53 | 6.00 | 6.40 | 6.61 |
| Curing Tem. | Minerals | I/% | Unreacted Silica I/% | Q3/Q2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Q1a | Q1b | Q2 | Q3a | Q3b | Q3 | Q4 | |||
| SiO2 | Serpentine | 31.4 | 68.6 | ||||||
| Olivine | 31.8 | 68.2 | |||||||
| Sepiolite | 30.9 | 69.1 | |||||||
| Chlorite | 32.4 | 67.6 | |||||||
| 50 °C | Serpentine | 4.6 | 7.8 | 41.1 | 32.6 | 10.1 | 3.8 | 1.04 | |
| Olivine | 5.8 | 7.3 | 43.8 | 35.6 | 7.5 | 0 | 0.98 | ||
| Sepiolite | 1.2 | 4.2 | 36.1 | 43.7 | 5.1 | 9.7 | 1.35 | ||
| Chlorite | 0 | 23.8 | 40.7 | 18.9 | 14.7 | 1.9 | 0.83 | ||
| 80 °C | Serpentine | 4.3 | 7.1 | 43.6 | 35.9 | 7.9 | 1.2 | 1.00 | |
| Olivine | 4.1 | 6.1 | 46.4 | 37.1 | 6.3 | 0 | 0.94 | ||
| Sepiolite | 0.7 | 5.4 | 38.9 | 45.9 | 5.2 | 3.9 | 1.31 | ||
| Chlorite | 0 | 24.4 | 43.4 | 20.2 | 11.6 | 0.4 | 0.73 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Q.; He, Q.; Nie, J.; Song, T.; Zhou, H.; Hu, Y.; Chen, Y.; Deng, Y.; Cheng, F. The Properties of Magnesium Silicate Hydrate Prepared from the Magnesium Silicate Minerals in the Earth’s Crust. Buildings 2024, 14, 1188. https://doi.org/10.3390/buildings14051188
Song Q, He Q, Nie J, Song T, Zhou H, Hu Y, Chen Y, Deng Y, Cheng F. The Properties of Magnesium Silicate Hydrate Prepared from the Magnesium Silicate Minerals in the Earth’s Crust. Buildings. 2024; 14(5):1188. https://doi.org/10.3390/buildings14051188
Chicago/Turabian StyleSong, Qiang, Qian He, Jiao Nie, Tiantian Song, Hong Zhou, Yaru Hu, Yanxin Chen, Yang Deng, and Fuan Cheng. 2024. "The Properties of Magnesium Silicate Hydrate Prepared from the Magnesium Silicate Minerals in the Earth’s Crust" Buildings 14, no. 5: 1188. https://doi.org/10.3390/buildings14051188




