Coronavirus Disease 2019 on the Heels of Ebola Virus Disease in West Africa
Abstract
:1. Introduction
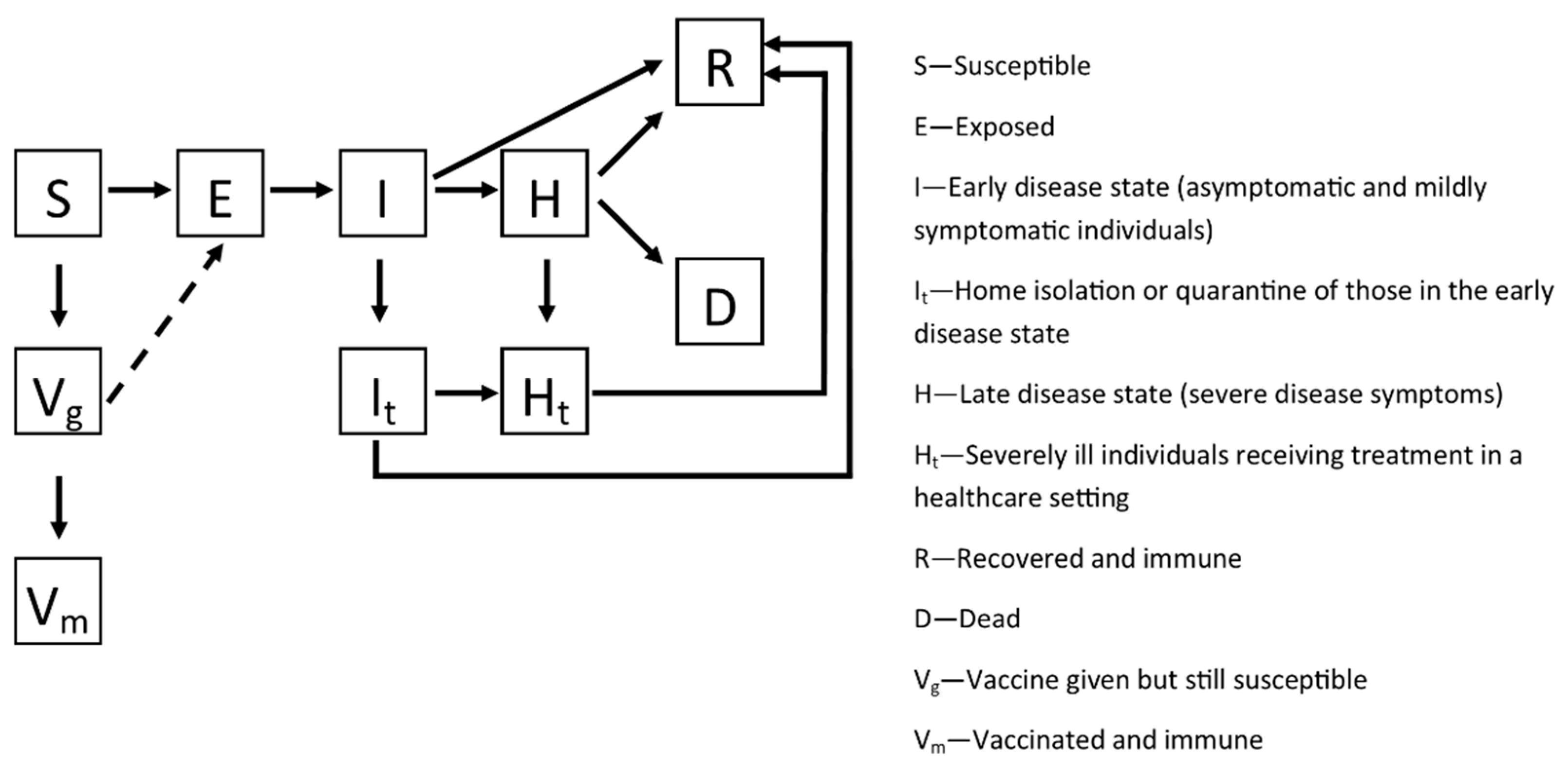
2. Methods
2.1. Country-Specific Models
2.2. Model Calibration
2.3. Vaccination
2.4. Simulations
2.5. Geographic Data
3. Results
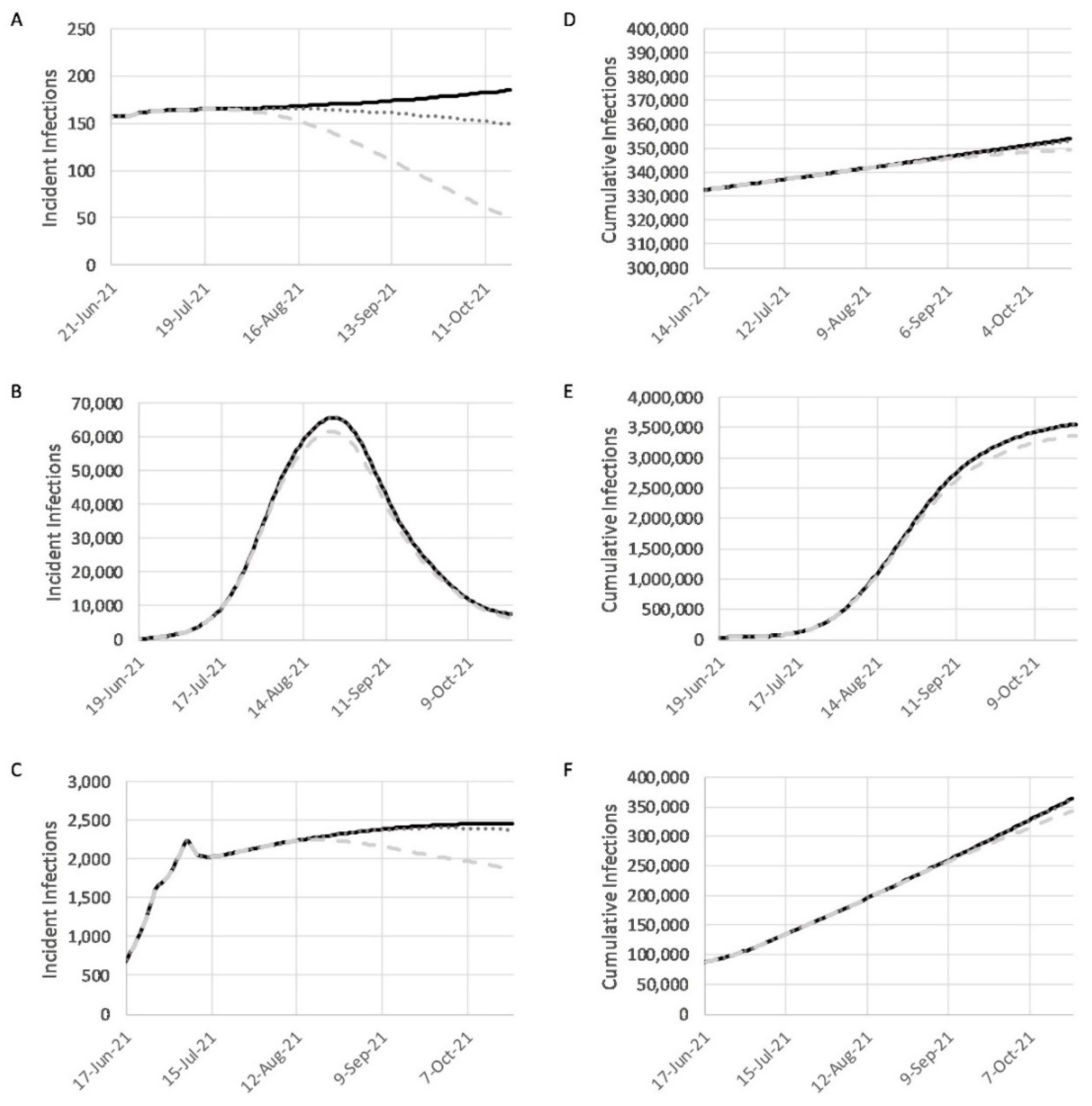
3.1. Model Calibration
3.2. Geographic Distribution
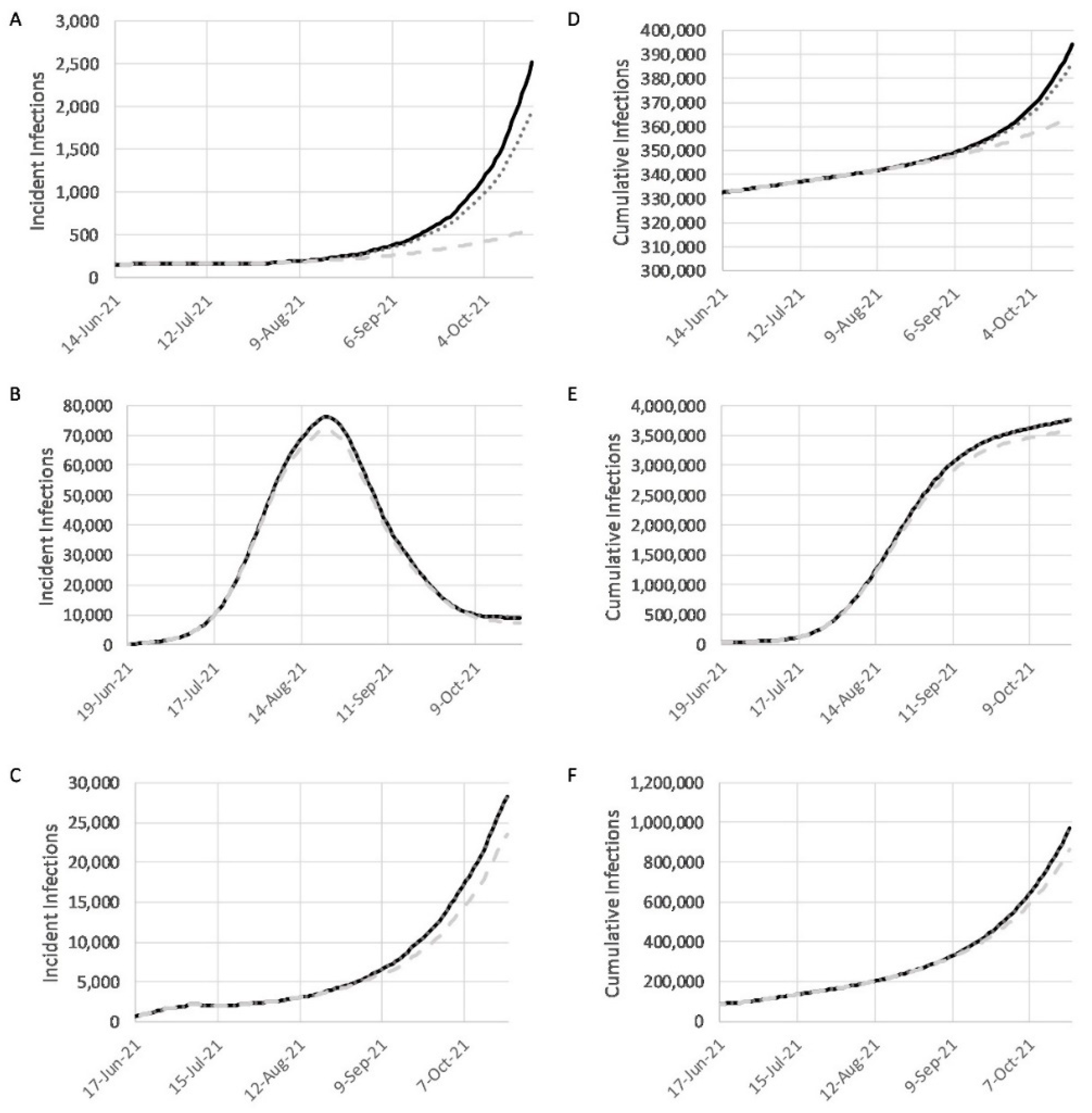
3.3. Simulations on the Effects of Vaccination
3.4. Combined Effects of Vaccination and Relaxed NPIs under Baseline, Realistic, and Optimistic Scenarios
4. Discussion
4.1. Vaccination
4.2. Non-Pharmaceutical Interventions (NPIs)
4.3. Modeling Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Standley, C.J.; Carlin, E.P.; Sorrell, E.M.; Barry, A.M.; Bile, E.; Diakite, A.S.; Keita, M.S.; Koivogui, L.; Mane, S.; Martel, L.D.; et al. Assessing Health Systems in Guinea for Prevention and Control of Priority Zoonotic Diseases: A One Health Approach. One Health 2019, 7, 100093. [Google Scholar] [CrossRef] [PubMed]
- 2014–2016 Ebola Outbreak in West Africa. Available online: https://www.cdc.gov/vhf/ebola/history/2014-2016-outbreak/index.html (accessed on 15 June 2021).
- Salaam-Blyther, T. The 2014 Ebola Outbreak: International and U.S. Responses; Congressional Research Service: Washington, DC, USA, 2014.
- African Countries that Faced Ebola Outbreaks Use Lessons to Fight COVID-19, Experts Say. Available online: https://www.nbcnews.com/news/world/african-countries-faced-ebola-outbreaks-use-lessons-fight-covid-19-n1181156 (accessed on 15 June 2021).
- Dembek, Z.F.; Mothershead, J.L.; Chekol, T.; Myers, D.B.; Meris, R.G.; Meranus, D.; Wu, A. Operational perspective of lessons learned from the Ebola crisis. Mil. Med. 2017, 182, e1507–e1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenya Third Wave Response: A Time to Synergize and Re-Energize. Available online: https://www.afro.who.int/news/kenya-third-wave-response-time-synergize-and-re-energize (accessed on 15 June 2021).
- Charts Show South Africa Coronavirus Resurgence Is Imminent. Available online: https://www.bloomberg.com/news/articles/2021-05-13/charts-show-south-africa-coronavirus-resurgence-is-imminent (accessed on 15 June 2021).
- Guinea, World Health Organization. Available online: https://covid19.who.int/region/afro/country/gn (accessed on 15 June 2021).
- Worldometer, Guinea. Available online: https://www.worldometers.info/coronavirus/country/guinea/ (accessed on 14 June 2021).
- Liberia, World Health Organization. Available online: https://covid19.who.int/region/afro/country/lr (accessed on 14 June 2021).
- World Health Organization. Sierra Leone. Available online: https://covid19.who.int/region/afro/country/sl (accessed on 14 June 2021).
- Maxmen, A. Ebola prepared these countries for coronavirus–but now they are floundering. Nature 2020, 583, 667–668. [Google Scholar] [CrossRef]
- Ministry of Health Starts Vaccination Campaign Against COVID-19. Available online: https://www.unicef.org/liberia/press-releases/ministry-health-starts-vaccination-campaign-against-covid19-0 (accessed on 10 June 2021).
- Liberia: COVID-19 Vaccine Is Finally Here. Available online: https://frontpageafricaonline.com/editorial/liberia-covid-19-vaccine-is-finally-here/ (accessed on 10 June 2021).
- COVAX Vaccine Roll-Out–Guinea. Available online: https://www.gavi.org/covax-vaccine-roll-out/guinea (accessed on 10 June 2021).
- Guinea Receives Purchase of 300,000 Sinovac COVID-19 Vaccines. Available online: https://www.reuters.com/business/healthcare-pharmaceuticals/guinea-receives-purchase-300000-sinovac-covid-19-vaccines-2021-04-18/ (accessed on 10 June 2021).
- COVAX Vaccine Roll-Out–Sierra Leone. Available online: https://www.gavi.org/covax-vaccine-roll-out/sierra-leone (accessed on 10 June 2021).
- China Donates 200,000 Doses of COVID-19 Vaccine to Sierra Leone. Available online: http://www.xinhuanet.com/english/2021-02/26/c_139767646.htm (accessed on 10 June 2021).
- Mourant, J.R.; Fenimore, P.W.; Manore, C.A.; McMahon, B.H. Decision Support for Mitigation of Livestock Disease: Rinderpest as a Case Study. Front. Vet. Sci. 2018, 5, 182. [Google Scholar] [CrossRef] [PubMed]
- Tyler, C. What Happens Next, Los Alamos National Laboratory. Available online: https://www.lanl.gov/discover/publications/1663/2021-february/what-happens-next.shtml (accessed on 31 August 2021).
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Lavezzo, E.; Franchin, E.; Ciavarella, C.; Cuomo-Dannenburg, G.; Barzon, L.; Del Vecchio, C.; Rossi, L.; Manganelli, R.; Loregian, A.; Navarin, N.; et al. Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo’. Nature 2020, 584, 425–429. [Google Scholar] [CrossRef]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.M.; Lau, E.H.Y.; Wong, J.Y.; et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- Arons, M.M.; Hatfield, K.M.; Reddy, S.C.; Kimball, A.; James, A.; Jacobs, J.R.; Taylor, J.; Spicer, K.; Bardossy, A.C.; Oakley, L.P.; et al. Presymptomatic SARS-CoV-2 Infections and Transmission in a Skilled Nursing Facility. NEJM 2020, 382, 2081–2090. [Google Scholar] [CrossRef]
- Bullard, J.; Dust, K.; Funk, D.; Strong, J.E.; Alexander, D.; Garnett, L.; Boodman, C.; Bello, A.; Hedley, A.; Schffman, Z.; et al. Predicting Infectious Severe Acute Respiratory Syndrome Coronavirus 2 from Diagnostic Samples. Clin. Infect. Dis. 2020, 71, 2663–2666. [Google Scholar] [CrossRef]
- van Kampen, J.J.A.; van de Vijver, D.A.M.C.; Fraaij, P.L.A.; Haagmans, B.L.; Lamers, M.M.; Okba, N.; van den Akker, J.P.C.; Endeman, H.; Gommers, D.A.M.P.J.; Cornelissen, J.J.; et al. Duration and Key Determinants of Infectious Virus Shedding in Hospitalized Patients with Coronavirus Disease-2019 (COVID- 19). Nat. Commun. 2021, 12, 267. [Google Scholar] [CrossRef] [PubMed]
- New Mexico Department of Health, COVID-19 in New Mexico. Available online: https://cvprovider.nmhealth.org/public-dashboard.html (accessed on 27 August 2021).
- Adiga, A.; Venkatramanan, S.; Schlitt, J.; Peddireddy, A.; Dickerman, A.; Bura, A.; Warren, A.; Klahn, B.D.; Mao, C.; Xie, D.; et al. Evaluating the Impact of International Airline Suspensions on the Early Global Spread of COVID-19. medRxiv 2020. [Google Scholar] [CrossRef]
- Brockmann, D.; Helbing, D. The hidden geometry of complex, network-driven contagion phenomena. Science 2013, 342, 1337–1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, M.G.; Taylor, M.M.; Dee, J.; Hakim, A.; Cantey, P.; Lim, T.; Bah, H.; Camara, S.M.; Ndongmo, C.B.; Togba, M.; et al. Contact Tracing Activities during the Ebola Virus Disease Epidemic in Kindia and Faranah, Guinea, 2014. Emerg. Infect. Dis. 2015, 21, 2022–2028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olu, O.O.; Lamunu, M.; Nanyunja, M.; Dafae, F.; Samba, T.; Sempiira, N.; Kuti-George, F.; Abebe, F.Z.; Sensasi, B.; Chimbaru, A.; et al. Contact Tracing during an Outbreak of Ebola Virus Disease in the Western Area Districts of Sierra Leone: Lessons for Future Ebola Outbreak Response. Front. Public Health 2016, 4, 130. [Google Scholar] [CrossRef] [Green Version]
- Sierra Leone Ebola Lessons Shaping Massachusetts COVID-19 Response. Available online: https://www.pih.org/article/sierra-leone-ebola-lessons-shaping-massachusetts-covid-19-response (accessed on 15 July 2020).
- The World Factbook. Available online: https://www.cia.gov/the-world-factbook/ (accessed on 10 July 2020).
- Hospital Beds (per 1,000 People). Available online: https://data.worldbank.org/indicator/SH.MED.BEDS.ZS (accessed on 10 July 2020).
- Moore, M.; Gelfeld, B.; Okunogbe, A.T.; Paul, C. Identifying Future Disease Hot Spots: Infectious Disease Vulnerability Index; RAND Corporation: Santa Monica, CA, USA, 2016; Available online: https://www.rand.org/pubs/research_reports/RR1605.html (accessed on 1 June 2020).
- Republic of Sierra Leone, Ministry of Health Website. Available online: https://mohs.gov.sl/covid-19/. (accessed on 22 September 2020).
- Skrip, L.A.; Selvaraj, P.; Hagedorn, B.; Ouédraogo, A.L.; Noori, N.; Orcutt, A.; Mistry, D.; Bedson, J.; Hébert-Dufresne, L.; Scarpino, S.V.; et al. Seeding COVID-19 across Sub-Saharan Africa: An Analysis of Reported Importation Events across 49 Countries. Am. J. Trop. Med. Hyg. 2021, 104, 1694–1702. [Google Scholar] [CrossRef]
- COVID-19 Prevalence Calculator. Available online: https://preventepidemics.org/covid19/resources/prevalence-calculator/#how-to-use-the-calculator (accessed on 3 June 2020).
- Update on COVID-19 in Africa. Available online: https://preventepidemics.org/covid19/science/insights/update-on-covid-19-in-africa/ (accessed on 5 September 2021).
- IHME, COVID-19 Results Briefing: Global. Available online: http://www.healthdata.org/sites/default/files/files/Projects/COVID/2021/briefing_Global_20210122.pdf/ (accessed on 5 September 2021).
- Partnership for Evidence-Based Response to COVID-19 (PERC)-Effective Implementation of Public Health and Social Measures in Liberia: Situational Analysis Data, 30 April 2020. Available online: https://preventepidemics.org/wp-content/uploads/2020/05/Liberia_perc-countrybrief_mobility.pdf (accessed on 3 June 2020).
- Cuccaro, F.; Debenedetti, L.; Holzinger, A. New RECOVR Survey Findings in Sierra Leone Highlight Socio-Economic Fallout from COVID-19 as New Restrictions Are Put in Place. Available online: https://www.poverty-action.org/blog/new-recovr-survey-socio-economic-fallout (accessed on 22 September 2020).
- Ebola Crisis: Guinea Schools Reopen after Five-Month Closure. Available online: https://www.bbc.com/news/world-africa-30879937 (accessed on 22 September 2021).
- Rodriguez, M. 5 Tips for Reopening after COVID-19 School Closures from a Liberian School that Made It through Ebola. Available online: https://www.nwea.org/blog/2020/5-tips-reopening-after-covid-19-from-liberian-school-made-through-ebola/ (accessed on 6 June 2021).
- Fofana, U. Sierra Leone Schools Reopen after Long Closure Due to Ebola. Available online: https://www.reuters.com/article/us-health-ebola-leone-education/sierra-leone-schools-reopen-after-long-closure-due-to-ebola-idUSKBN0N51JY20150414 (accessed on 6 June 2021).
- ‘Make Ebola a Thing of the Past’: First Vaccine against Deadly Virus Approved. Available online: https://www.nature.com/articles/d41586-019-03490-8 (accessed on 27 August 2021).
- Ebola Vaccination Starts in Guinea to Curb New Outbreak. Available online: https://www.afro.who.int/news/ebola-vaccination-starts-guinea-curb-new-outbreak (accessed on 27 August 2021).
- Coronavirus (COVID-19) Vaccinations-Statistics and Research. Available online: https://ourworldindata.org/covid-vaccinations (accessed on 14 June 2021).
- United Nations Office for the Coordination of Humanitarian Affairs: HDX Human Data Set. Available online: https://data.humdata.org/dataset/ (accessed on 22 September 2020).
- LandScan Datasets. Available online: https://landscan.ornl.gov/landscan-datasets (accessed on 15 July 2021).
- COVAX, Working for a Global Equitable Access to COVID-19 Vaccines. Available online: https://www.who.int/initiatives/act-accelerator/covax (accessed on 15 July 2021).
- Berkley, S. COVAX Explained. Available online: https://www.gavi.org/vaccineswork/covax-explained (accessed on 10 June 2021).
- Africa CDC, African Union. Available online: https://africacdc.org/ (accessed on 6 June 2020).
- Guinea Gets Donation of 200,000 Doses of COVID-19 Vaccine from China. Available online: https://www.reuters.com/article/health-coronavirus-guinea/guinea-gets-donation-of-200000-covid-19-vaccine-doses-from-china-idUSL5N2L1637 (accessed on 4 June 2021).
- Liberia: MTN Group Donates 27,000 COVID-19 Vaccines to Government. Available online: https://frontpageafricaonline.com/news/liberia-mtn-group-donates-27000-covid-19-vaccines-to-government/ (accessed on 4 June 2021).
- Sierra Leone Thanks China for Vaccine Donation. Available online: https://news.cgtn.com/news/2021-03-02/Sierra-Leone-thanks-China-for-vaccine-donation-YjfdPpiABq/index.html (accessed on 4 June 2021).
- Roberts, M.; BBC Visual Journalism Team. Vaccines Alone Will Not Stop Covid Spreading–Here’s Why. Available online: https://www.bbc.co.uk/news/resources/idt-40ac92b1-1750-4e86-9936-2cda6b0acb3f (accessed on 15 July 2021).
- Nachega, J.B.; Sam-Agudu, N.A.; Masekela, R.; van der Zalm, M.M.; Nsanzimana, S.; Condo, J.; Ntoumi, F.; Rabie, H.; Kruger, M.; Wiysonge, C.S.; et al. Addressing challenges to rolling out COVID-19 vaccines in African countries. Lancet Glob. Health 2021, 9, e746–e748. [Google Scholar] [CrossRef]
- Seydou, A. AD432: Who Wants COVID-19 Vaccination? In 5 West African Countries, Vaccine Hesitancy Is High, Trust Is Low. Available online: https://afrobarometer.org/publications/ad432-who-wants-covid-19-vaccination-5-west-african-countries-hesitancy-high-trust-low (accessed on 15 July 2021).
- AstraZeneca Vaccine: Denmark Stops Rollout Completely. Available online: https://www.bbc.com/news/world-europe-56744474 (accessed on 15 July 2021).
- Mueller, B. AstraZeneca Vaccine Faces New Setbacks in UK and European Union. Available online: https://www.nytimes.com/2021/04/07/world/europe/astrazeneca-uk-european-union.html (accessed on 15 July 2021).
- Jeong, M. Oxford-AstraZeneca Vaccine: What to Know about Side Effects. Available online: https://www.medicalnewstoday.com/articles/oxford-astrazeneca-vaccine-what-to-know-about-side-effects (accessed on 15 July 2021).
- Samarasekera, U. Feelings toward COVID-19 vaccination in Africa. Lancet Infect. Dis. 2021, 21, 324. [Google Scholar] [CrossRef]
- DeFeudis, N. COVID-19 Roundup: Study Suggests AstraZeneca/Oxford Booster Is Effective against Variants–Report; IFPMA Issues Guidance on Vaccine Inequity. Available online: https://endpts.com/covid-19-roundup-study-suggests-astrazeneca-oxford-booster-is-effective-against-variants-report-ifpma-issues-guidance-on-vaccine-inequity/ (accessed on 15 July 2021).
- Weyer, J. A Recent Ebola Outbreak Puts Guinea’s “Muscle Memory” to the Test. Available online: https://qz.com/africa/1977293/why-has-ebola-returned/ (accessed on 15 July 2021).
- Pandemic Planning Scenarios. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios.html (accessed on 15 July 2021).
- Coronavirus 19: How COVID-19 Spreads. Available online: https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html (accessed on 22 September 2020).
- Jefferson, T.; Del Mar, C.B.; Dooley, L.; Ferroni, E.; Al-Ansary, L.A.; Bawazeer, G.A.; van Driel, M.L.; Jones, M.A.; Thorning, S.; Beller, E.M.; et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database Syst. Rev. 2020, 11, CD006207. [Google Scholar] [CrossRef]
- Seale, H.; Dyer, C.E.F.; Abdi, I.; Rahman, K.M.; Sun, Y.; Qureshi, M.O.; Dowell-Day, A.; Sward, J.; Islam, M.S. Improving the impact of non-pharmaceutical interventions during COVID-19: Examining the factors that influence engagement and the impact of individuals. BMC Infect. Dis. 2020, 20, 607. [Google Scholar] [CrossRef]
- Africa Faces First Recession in 25 Years Amid COVID-19 Pandemic: UN Report. Available online: http://www.xinhuanet.com/english/africa/2021-03/02/c_139778665.htm (accessed on 15 July 2021).
- Stoop, N.; Desbureaux, S.; Kaota, A.; Verpoorten, M. COVID-19 vs. Ebola: Impact on households and small businesses in North Kivu, Democratic Republic of Congo. World Dev. 2021, 140, 105352. [Google Scholar] [CrossRef] [PubMed]
- Egger, D.; Miguel, E.; Warren, S.S.; Shenoy, A.; Collins, E.; Karlan, D.; Parkerson, D.; Mobarak, A.M.; Fink, G.; Udry, C.; et al. Falling living standards during the COVID-19 crisis: Quantitative evidence from nine developing countries. Sci. Adv. 2021, 7, eabe0997. [Google Scholar] [CrossRef] [PubMed]
- Aktar, M.A.; Alam, M.M.; Al-Amin, A.Q. Global economic crisis, energy use, CO2 emissions, and policy roadmap amid COVID-19. Sustain. Prod. Consum. 2021, 26, 770–781. [Google Scholar] [CrossRef] [PubMed]

| Percent of Total Population | |||
|---|---|---|---|
| Guinea | Liberia | Sierra Leone | |
| Baseline Vaccination | 3.5 | 2.2 | 1.7 |
| Realistic Vaccination | 8.6 | 1.7 | 5.3 |
| Optimistic Vaccination | 32 | 24 | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dembek, Z.F.; Schwartz-Watjen, K.T.; Swiatecka, A.L.; Broadway, K.M.; Hadeed, S.J.; Mothershead, J.L.; Chekol, T.; Owens, A.N.; Wu, A. Coronavirus Disease 2019 on the Heels of Ebola Virus Disease in West Africa. Pathogens 2021, 10, 1266. https://doi.org/10.3390/pathogens10101266
Dembek ZF, Schwartz-Watjen KT, Swiatecka AL, Broadway KM, Hadeed SJ, Mothershead JL, Chekol T, Owens AN, Wu A. Coronavirus Disease 2019 on the Heels of Ebola Virus Disease in West Africa. Pathogens. 2021; 10(10):1266. https://doi.org/10.3390/pathogens10101266
Chicago/Turabian StyleDembek, Zygmunt F., Kierstyn T. Schwartz-Watjen, Anna L. Swiatecka, Katherine M. Broadway, Steven J. Hadeed, Jerry L. Mothershead, Tesema Chekol, Akeisha N. Owens, and Aiguo Wu. 2021. "Coronavirus Disease 2019 on the Heels of Ebola Virus Disease in West Africa" Pathogens 10, no. 10: 1266. https://doi.org/10.3390/pathogens10101266






