Retrospective Genotyping and Whole Genome Sequencing of a Canine Parvovirus Outbreak in Bangladesh
Abstract
:1. Introduction
2. Results and Discussion
2.1. WGS and Metagenomic Investigation of CPV-2 Outbreak
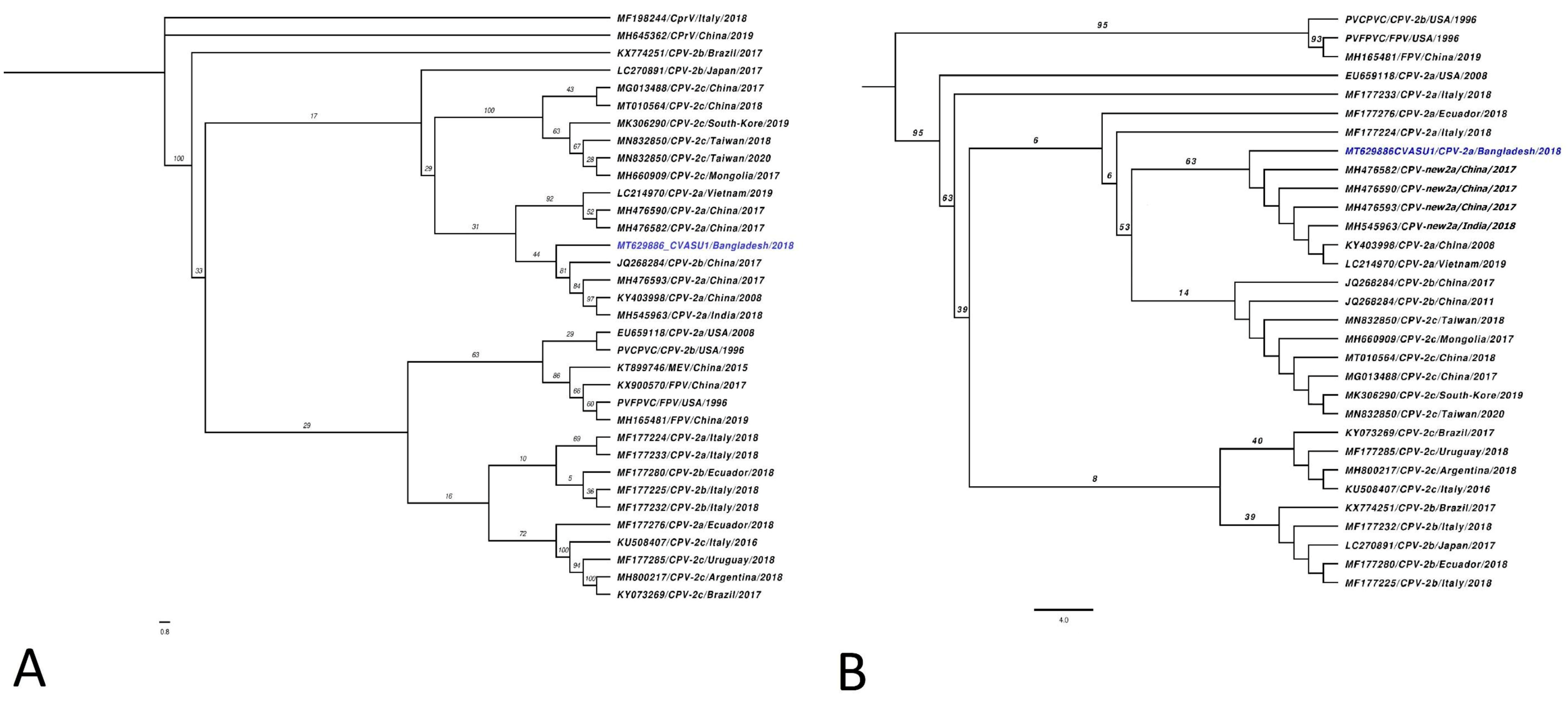
2.2. Phylogenetic Reconstruction and Analysis of the Complete Genome
2.3. PCR-HRM-Based Genotyping and Investigation for Community Transmission
3. Conclusions
4. Materials and Methods
4.1. Samples
4.2. Shotgun Metagenomics by Second-Generation Sequencing
4.2.1. DNA Extraction and Library Construction for NGS
4.2.2. Metagenomic Screening from the Pooled gDNA
4.2.3. CPV Whole Genome Assembly
4.3. CPV Targeted PCR Amplification and HRM-Based Strain Screening
4.4. Phylogenetic Reconstruction
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Giraldo-Ramirez, S.; Rendon-Marin, S.; Ruiz-Saenz, J. Phylogenetic, evolutionary and structural analysis of Canine Parvovirus (CPV-2) antigenic variants circulating in Colombia. Viruses 2020, 12, 500. [Google Scholar] [CrossRef]
- Barrs, V.R. Feline panleukopenia: A re-emergent disease. Vet. Clin. Small Anim. Pract. 2019, 49, 651–670. [Google Scholar]
- Parrish, C.R.; Aquadro, C.F.; Carmichael, L.E. Canine host range and a specific epitope map along with variant sequences in the capsid protein gene of canine parvovirus and related feline, mink, and raccoon parvoviruses. Virology 1988, 166, 293–307. [Google Scholar] [CrossRef]
- Reed, A.P.; Jones, E.V.; Miller, T.J. Nucleotide sequence and genome organization of canine parvovirus. J. Virol. 1988, 62, 266–276. [Google Scholar] [CrossRef] [Green Version]
- Silva, S.; Silva, L.; Rodrigues, E.; Cardoso, J.; Tavares Neto, F.; Souza, W.; Santos, C.; Martins, F.; Jesus, I.; Brito, T. Full-length genomic and molecular characterization of Canine parvovirus in dogs from North of Brazil. Genet. Mol. Res. 2017, 16, gmr16039719. [Google Scholar] [CrossRef]
- Parrish, C.R.; Aquadro, C.F.; Strassheim, M.; Evermann, J.; Sgro, J.; Mohammed, H. Rapid antigenic-type replacement and DNA sequence evolution of canine parvovirus. J. Virol. 1991, 65, 6544–6552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parrish, C.R.; Kawaoka, Y. The origins of new pandemic viruses: The acquisition of new host ranges by canine parvovirus and influenza A viruses. Annu. Rev. Microbiol. 2005, 59, 553–586. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, M.; Majer-Dziedzic, B.; Kostro, K.; Szabelak, A.; ZIFTEK, J.; Gryzinska, M.; Jakubczak, A. Diagnostics and genotyping of canine parvovirus type 2 (CPV-2) from disease cases in South-Eastern Poland. Acta Vet. 2019, 69, 32–46. [Google Scholar] [CrossRef] [Green Version]
- Mira, F.; Dowgier, G.; Purpari, G.; Vicari, D.; Di Bella, S.; Macaluso, G.; Gucciardi, F.; Randazzo, V.; Decaro, N.; Guercio, A. Molecular typing of a novel canine parvovirus type 2a mutant circulating in Italy. Infect. Genet. Evol. 2018, 61, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, T.; Hisaka, M.; Kawakami, K.; Kishi, M.; Tohya, Y.; Mochizuki, M. Chronological analysis of canine parvovirus type 2 isolates in Japan. J. Vet. Med. Sci. 2008, 70, 769–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battilani, M.; Modugno, F.; Mira, F.; Purpari, G.; Di Bella, S.; Guercio, A.; Balboni, A. Molecular epidemiology of canine parvovirus type 2 in Italy from 1994 to 2017: Recurrence of the CPV-2b variant. BMC Vet. Res. 2019, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Jalal, M.; Bayzid, M.; Sharif, M.; Masuduzzaman, M. A comparative study on canine parvovirus infection of dog in Bangladesh and India. Bangladesh J. Vet. Med. 2016, 14, 237–241. [Google Scholar] [CrossRef] [Green Version]
- Hasib, F.; Akter, S.; Chowdhury, S. First report of canine parvovirus molecular detection in Bangladesh. Vet. World 2021, 14, 1038–1043. [Google Scholar] [CrossRef]
- Islam, M.R.; Islam, M.A.; Rahman, M.S.; Uddin, M.J.; Sarker, M.A.S.; Akter, L.; Alam, E. Prevalence of canine parvovirus infection in street dogs in Mymensingh Municipality area, Bangladesh. Microbes Health 2014, 3, 5–6. [Google Scholar] [CrossRef] [Green Version]
- Rosario, K.; Breitbart, M. Exploring the viral world through metagenomics. Curr. Opin. Virol. 2011, 1, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.; Batinovic, S.; Talukder, S.; Das, S.; Park, F.; Petrovski, S.; Forwood, J.K.; Helbig, K.J.; Raidal, S.R. Molecular characterisation of a novel pathogenic avipoxvirus from the Australian magpie (Gymnorhina tibicen). Virology 2020, 540, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mokili, J.L.; Rohwer, F.; Dutilh, B.E. Metagenomics and future perspectives in virus discovery. Curr. Opin. Virol. 2012, 2, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Sarker, S.; Ghorashi, S.A.; Forwood, J.K.; Raidal, S.R. A comparison of PCR assays for beak and feather disease virus and high resolution melt (HRM) curve analysis of replicase associated protein and capsid genes. J. Virol. Methods 2016, 237, 47–57. [Google Scholar] [CrossRef]
- Bingga, G.; Liu, Z.; Zhang, J.; Zhu, Y.; Lin, L.; Ding, S.; Guo, P. High resolution melting curve analysis as a new tool for rapid identification of canine parvovirus type 2 strains. Mol. Cell. Probes 2014, 28, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Słomka, M.; Sobalska-Kwapis, M.; Wachulec, M.; Bartosz, G.; Strapagiel, D. High resolution melting (HRM) for high-throughput genotyping—limitations and caveats in practical case studies. Int. J. Mol. Sci. 2017, 18, 2316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sykes, J.; Greene, C.E. Feline enteric viral infections. Infectious Diseases of the Dog and Cat, 4th ed.; Elsevier: Amsterdam, the Netherlands, 2013; pp. 80–91. [Google Scholar]
- Miranda, C.; Thompson, G. Canine parvovirus: The worldwide occurrence of antigenic variants. J. Gen. Virol. 2016, 97, 2043–2057. [Google Scholar] [CrossRef]
- Geng, Y.; Guo, D.; Li, C.; Wang, E.; Wei, S.; Wang, Z.; Yao, S.; Zhao, X.; Su, M.; Wang, X. Co-circulation of the rare CPV-2c with unique Gln370Arg substitution, new CPV-2b with unique Thr440Ala substitution, and new CPV-2a with high prevalence and variation in Heilongjiang Province, Northeast China. PLoS ONE 2015, 10, e0137288. [Google Scholar] [CrossRef]
- Raj, J.M.; Mukhopadhyay, H.; Thanislass, J.; Antony, P.; Pillai, R. Isolation, molecular characterization and phylogenetic analysis of canine parvovirus. Infect. Genet. Evol. 2010, 10, 1237–1241. [Google Scholar]
- Truyen, U. Evolution of canine parvovirus—a need for new vaccines? Vet. Microbiol. 2006, 117, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Chinchkar, S.; Subramanian, B.M.; Rao, N.H.; Rangarajan, P.; Thiagarajan, D.; Srinivasan, V. Analysis of VP2 gene sequences of canine parvovirus isolates in India. Arch. Virol. 2006, 151, 1881–1887. [Google Scholar] [CrossRef] [PubMed]
- Raja, P.; Parthiban, M.; Maha Prabhu, M. Complete genome sequence analysis of canine Parvovirus new 2a variant strain isolated from a dog with severe haemarrogic gastroenteritis in South India. In Proceedings of the 20th Indian Veterinary Congress, Annual Conference of IAAVR, TANUVAS, Chennai, India, 21–22 February 2020. [Google Scholar]
- Ikeda, Y.; Mochizuki, M.; Naito, R.; Nakamura, K.; Miyazawa, T.; Mikami, T.; Takahashi, E. Predominance of canine parvovirus (CPV) in unvaccinated cat populations and emergence of new antigenic types of CPVs in cats. Virology 2000, 278, 13–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battilani, M.; Scagliarini, A.; Tisato, E.; Turilli, C.; Jacoboni, I.; Casadio, R.; Prosperi, S. Analysis of canine parvovirus sequences from wolves and dogs isolated in Italy. J. Gen. Virol. 2001, 82, 1555–1560. [Google Scholar] [CrossRef] [PubMed]
- Battilani, M.; Gallina, L.; Vaccari, F.; Morganti, L. Co-infection with multiple variants of canine parvovirus type 2 (CPV-2). Vet. Res. Commun. 2007, 31, 209–212. [Google Scholar] [CrossRef]
- Viscardi, M.; Santoro, M.; Clausi, M.T.; Cozzolino, L.; Decaro, N.; Colaianni, M.L.; Fusco, G. Molecular detection and characterization of carnivore parvoviruses in free-ranging Eurasian otters (Lutra lutra) in southern Italy. Transbound. Emerg. Dis. 2019, 66, 1864–1872. [Google Scholar] [CrossRef] [PubMed]
- Decaro, N.; Buonavoglia, C. Canine parvovirus—a review of epidemiological and diagnostic aspects, with emphasis on type 2c. Vet. Microbiol. 2012, 155, 1–12. [Google Scholar] [CrossRef]
- Pérez, R.; Bianchi, P.; Calleros, L.; Francia, L.; Hernández, M.; Maya, L.; Panzera, Y.; Sosa, K.; Zoller, S. Recent spreading of a divergent canine parvovirus type 2a (CPV-2a) strain in a CPV-2c homogenous population. Vet. Microbiol. 2012, 155, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Zhao, J.; Guo, D.; Sun, D. A mini-review on the epidemiology of canine parvovirus in China. Front. Vet. Sci. 2020, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Chinchkar, S.R.; Subramanian, B.M.; Naidu, H.; Thiagarajan, D.; Srinivasan, V.A. Canine parvovirus isolates of India and the relevance of canine parvovirus type-2 vaccines. J. Adv. Vet. Res. 2014, 4, 34–41. [Google Scholar]
- Decaro, N.; Buonavoglia, C.; Barrs, V.R. Canine parvovirus vaccination and immunisation failures: Are we far from disease eradication? Vet. Microbiol. 2020, 247, 108760. [Google Scholar] [CrossRef] [PubMed]
- Itokawa, K.; Sekizuka, T.; Maekawa, Y.; Yatsu, K.; Komagata, O.; Sugiura, M.; Sasaki, T.; Tomita, T.; Kuroda, M.; Sawabe, K. High-throughput genotyping of a full voltage-gated sodium channel gene via genomic DNA using target capture sequencing and analytical pipeline MoNaS to discover novel insecticide resistance mutations. PLoS Negl. Trop. 2019, 13, e0007818. [Google Scholar] [CrossRef] [PubMed]
- Kaper, F.; Swamy, S.; Klotzle, B.; Munchel, S.; Cottrell, J.; Bibikova, M.; Chuang, H.-Y.; Kruglyak, S.; Ronaghi, M.; Eberle, M.A. Whole-genome haplotyping by dilution, amplification, and sequencing. Proc. Natl. Acad. Sci. USA 2013, 110, 5552–5557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.; Fearnside, K.; Sarker, S.; Forwood, J.K.; Raidal, S.R. A novel pathogenic aviadenovirus from red-bellied parrots (Poicephalus rufiventris) unveils deep recombination events among avian host lineages. Virology 2017, 502, 188–197. [Google Scholar] [CrossRef]
- Takeuchi, F.; Sekizuka, T.; Yamashita, A.; Ogasawara, Y.; Mizuta, K.; Kuroda, M. MePIC, metagenomic pathogen identification for clinical specimens. Jpn. J. Infect. Dis. 2014, 67, 62–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ondov, B.D.; Bergman, N.H.; Phillippy, A.M. Interactive metagenomic visualization in a Web browser. BMC Bioinform. 2011, 12, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delcher, A.L.; Bratke, K.A.; Powers, E.C.; Salzberg, S.L. Identifying bacterial genes and endosymbiont DNA with Glimmer. Bioinformatics 2007, 23, 673–679. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.i.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013 30, 772–780. [CrossRef] [Green Version]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guindon, S.; Gascuel, O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef] [Green Version]
- Boc, A.; Diallo, A.B.; Makarenkov, V. T-REX: A web server for inferring, validating and visualizing phylogenetic trees and networks. Nucleic Acids Res. 2012, 40, W573–W579. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakib, T.M.; Nath, B.K.; Das, T.; Yadav, S.K.; Raidal, S.R.; Das, S. Retrospective Genotyping and Whole Genome Sequencing of a Canine Parvovirus Outbreak in Bangladesh. Pathogens 2021, 10, 1373. https://doi.org/10.3390/pathogens10111373
Rakib TM, Nath BK, Das T, Yadav SK, Raidal SR, Das S. Retrospective Genotyping and Whole Genome Sequencing of a Canine Parvovirus Outbreak in Bangladesh. Pathogens. 2021; 10(11):1373. https://doi.org/10.3390/pathogens10111373
Chicago/Turabian StyleRakib, Tofazzal Md, Babu Kanti Nath, Tridip Das, Saroj Kumar Yadav, Shane R. Raidal, and Shubhagata Das. 2021. "Retrospective Genotyping and Whole Genome Sequencing of a Canine Parvovirus Outbreak in Bangladesh" Pathogens 10, no. 11: 1373. https://doi.org/10.3390/pathogens10111373







