Borrelia burgdorferi Surface Exposed GroEL Is a Multifunctional Protein
Abstract
:1. Introduction
2. Results
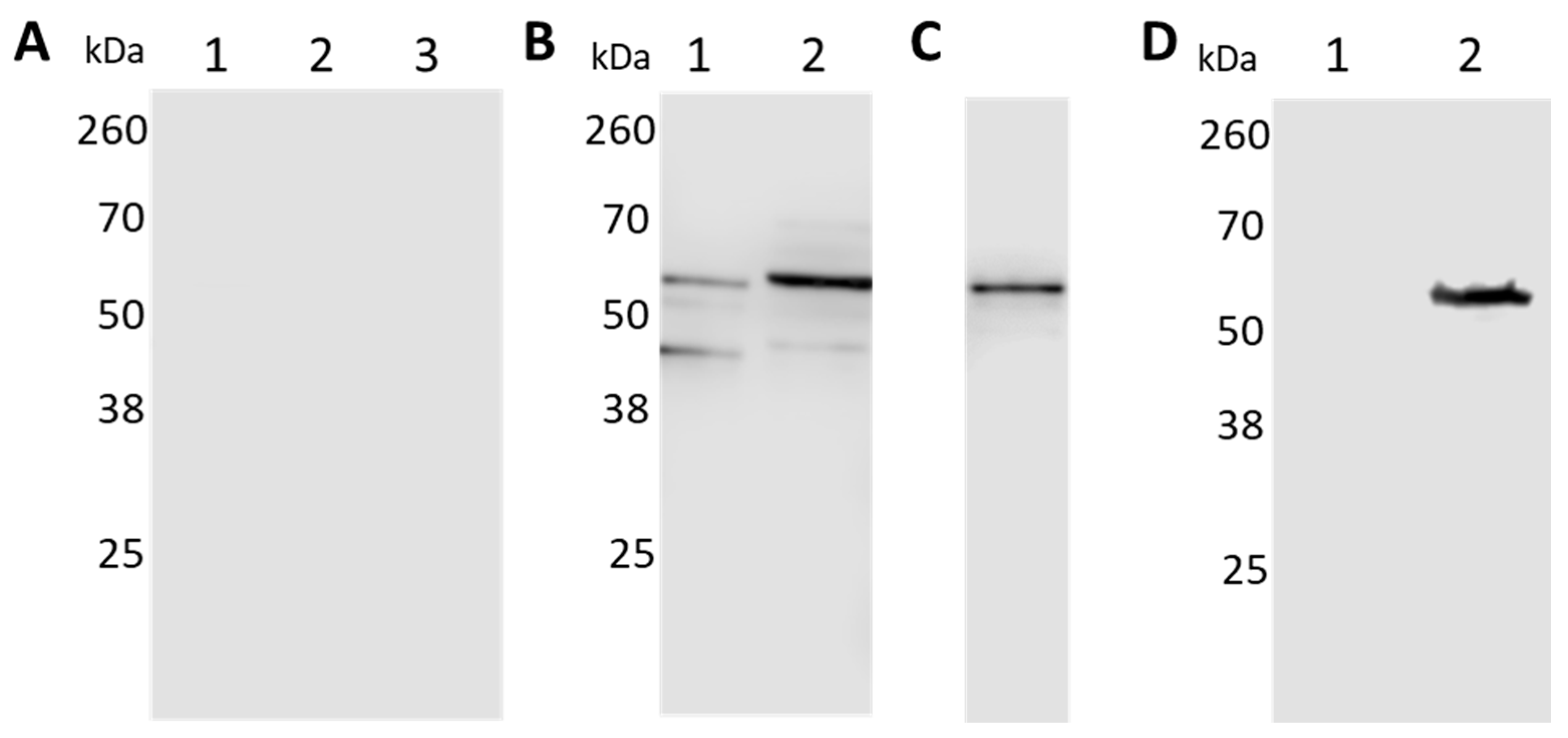
2.1. Purification of Recombinant BbGroEL from Escherichia coli Strain Rosetta (DE3)
2.2. Generation of a Polyclonal Anti-BbGroEL Antibody
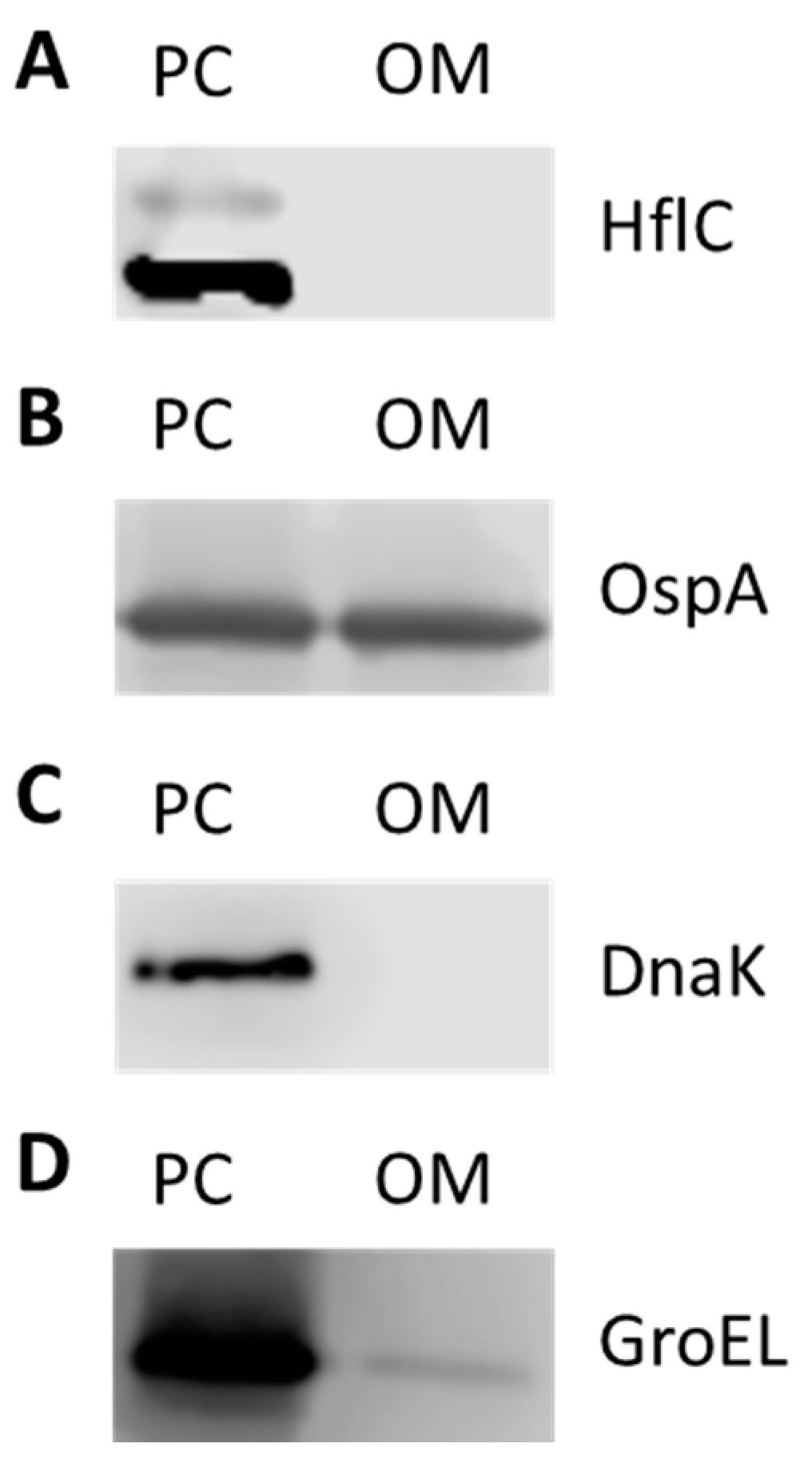
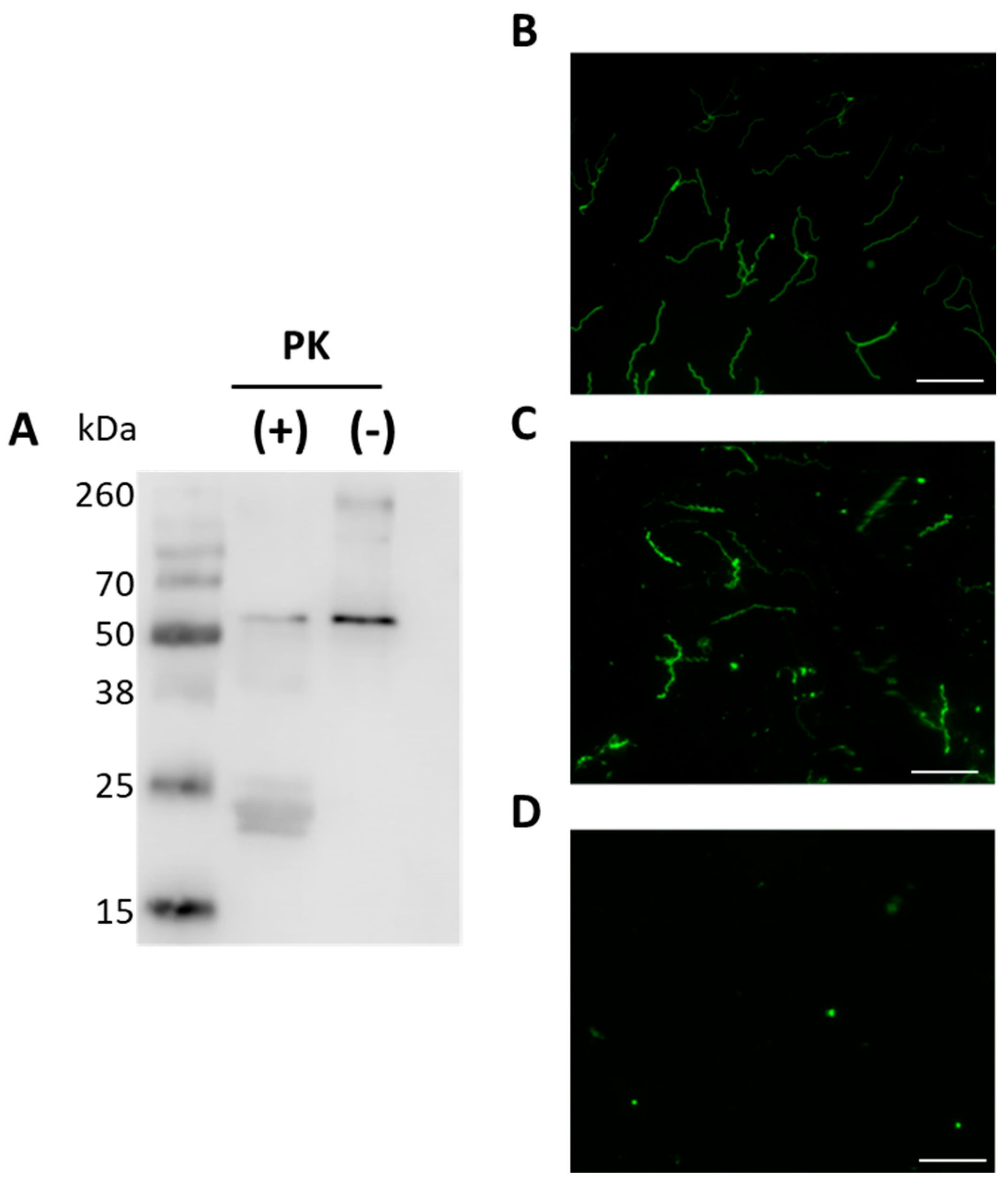
2.3. BbGroEL Is Surface Exposed in the OM of B. burgdorferi
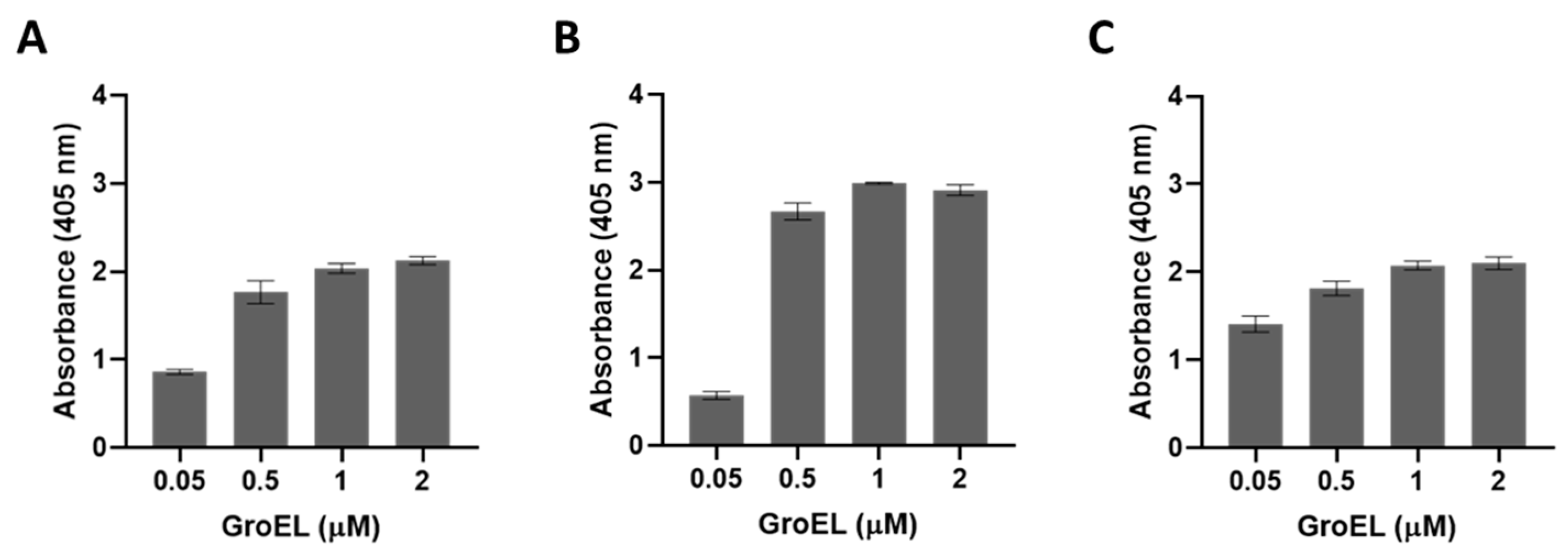
2.4. BbGroEL Binds PLG, and ECM Proteins in a Dose-Dependent Manner
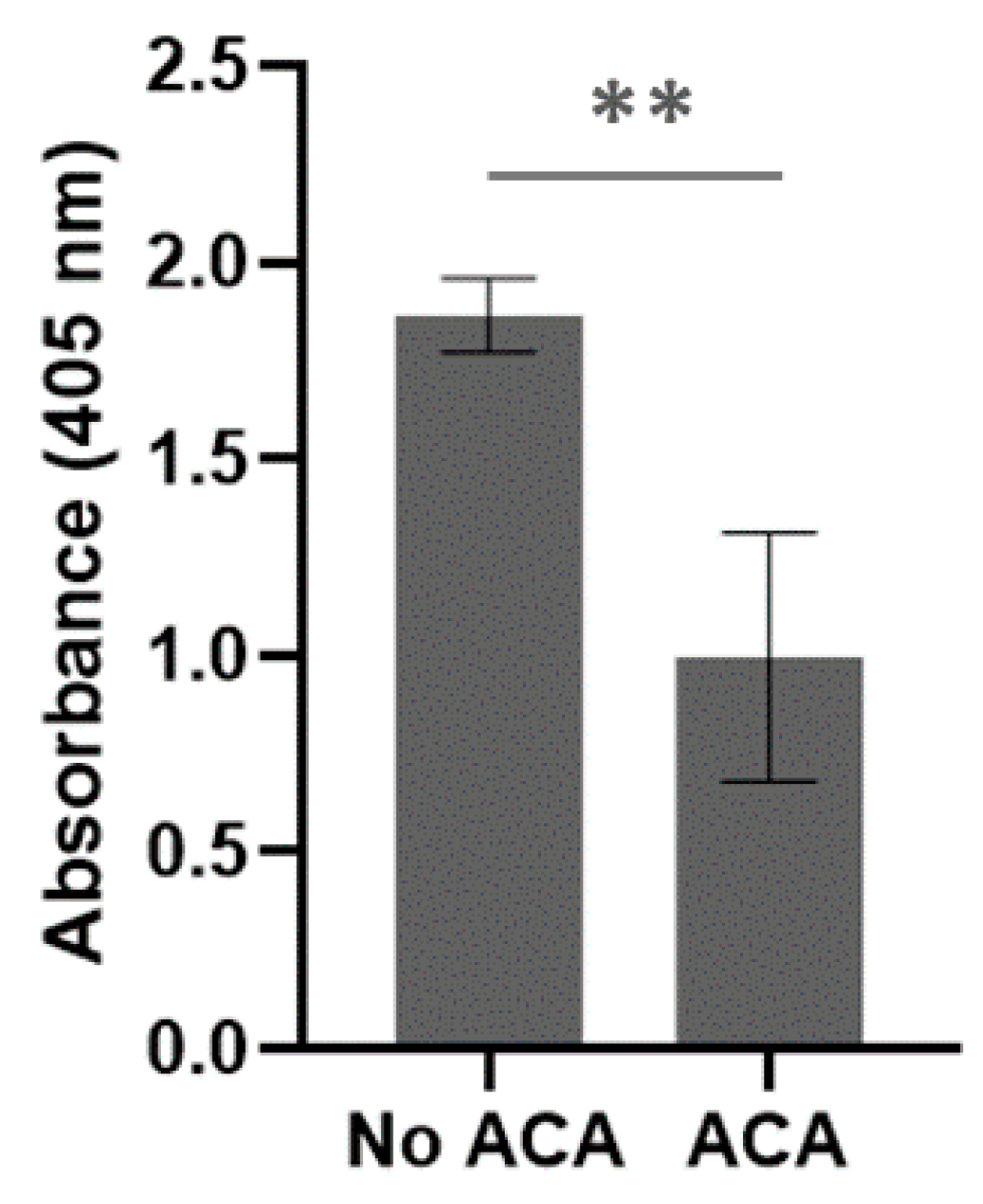
2.5. BbGroEL Is an Immunogenic Protein
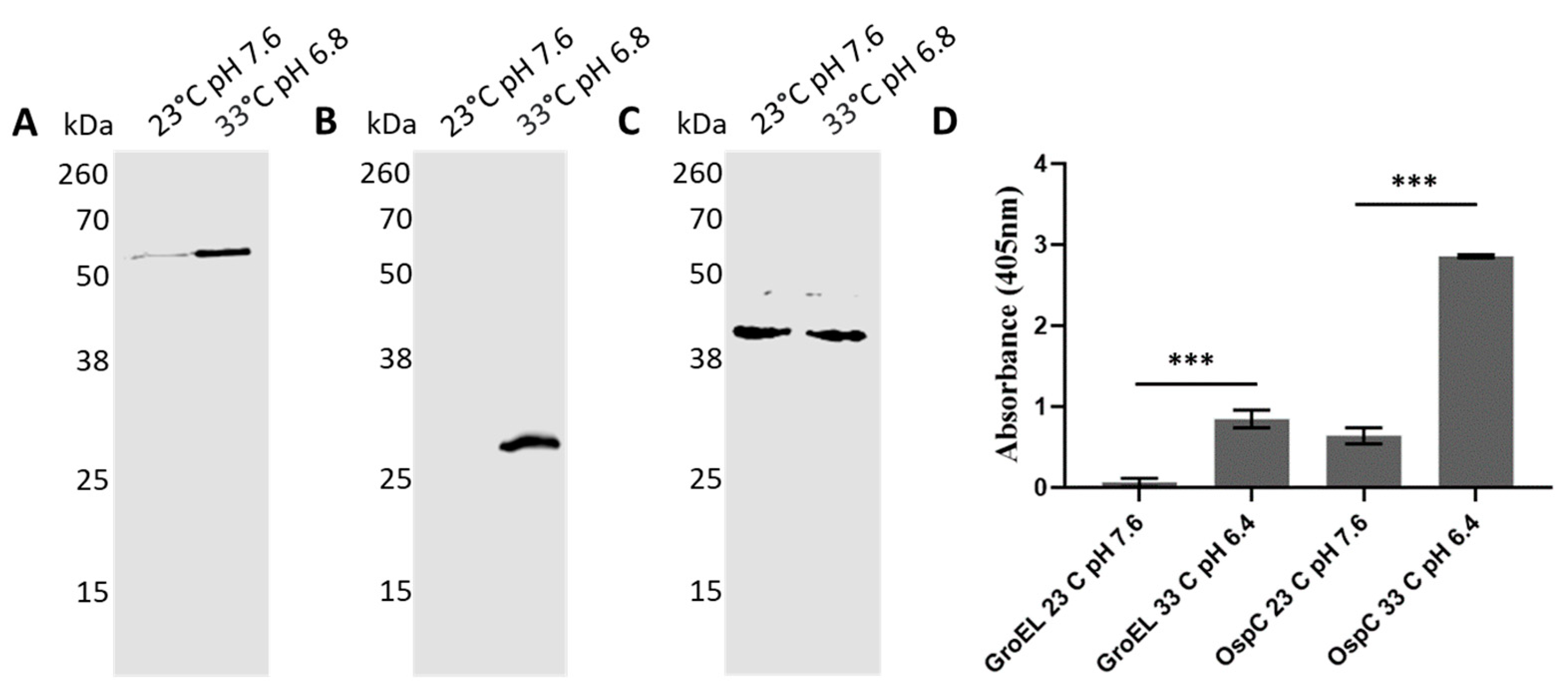
2.6. BbGroEL Protein Production
3. Discussion
4. Materials and Methods
4.1. Bacteria, Cultures, and Sera from Laboratory Animals
4.2. Purification of the OM and PC from B. burgdorferi
4.3. Recombinant Protein Expression and Purification
4.4. Western Blot Analysis
4.5. ELISA
4.6. Immunofluorescence Assay
4.7. PK Treatments
4.8. Effect of Temperature and pH Shift on the Protein Expression of BbGroEL
4.9. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burgdorfer, W.; Barbour, A.G.; Hayes, S.F.; Benach, J.L.; Grunwaldt, E.; Davis, J.P. Lyme Disease—A Tick-Borne Spirochetosis? Science 1982, 216, 1317–1319. [Google Scholar] [CrossRef] [Green Version]
- Benach, J.L.; Bosler, E.M.; Hanrahan, J.P.; Coleman, J.L.; Habicht, G.S.; Bast, T.F.; Cameron, D.J.; Ziegler, J.L.; Barbour, A.G.; Burgdorfer, W.; et al. Spirochetes Isolated from the Blood of Two Patients with Lyme Disease. N. Engl. J. Med. 1983, 308, 740–742. [Google Scholar] [CrossRef]
- Coleman, J.L.; Sellati, T.J.; Testa, J.E.; Kew, R.R.; Furie, M.B.; Benach, J.L. Borrelia burgdorferi Binds Plasminogen, Resulting in Enhanced Penetration of Endothelial Monolayers. Infect. Immun. 1995, 63, 2478–2484. [Google Scholar] [CrossRef] [Green Version]
- Coleman, J.L.; Gebbia, J.A.; Piesman, J.; Degen, J.L.; Bugge, T.H.; Benach, J.L. Plasminogen Is Required for Efficient Dissemination of B. Burgdorferi in Ticks and for Enhancement of Spirochetemia in Mice. Cell 1997, 89, 1111–1119. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, H.; Wallich, R.; Simon, M.M.; Kramer, M.D. The Outer Surface Protein A of the Spirochete Borrelia burgdorferi Is a plasmin(ogen) Receptor. Proc. Natl. Acad. Sci. USA 1994, 91, 12594–12598. [Google Scholar] [CrossRef] [Green Version]
- Lagal, V.; Portnoï, D.; Faure, G.; Postic, D.; Baranton, G. Borrelia burgdorferi Sensu Stricto Invasiveness Is Correlated With OspC–plasminogen Affinity. Microbes Infect. 2006, 8, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Onder, O.; Humphrey, P.T.; McOmber, B.; Korobova, F.; Francella, N.; Greenbaum, D.C.; Brisson, D. OspC Is Potent Plasminogen Receptor on Surface of Borrelia Burgdorferi. J. Biol. Chem. 2012, 287, 16860–16868. [Google Scholar] [CrossRef] [Green Version]
- Koenigs, A.; Hammerschmidt, C.; Jutras, B.L.; Pogoryelov, D.; Barthel, D.; Skerka, C.; Kugelstadt, D.; Wallich, R.; Stevenson, B.; Zipfel, P.F.; et al. BBA70 of Borrelia burgdorferi Is a Novel Plasminogen-Binding Protein. J. Biol. Chem. 2013, 288, 25229–25243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brissette, C.A.; Haupt, K.; Barthel, D.; Cooley, A.E.; Bowman, A.; Skerka, C.; Wallich, R.; Zipfel, P.F.; Kraiczy, P.; Stevenson, B. Borrelia burgdorferi Infection-Associated Surface Proteins ErpP, ErpA, and ErpC Bind Human Plasminogen. Infect. Immun. 2008, 77, 300–306. [Google Scholar] [CrossRef] [Green Version]
- Klempner, M.S.; Noring, R.; Epstein, M.P.; McCloud, B.; Hu, R.; Limentani, S.A.; Rogers, R.A. Binding of Human Plasminogen and Urokinase-Type Plasminogen Activator to the Lyme Disease Spirochete, Borrelia Burgdorferi. J. Infect. Dis. 1995, 171, 1258–1265. [Google Scholar] [CrossRef]
- Fuchs, H.; Simon, M.M.; Wallich, R.; Bechtel, M.; Kramer, M.D. Borrelia burgdorferi induces secretion of pro-urokinase-type plasminogen activator by human monocytes. Infect. Immun. 1996, 64, 4307–4312. [Google Scholar] [CrossRef] [Green Version]
- Klempner, M.S.; Noring, R.; Epstein, M.P.; McCloud, B.; Rogers, R.A. Binding of Human Urokinase Type Plasminogen Activator and Plasminogen to Borrelia Species. J. Infect. Dis. 1996, 174, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Castellino, F.J.; McCance, S.G. The Kringle Domains of Human Plasminogen. Novartis Found. Symp. 2007, 212, 46–65. [Google Scholar] [CrossRef]
- Brissette, C.A.; Gaultney, R.A. That’s My Story, and I’m Sticking to it—An Update on B. burgdorferi Adhesins. Front. Cell. Infect. Microbiol. 2014, 4, 41. [Google Scholar] [CrossRef] [PubMed]
- Antonara, S.; Ristow, L.; Coburn, J. Adhesion Mechanisms of Borrelia burgdorferi. Adv. Exp. Med. Biol. 2011, 715, 35–49. [Google Scholar] [CrossRef] [Green Version]
- Hallström, T.; Haupt, K.; Kraiczy, P.; Hortschansky, P.; Wallich, R.; Skerka, C.; Zipfel, P.F. Complement Regulator–Acquiring Surface Protein 1 OfBorrelia BurgdorferiBinds to Human Bone Morphogenic Protein 2, Several Extracellular Matrix Proteins, and Plasminogen. J. Infect. Dis. 2010, 202, 490–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.P.; Chen, Q.; Ritchie, J.A.; Dufour, N.P.; Fischer, J.R.; Coburn, J.; Leong, J.M. Glycosaminoglycan Binding ByBorrelia Burgdorferiadhesin BBK32 Specifically and Uniquely Promotes Joint Colonization. Cell. Microbiol. 2015, 17, 860–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, G.; Ma, W.; Maurer, L.M.; Potts, J.R.; Mosher, D.F. Borrelia burgdorferi Protein BBK32 Binds to Soluble Fibronectin via the N-Terminal 70-KDa Region, Causing Fibronectin to Undergo Conformational Extension. J. Biol. Chem. 2014, 289, 22490–22499. [Google Scholar] [CrossRef] [Green Version]
- Zhi, H.; Weening, E.H.; Barbu, E.M.; Hyde, J.A.; Hook, M.; Skare, J.T. The BBA33 Lipoprotein Binds Collagen and ImpactsBorrelia Burgdorferipathogenesis. Mol. Microbiol. 2015, 96, 68–83. [Google Scholar] [CrossRef] [Green Version]
- Schlachter, S.; Seshu, J.; Lin, T.; Norris, S.; Parveen, N. The Borrelia burgdorferi Glycosaminoglycan Binding Protein Bgp in the B31 Strain Is Not Essential for Infectivity Despite Facilitating Adherence and Tissue Colonization. Infect. Immun. 2017, 86, e00667-17. [Google Scholar] [CrossRef] [Green Version]
- Toledo, A.; Coleman, J.L.; Kuhlow, C.J.; Crowley, J.T.; Benach, J.L. The Enolase of Borrelia burgdorferi Is a Plasminogen Receptor Released in Outer Membrane Vesicles. Infect. Immun. 2011, 80, 359–368. [Google Scholar] [CrossRef] [Green Version]
- Nogueira, S.V.; Smith, A.A.; Qin, J.H.; Pal, U. A Surface Enolase Participates in Borrelia Burgdorferi-Plasminogen Interaction and Contributes to Pathogen Survival Within Feeding Ticks. Infect. Immun. 2011, 80, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Floden, A.M.; Watt, J.A.; Brissette, C.A. Borrelia burgdorferi Enolase Is a Surface-Exposed Plasminogen Binding Protein. PLoS ONE 2011, 6, e27502. [Google Scholar] [CrossRef] [Green Version]
- Nowalk, A.J.; Nolder, C.; Clifton, D.R.; Carroll, J.A. Comparative Proteome Analysis of Subcellular Fractions from Borrelia burgdorferi by NEPHGE and IPG. Proteomics 2006, 6, 2121–2134. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.L.; Crowley, J.T.; Toledo, A.M.; Benach, J.L. The HtrA Protease of B Orrelia Burgdorferi Degrades Outer Membrane Protein BmpD and Chemotaxis Phosphatase CheX. Mol. Microbiol. 2013, 88, 619–633. [Google Scholar] [CrossRef]
- Ye, M.; Sharma, K.; Thakur, M.; Smith, A.A.; Buyuktanir, O.; Xiang, X.; Yang, X.; Promnares, K.; Lou, Y.; Pal, U.; et al. HtrA, a Temperature- and Stationary Phase-Activated Protease Involved in Maturation of a Key Microbial Virulence Determinant, Facilitates Borrelia burgdorferi Infection in Mammalian Hosts. Infect. Immun. 2016, 84, 2372–2381. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Yang, X.; Kumar, M.; Pal, U. BB0323 Function Is Essential for Borrelia BurgdorferiVirulence and Persistence through Tick-Rodent Transmission Cycle. J. Infect. Dis. 2009, 200, 1318–1330. [Google Scholar] [CrossRef] [Green Version]
- Russell, T.M.; DeLorey, M.J.; Johnson, B.J.B. Borrelia burgdorferi BbHtrA Degrades Host ECM Proteins and Stimulates Release of Inflammatory Cytokines In Vitro. Mol. Microbiol. 2013, 90, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, S.; Rohde, M.; Hammerschmidt, S. Glyceraldehyde-3-Phosphate Dehydrogenase of Streptococcus Pneumoniae Is a Surface-Displayed Plasminogen-Binding Protein. Infect. Immun. 2004, 72, 2416–2419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiting, G.; Evans, J.; Patel, S.; Gillespie, S. Purification of Native α-Enolase from Streptococcus Pneumoniae That Binds Plasminogen and Is Immunogenic. J. Med. Microbiol. 2002, 51, 837–843. [Google Scholar] [CrossRef] [Green Version]
- Boone, T.J.; Burnham, C.A.D.; Tyrrell, G.J. Binding of Group B Streptococcal Phosphoglycerate Kinase to Plasminogen and Actin. Microb. Pathog. 2011, 51, 255–261. [Google Scholar] [CrossRef]
- Bergmann, S.; Schoenen, H.; Hammerschmidt, S. The Interaction Between Bacterial Enolase and Plasminogen Promotes Adherence of Streptococcus Pneumoniae to Epithelial and Endothelial Cells. Int. J. Med. Microbiol. 2013, 303, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Hammerschmidt, S.; Malm, S.; Bergmann, S.; Riesbeck, K.; Blom, A.M. Enolase OfStreptococcus PneumoniaeBinds Human Complement Inhibitor C4b-Binding Protein and Contributes to Complement Evasion. J. Immunol. 2012, 189, 3575–3584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagemann, L.; Gründel, A.; Jacobs, E.; Dumke, R. The Surface-Displayed Chaperones GroEL and DnaK of Mycoplasma Pneumoniae Interact with Human Plasminogen and Components of the Extracellular Matrix. Pathog. Dis. 2017, 75. [Google Scholar] [CrossRef]
- Chung, M.C.; Tonry, J.H.; Narayanan, A.; Manes, N.P.; Mackie, R.S.; Gutting, B.; Mukherjee, D.V.; Popova, T.G.; Kashanchi, F.; Bailey, C.L.; et al. Bacillus Anthracis Interacts With Plasmin(ogen) to Evade C3b-Dependent Innate Immunity. PLoS ONE 2011, 6, e18119. [Google Scholar] [CrossRef] [Green Version]
- Scopio, A.; Johnson, P.; Laquerre, A.; Nelson, D.R. Subcellular Localization and Chaperone Activities of Borrelia burgdorferi Hsp60 and Hsp70. J. Bacteriol. 1994, 176, 6449–6456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toledo, A.; Huang, Z.; Coleman, J.L.; London, E.; Benach, J.L. Lipid Rafts Can Form in the Inner and Outer Membranes OfBorrelia Burgdorferiand Have Different Properties and Associated Proteins. Mol. Microbiol. 2018, 108, 63–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toledo, A.; Pérez, A.; Coleman, J.L.; Benach, J.L. The Lipid Raft Proteome OfBorrelia Burgdorferi. Proteomics 2015, 15, 3662–3675. [Google Scholar] [CrossRef]
- Tokarz, R.; Anderton, J.M.; Katona, L.I.; Benach, J.L. Combined Effects of Blood and Temperature Shift on Borrelia burgdorferi Gene Expression As Determined by Whole Genome DNA Array. Infect. Immun. 2004, 72, 5419–5432. [Google Scholar] [CrossRef] [Green Version]
- Grimm, D.; Tilly, K.; Byram, R.; Stewart, P.E.; Krum, J.G.; Bueschel, D.M.; Schwan, T.G.; Policastro, P.F.; Elias, A.F.; Rosa, P.A. Outer-Surface Protein C of the Lyme Disease Spirochete: A Protein Induced in Ticks for Infection of Mammals. Proc. Natl. Acad. Sci. USA 2004, 101, 3142–3147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilly, K.; Bestor, A.; Jewett, M.W.; Rosa, P. Rapid Clearance of Lyme Disease Spirochetes Lacking OspC from Skin. Infect. Immun. 2006, 75, 1517–1519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilly, K.; Krum, J.G.; Bestor, A.; Jewett, M.W.; Grimm, D.; Bueschel, D.; Byram, R.; Dorward, D.; VanRaden, M.J.; Stewart, P.; et al. Borrelia burgdorferi OspC Protein Required Exclusively in a Crucial Early Stage of Mammalian Infection. Infect. Immun. 2006, 74, 3554–3564. [Google Scholar] [CrossRef] [Green Version]
- Caine, J.A.; Coburn, J. Multifunctional and Redundant Roles of Borrelia burgdorferi Outer Surface Proteins in Tissue Adhesion, Colonization, and Complement Evasion. Front. Immunol. 2016, 7, 442. [Google Scholar] [CrossRef] [Green Version]
- Henderson, B. An Overview of Protein Moonlighting in Bacterial Infection. Biochem. Soc. Trans. 2014, 42, 1720–1727. [Google Scholar] [CrossRef]
- Jeffery, C.J. Protein Moonlighting: What Is It, and Why Is It Important? Philos. Trans. R. Soc. B Biol. Sci. 2018, 373. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.; Martin, A. Bacterial Virulence in the Moonlight: Multitasking Bacterial Moonlighting Proteins Are Virulence Determinants in Infectious Disease. Infect. Immun. 2011, 79, 3476–3491. [Google Scholar] [CrossRef] [Green Version]
- Jeffery, C.J. Intracellular/Surface Moonlighting Proteins That Aid in the Attachment of Gut Microbiota to the Host. AIMS Microbiol. 2019, 5, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, C.J. Why Study Moonlighting Proteins? Front. Genet. 2015, 6, 211. [Google Scholar] [CrossRef] [Green Version]
- Shoberg, R.J.; Thomas, D.D. Borrelia burgdorferi Vesicle Production Occurs via a Mechanism Independent of Immunoglobulin M Involvement. Infect. Immun. 1995, 63, 4857–4861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoberg, R.J.; Thomas, D.D. Specific Adherence of Borrelia burgdorferi Extracellular Vesicles to Human Endothelial Cells in Culture. Infect. Immun. 1993, 61, 3892–3900. [Google Scholar] [CrossRef] [Green Version]
- Ellis, T.N.; Kuehn, M.J. Virulence and Immunomodulatory Roles of Bacterial Outer Membrane Vesicles. Microbiol. Mol. Biol. Rev. 2010, 74, 81–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Promnares, K.; Qin, J.; He, M.; Shroder, D.Y.; Kariu, T.; Wang, Y.; Pal, U. Characterization of Multiprotein Complexes of TheBorrelia BurgdorferiOuter Membrane Vesicles. J. Proteome Res. 2011, 10, 4556–4566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, J. Protein Folding Assisted by the GroEL/GroES Chaperonin System. Biochemistry 1998, 63, 374–381. [Google Scholar]
- Bergonzelli, G.E.; Granato, D.; Pridmore, R.D.; Marvin-Guy, L.F.; Donnicola, D.; Corthésy-TheulazI, E. GroEL of Lactobacillus Johnsonii La1 (NCC 533) Is Cell Surface Associated: Potential Role in Interactions With the Host and the Gastric Pathogen Helicobacter Pylori. Infect. Immun. 2006, 74, 425–434. [Google Scholar] [CrossRef] [Green Version]
- Hennequin, C.; Collignon, A.; Bourlioux, P.; Waligora-Dupriet, A.J.; Karjalainen, T.; Barc, M.C.; Porcheray, F. GroEL (Hsp60) of Clostridium Difficile Is Involved in Cell Adherence. Microbiol. 2001, 147, 87–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ensgraber, M.; Loos, M. A 66-Kilodalton Heat Shock Protein of Salmonella Typhimurium Is Responsible for Binding of the Bacterium to Intestinal Mucus. Infect. Immun. 1992, 60, 3072–3078. [Google Scholar] [CrossRef] [Green Version]
- Wampler, J.L.; Kim, K.P.; Jaradat, Z.; Bhunia, A.K. Heat Shock Protein 60 Acts As a Receptor for the Listeria Adhesion Protein in Caco-2 Cells. Infect. Immun. 2004, 72, 931–936. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, P.S.; Garduno, R.A. Surface-Associated Heat Shock Proteins of Legionella Pneumophila and Helicobacter Pylori: Roles in Pathogenesis and Immunity. Infect. Dis. Obstet. Gynecol. 1999, 7, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, H.; Osaki, T.; Kurihara, N.; Taguchi, H.; Hanawa, T.; Yamamoto, T.; Kamiya, S. Heat-Shock Protein 60 Homologue of Helicobacter Pylori Is Associated With Adhesion of H. Pylori to Human Gastric Epithelial Cells. J. Med. Microbiol. 1997, 46, 825–831. [Google Scholar] [CrossRef]
- Pechine, S.; Hennequin, C.; Boursier, C.; Hoÿs, S.; Collignon, A. Immunization Using GroEL Decreases Clostridium Difficile Intestinal Colonization. PLoS ONE 2013, 8, e81112. [Google Scholar] [CrossRef]
- Nordstrand, A.; Shamaei-Tousi, A.; Ny, A.; Bergström, S. Delayed Invasion of the Kidney and Brain by Borrelia Crocidurae in Plasminogen-Deficient Mice. Infect. Immun. 2001, 69, 5832–5839. [Google Scholar] [CrossRef] [Green Version]
- Coleman, J.L.; Benach, J.L. Use of the Plasminogen Activation System by Microorganisms. J. Lab. Clin. Med. 1999, 134, 567–576. [Google Scholar] [CrossRef]
- Gebbia, J.A.; Monco, J.C.G.; Degen, J.L.; Bugge, T.H.; Benach, J.L. The Plasminogen Activation System Enhances Brain and Heart Invasion in Murine Relapsing Fever Borreliosis. J. Clin. Investig. 1999, 103, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Coleman, J.L.; Benach, J.L. The Urokinase Receptor Can Be Induced by Borrelia burgdorferi through Receptors of the Innate Immune System. Infect. Immun. 2003, 71, 5556–5564. [Google Scholar] [CrossRef] [Green Version]
- Coleman, J.L.; Gebbia, J.A.; Benach, J.L. Borrelia burgdorferi and other bacterial products induce expression and release of the urokinase receptor (CD87). J. Immunol. 2001, 166, 473–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hovius, J.W.R.; Bijlsma, M.F.; Van Der Windt, G.J.W.; Wiersinga, W.J.; Boukens, B.J.D.; Coumou, J.; Oei, A.; De Beer, R.; De Vos, A.F.; Veer, C.V.T.; et al. The Urokinase Receptor (uPAR) Facilitates Clearance of Borrelia Burgdorferi. PLoS Pathog. 2009, 5, e1000447. [Google Scholar] [CrossRef] [PubMed]
- Sanderson-Smith, M.L.; De Oliveira, D.M.P.; Ranson, M.; McArthur, J.D. Bacterial Plasminogen Receptors: Mediators of a Multifaceted Relationship. J. Biomed. Biotechnol. 2012, 2012, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanegas, G.; Quiñones, W.; Carrasco-López, C.; Concepción, J.L.; Albericio, F.; Avilán, L. Enolase As a Plasminogen Binding Protein in Leishmania Mexicana. Parasitol. Res. 2007, 101, 1511–1516. [Google Scholar] [CrossRef]
- Stevenson, B.; Schwan, T.G.; Rosa, P.A. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect. Immun. 1995, 63, 4535–4539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carroll, J.A.; Garon, C.F.; Schwan, T.G. Effects of environmental pH on membrane proteins in Borrelia burgdorferi. Infect. Immun. 1999, 67, 3181–3187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roncarati, D.; Scarlato, V. Regulation of Heat-Shock Genes in Bacteria: From Signal Sensing to Gene Expression Output. FEMS Microbiol. Rev. 2017, 41, 549–574. [Google Scholar] [CrossRef] [Green Version]
- Slamti, L.; Livny, J.; Waldor, M.K. Global Gene Expression and Phenotypic Analysis of a Vibrio Cholerae RpoH Deletion Mutant. J. Bacteriol. 2006, 189, 351–362. [Google Scholar] [CrossRef] [Green Version]
- Nagai, H.; Yano, R.; Erickson, J.W.; Yura, T. Transcriptional Regulation of the Heat Shock Regulatory Gene RpoH in Escherichia Coli: Involvement of a Novel Catabolite-Sensitive Promoter. J. Bacteriol. 1990, 172, 2710–2715. [Google Scholar] [CrossRef] [Green Version]
- Allan, B.; Linseman, M.; Macdonald, L.A.; Lam, J.S.; Kropinski, A.M. Heat Shock Response of Pseudomonas Aeruginosa. J. Bacteriol. 1988, 170, 3668–3674. [Google Scholar] [CrossRef] [Green Version]
- Ojaimi, C.; Brooks, C.; Casjens, S.; Rosa, P.; Elias, A.; Barbour, A.; Jasinskas, A.; Benach, J.; Katona, L.; Radolf, J.; et al. Profiling of Temperature-Induced Changes in Borrelia burgdorferi Gene Expression by Using Whole Genome Arrays. Infect. Immun. 2003, 71, 1689–1705. [Google Scholar] [CrossRef] [Green Version]
- Guerreiro, H.; Croda, J.; Flannery, B.; Mazel, M.; Matsunaga, J.; Reis, M.G.; Levett, P.N.; Ko, A.I.; Haake, D.A. Leptospiral Proteins Recognized During the Humoral Immune Response to Leptospirosis in Humans. Infect. Immun. 2001, 69, 4958–4968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKevitt, M.; Brinkman, M.B.; McLoughlin, M.; Perez, C.; Howell, J.K.; Weinstock, G.M.; Norris, S.J.; Palzkill, T. Genome Scale Identification of Treponema Pallidum Antigens. Infect. Immun. 2005, 73, 4445–4450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haake, D.A.; Matsunaga, J. Characterization of the Leptospiral Outer Membrane and Description of Three Novel Leptospiral Membrane Proteins. Infect. Immun. 2002, 70, 4936–4945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, M.L.; Atzingen, M.V.; Oliveira, R.; Mendes, R.S.; Domingos, R.F.; Vasconcellos, S.A.; Nascimento, A.L.T.O. Plasminogen Binding Proteins and Plasmin Generation on the Surface ofLeptospiraspp.: The Contribution to the Bacteria-Host Interactions. J. Biomed. Biotechnol. 2012, 2012, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Skare, J.T.; Shang, E.S.; Foley, D.M.; Blanco, D.R.; Champion, C.I.; Mirzabekov, T.; Sokolov, Y.; Kagan, B.L.; Miller, J.N.; Lovett, M.A. Virulent Strain Associated Outer Membrane Proteins of Borrelia Burgdorferi. J. Clin. Investig. 1995, 96, 2380–2392. [Google Scholar] [CrossRef]
- Lenhart, T.R.; Akins, D.R. Borrelia burgdorferi Locus BB0795 Encodes a BamA Orthologue Required for Growth and Efficient Localization of Outer Membrane Proteins. Mol. Microbiol. 2010, 75, 692–709. [Google Scholar] [CrossRef] [Green Version]
- Barbour, A.G.; Tessier, S.L.; Todd, W.J. Lyme Disease Spirochetes and Ixodid Tick Spirochetes Share a Common Surface Antigenic Determinant Defined by a Monoclonal Antibody. Infect. Immun. 1983, 41, 795–804. [Google Scholar] [CrossRef] [Green Version]
- Chu, C.Y.; Stewart, P.E.; Bestor, A.; Hansen, B.; Lin, T.; Gao, L.; Norris, S.J.; Rosa, P.A. Function of the Borrelia burgdorferi FtsH Homolog Is Essential for Viability Both In Vitro and In Vivo and Independent of HflK/C. MBio 2016, 7, e00404-16. [Google Scholar] [CrossRef] [Green Version]
- Tilly, K.; Hauser, R.; Campbell, J.; Ostheimer, G.J. Isolation of DnaJ, DnaK, and GrpE Homologues from Borrelia burgdorferi and Complementation of Escherichia Coli Mutants. Mol. Microbiol. 1993, 7, 359–369. [Google Scholar] [CrossRef]
- Coleman, J.L.; Benach, J.L. Characterization of Antigenic Determinants of Borrelia burgdorferi Shared by Other Bacteria. J. Infect. Dis. 1992, 165, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.L.; Benach, J.L. Identification and Characterization of an Endoflagellar Antigen of Borrelia Burgdorferi. J. Clin. Investig. 1989, 84, 322–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Hage, N.; Babb, K.; Carroll, J.A.; Lindstrom, N.; Fischer, E.R.; Miller, J.C.; Gilmore, R.D., Jr.; Mbow, M.L.; Stevenson, B. Surface exposure and protease insensitivity of Borrelia burgdorferi Erp (OspEF-related) lipoproteins. Microbiology 2001, 147, 821–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, D.L.; Akins, D.R.; Bourell, K.W.; Lahdenne, P.; Norgard, M.V.; Radolf, J.D. Limited Surface Exposure of Borrelia burgdorferi Outer Surface Lipoproteins. Proc. Natl. Acad. Sci. USA 1996, 93, 7973–7978. [Google Scholar] [CrossRef] [Green Version]
- Bunikis, J.; Barbour, A.G. Access of antibody or trypsin to an integral outer membrane protein (P66) of Borrelia burgdorferi is hindered by Osp lipoproteins. Infect. Immun. 1999, 67, 2874–2883. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cafiero, T.; Toledo, A. Borrelia burgdorferi Surface Exposed GroEL Is a Multifunctional Protein. Pathogens 2021, 10, 226. https://doi.org/10.3390/pathogens10020226
Cafiero T, Toledo A. Borrelia burgdorferi Surface Exposed GroEL Is a Multifunctional Protein. Pathogens. 2021; 10(2):226. https://doi.org/10.3390/pathogens10020226
Chicago/Turabian StyleCafiero, Thomas, and Alvaro Toledo. 2021. "Borrelia burgdorferi Surface Exposed GroEL Is a Multifunctional Protein" Pathogens 10, no. 2: 226. https://doi.org/10.3390/pathogens10020226





