The First Record of Echinococcus ortleppi (G5) Tapeworms in Grey Wolf (Canis lupus)
Abstract
:1. Introduction
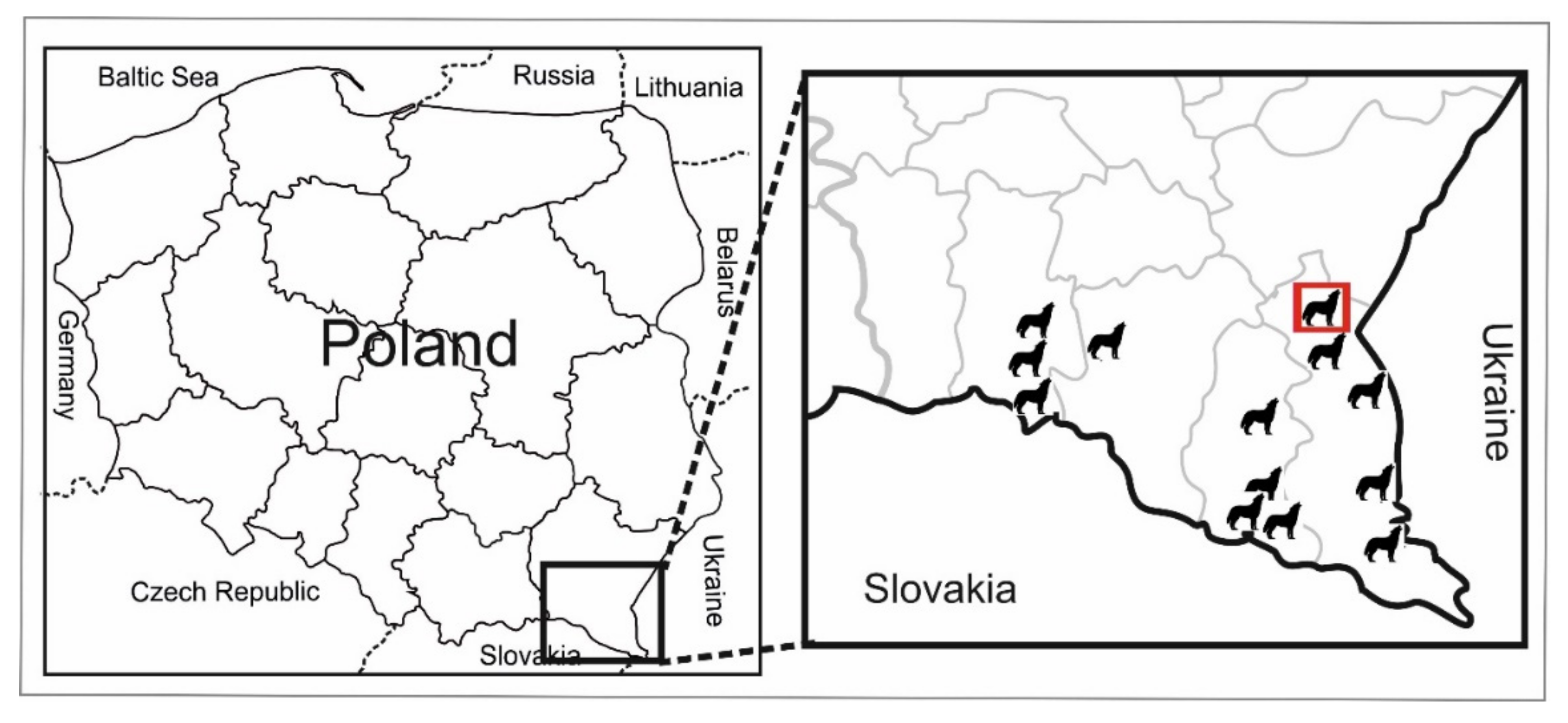
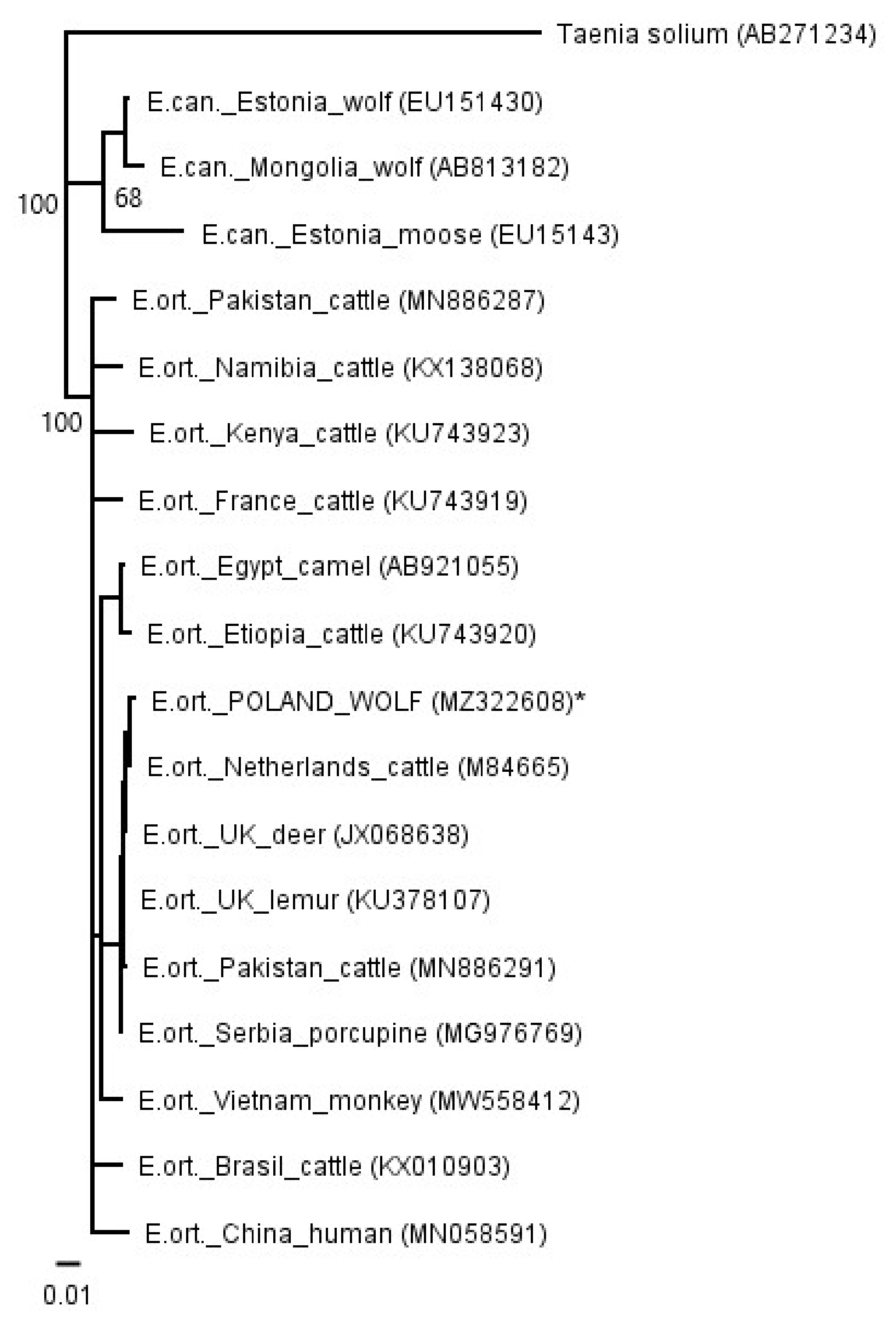
2. Results
3. Discussion
4. Materials and Methods
4.1. Wolves
4.2. Sedimentation and Counting Technique (SCT)
4.3. PCR and Sequencing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Romig, T.; Deplazes, P.; Jenkins, D.; Giraudoux, P.; Massolo, A.; Craig, P.S.; Wassermann, M.; Takahashi, K.; de la Rue, M. Chapter Five—Ecology and Life Cycle Patterns of Echinococcus Species. In Advances in Parasitology, Echinococcus and Echinococcosis; Pt, A., Thompson, R.C.A., Deplazes, P., Lymbery, A.J., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 95, pp. 213–314. [Google Scholar]
- Thompson, R.C.A.; McManus, D.P. Towards a taxonomic revision of the genus Echinococcus. Trends Parasitol. 2002, 18, 452–457. [Google Scholar] [CrossRef]
- Lymbery, A.J. Chapter three-Phylogenetic Pattern, Evolutionary Processes and Species Delimitation in the Genus Echinococcus. In Advances in Parasitology, Echinococcus and Echinococcosis; Pt, A., Thompson, R.C.A., Deplazes, P., Lymbery, A.J., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 95, pp. 111–145. [Google Scholar]
- Foreyt, W.J.; Drew, M.L.; Atkinson, M.; McCauley, D. Echinococcus granulosus in Gray Wolves and Ungulates in Idaho and Montana, USA. J. Wildl. Dis. 2009, 45, 1208–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pipas, M.J.; Fowler, D.R.; Bardsley, K.D.; Bangoura, B. Survey of coyotes, red foxes and wolves from Wyoming, USA, for Echinococcus granulosus s. l. Parasitol. Res. 2021, 120, 1335–1340. [Google Scholar] [CrossRef]
- Abdybekova, A.M.; Torgerson, P.R. Frequency distributions of helminths of wolves in Kazakhstan. Vet. Parasitol. 2012, 184, 348–351. [Google Scholar] [CrossRef] [Green Version]
- Ito, A.; Chuluunbaatar, G.; Yanagida, T.; Davaasuren, A.; Sumiya, B.; Asakawa, M.; Ki, T.; Nakaya, K.; Davaajav, A.; Dorjsuren, T.; et al. Echinococcus species from red foxes, corsac foxes, and wolves in Mongolia. Parasitology 2013, 140, 1648–1654. [Google Scholar] [CrossRef] [Green Version]
- Moks, E.; Jogisalu, I.; Saarma, U.; Talvik, H.; Jarvis, T.; Valdmann, H. Helminthologic survey of the wolf (Canis lupus) in Estonia, with an emphasis on Echinococcus granulosus. J. Wildl. Dis. 2006, 42, 359–365. [Google Scholar] [CrossRef] [Green Version]
- Kazlauskas, J.; Prusaite, J. Helminths of carnivores in Lithuania. Acta Parasitol. Lith. 1976, 12, 33–40. [Google Scholar]
- Marcinkute, A.; Sarkunas, M.; Moks, E.; Saarma, U.; Jokelainen, P.; Bagrade, G.; Laivacuma, S.; Strupas, K.; Sokolovas, V.; Deplazes, P. Echinococcus infections in the Baltic region. Vet. Parasitol. 2015, 213, 121–131. [Google Scholar] [CrossRef] [Green Version]
- Shimalov, V.V.; Shimalov, V.T. Helminth fauna of the wolf (Canis lupus Linnaeus, 1758) in Belorussian Polesie. Parasitol. Res. 2000, 86, 163–164. [Google Scholar] [CrossRef] [PubMed]
- Hirvela-Koski, V.; Haukisalmi, V.; Kilpela, S.S.; Nylund, M.; Koski, P. Echinococcus granulosus in Finland. Vet. Parasitol. 2003, 111, 175–192. [Google Scholar] [CrossRef]
- Guberti, V.; Bolognini, M.; Lanfranchi, P.; Battelli, G. Echinococcus granulosus in the wolf in Italy. Parassitologia 2004, 46, 425–427. [Google Scholar]
- Jarosova, J.; Antolova, D.; Snabel, V.; Guimaraes, N.; Stofik, J.; Urban, P.; Cavallero, S.; Miterpakova, M. The fox tapeworm, Echinococcus multilocularis, in grey wolves and dogs in Slovakia: Epidemiology and genetic analysis. J. Helminthol. 2020, 94, e168. [Google Scholar] [CrossRef] [PubMed]
- Nowak, S.; Mysłajek, R.W. Wolf recovery and population dynamics in Western Poland, 2001–2012. Mamm. Res. 2016, 61, 83–98. [Google Scholar] [CrossRef]
- Mysłajek, R.W.; Tracz, M.; Tracz, M.; Tomczak, P.; Szewczyk, M.; Niedźwiecka, N.; Nowak, S. Spatial organization in wolves Canis lupus recolonizing north-west Poland: Large territories at low population density. Mamm. Biol. 2018, 92, 37–44. [Google Scholar] [CrossRef]
- CSO. Environmental Protection—Report 2019; Polish Central Statistical Office: Warsaw, Poland, 2019.
- Kloch, A.; Bednarska, M.; Bajer, A. Intestinal macro- and microparasites of wolves (Canis lupus L.) from north-eastern Poland recovered by coprological study. Ann. Agric. Env. Med. 2005, 12, 237–245. [Google Scholar]
- Borecka, A.; Gawor, J.; Zieba, F. A survey of intestinal helminths in wild carnivores from the Tatra National Park, southern Poland. Ann. Parasit. 2013, 59, 169–172. [Google Scholar]
- Karamon, J.; Samorek-Pierog, M.; Kochanowski, M.; Dabrowska, J.; Sroka, J.; Golab, E.; Umhang, G.; Cencek, T. First detection of Echinococcus multilocularis in dogs in a highly endemic area of Poland. Folia Parasitol. 2016, 63, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karamon, J.; Sroka, J.; Dabrowska, J.; Bilska-Zajac, E.; Zdybel, J.; Kochanowski, M.; Rozycki, M.; Cencek, T. First report of Echinococcus multilocularis in cats in Poland: A monitoring study in cats and dogs from a rural area and animal shelter in a highly endemic region. Parasite Vector 2019, 12, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Machnicka–Rowińska, B.; Rocki, B.; Dziemian, E.; Kolodziej–Sobocinska, M. Raccoon dog (Nyctereutes procyonoides) the new host of Echinococcus multilocularis in Poland. Wiad. Parazytol. 2002, 48, 65–68. [Google Scholar] [PubMed]
- Malczewski, A. Helminth parasites in foxes and mink in Poland. Med. Weter. 1962, 18, 730–734. [Google Scholar]
- Grzywinski, L. The internal parasites of furred animals on farms in the western territories in Poland. Wiad. Parazytol. 1956, 2, 155–156. [Google Scholar]
- Guerra, D.; Armua-Fernandez, M.T.; Silva, M.; Bravo, I.; Santos, N.; Deplazes, P.; Madeira de Carvalho, L.M. Taeniid species of the Iberian wolf (Canis lupus signatus) in Portugal with special focus on Echinococcus spp. Int. J. Parasitol. Parasites Wildl. 2013, 2, 50–53. [Google Scholar] [CrossRef] [Green Version]
- Nakao, M.; Yanagida, T.; Konyaev, S.; Lavikainen, A.; Odnokurtsev, V.A.; Zaikov, V.A.; Ito, A. Mitochondrial phylogeny of the genus Echinococcus (Cestoda: Taeniidae) with emphasis on relationships among Echinococcus canadensis genotypes. Parasitology 2013, 140, 1625–1636. [Google Scholar] [CrossRef]
- Konyaev, S.V.; Yanagida, T.; Nakao, M.; Ingovatova, G.M.; Shoykhet, Y.N.; Bondarev, A.Y.; Odnokurtsev, V.A.; Loskutova, K.S.; Lukmanova, G.I.; Dokuchaev, N.E.; et al. Genetic diversity of Echinococcus spp. in Russia. Parasitology 2013, 140, 1637–1647. [Google Scholar] [CrossRef]
- Breyer, I.; Georgieva, D.; Kurdova, R.; Gottstein, B. Echinococcus granulosus strain typing in Bulgaria: The G1 genotype is predominant in intermediate and definitive wild hosts. Parasitol. Res. 2004, 93, 127–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tigre, W.; Deresa, B.; Haile, A.; Gabriel, S.; Victor, B.; Van Pelt, J.; Devleesschauwer, B.; Vercruysse, J.; Dorny, P. Molecular characterization of Echinococcus granulosus s.l. cysts from cattle, camels, goats and pigs in Ethiopia. Vet. Parasitol. 2016, 215, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Pednekar, R.P.; Gatne, M.L.; Thompson, R.C.A.; Traub, R.J. Molecular and morphological characterisation of Echinococcus from food producing animals in India. Vet. Parasitol. 2009, 165, 58–65. [Google Scholar] [CrossRef] [Green Version]
- Mbaya, H.; Magambo, J.; Njenga, S.; Zeyhle, E.; Mbae, C.; Mulinge, E.; Wassermann, M.; Kern, P.; Romig, T. Echinococcus spp. in central Kenya: A different story. Parasitol. Res. 2014, 113, 3789–3794. [Google Scholar] [CrossRef] [PubMed]
- Amer, S.; Helal, I.B.; Kamau, E.; Feng, Y.Y.; Xiao, L.H. Molecular Characterization of Echinococcus granulosus sensu lato from Farm Animals in Egypt. PLoS ONE 2015, 10, e0118509. [Google Scholar] [CrossRef]
- Hodzic, A.; Alic, A.; Supic, J.; Skapur, V.; Duscher, G.G. Echinococcus ortleppi, the cattle strain in a crested porcupine (Hystrix cristata): A new host record. Vet. Parasitol. 2018, 256, 32–34. [Google Scholar] [CrossRef]
- Boufana, B.; Stidworthy, M.F.; Bell, S.; Chantrey, J.; Masters, N.; Unwin, S.; Wood, R.; Lawrence, R.P.; Potter, A.; McGarry, J.; et al. Echinococcus and Taenia spp. from captive mammals in the United Kingdom. Vet. Parasitol. 2012, 190, 95–103. [Google Scholar] [CrossRef]
- Deplazes, P.; Rinaldi, L.; Alvarez Rojas, C.A.; Torgerson, P.R.; Harandi, M.F.; Romig, T.; Antolova, D.; Schurer, J.M.; Lahmar, S.; Cringoli, G.; et al. Global Distribution of Alveolar and Cystic Echinococcosis. Adv. Parasitol. 2017, 95, 315–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dybicz, M.; Borkowski, P.K.; Jonas, M.; Wasiak, D.; Malkowski, P. First Report of Echinococcus ortleppi in Human Cases of Cystic Echinococcosis in Poland. Biomed Res. Int. 2019, 2019, 2474839. [Google Scholar] [CrossRef]
- Bowles, J.; McManus, D.P. NADH dehydrogenase-1 gene-sequences compared for species and strains of the genus Echinococcus. Int. J. Parasit. 1993, 23, 969–972. [Google Scholar] [CrossRef]
- Trachsel, D.; Deplazes, P.; Mathis, A. Identification of taeniid eggs in the faeces from carnivores based on multiplex PCR using targets in mitochondrial DNA. Parasitology 2007, 134, 911–920. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, I.; Branzburg, A.; Campos–Ponce, M.; Abdel Hafez, S.K.; Raoul, F.; Craig, P.S.; Hamburger, J. Copro–diagnosis of Echinococcus granulosus infection in dogs by amplification of a newly identified repeated DNA sequence. Am. J. Trop. Med. Hyg. 2003, 69, 324–330. [Google Scholar] [CrossRef] [Green Version]
- Karamon, J.; Sroka, J.; Dabrowska, J.; Bilska-Zajac, E.; Skrzypek, K.; Rozycki, M.; Zdybel, J.; Cencek, T. Distribution of Parasitic Helminths in the Small Intestine of the Red Fox (Vulpes vulpes). Pathogens 2020, 9, 477. [Google Scholar] [CrossRef]
- Thompson, R.C.A.; Eckert, J. Observations on Echinococcus multilocularis in the definitive host. Parasitol. Res. 1983, 69, 335–345. [Google Scholar] [CrossRef]
- Umhang, G.; Woronoff-Rhen, N.; Combes, B.; Boue, F. Segmental Sedimentation and Counting Technique (SSCT): An adaptable method for qualitative diagnosis of Echinococcus multilocularis in fox intestines. Exp. Parasitol. 2011, 128, 57–60. [Google Scholar] [CrossRef]
- Jędrzejewski, W.; Niedziałkowska, M.; Hayward, M.W.; Goszczyński, J.; Jędrzejewska, B.; Borowik, T.; Bartoń, K.A.; Nowak, S.; Harmuszkiewicz, J.; Juszczyk, A.; et al. Prey choice and diet of wolves related to ungulate communities and wolf subpopulations in Poland. J. Mammal. 2012, 93, 1480–1492. [Google Scholar] [CrossRef]
- Grenouillet, F.; Umhang, G.; Arbez-Gindre, F.; Mantion, G.; Delabrousse, E.; Millon, L.; Boue, F. Echinococcus ortleppi Infections in Humans and Cattle, France. Emerg. Infect. Dis. 2014, 20, 2100–2102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, M.; Sehgal, R.; Fomda, B.A.; Malhotra, A.; Malla, N. Molecular Characterization of Echinococcus granulosus Cysts in North Indian Patients: Identification of G1, G3, G5 and G6 Genotypes. PLoS Negl. Trop. Dis. 2013, 7, e2262. [Google Scholar] [CrossRef] [Green Version]
- De, N.V.; Van, D.L. The first report of two cases of cystic echinococcosis in the lung by Echinococcus ortleppi infection, in Vietnam. Res. Rep. Trop. Med. 2017, 8, 45–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avila, H.G.; Santos, G.B.; Cucher, M.A.; Macchiaroli, N.; Perez, M.G.; Baldi, G.; Jensen, O.; Perez, V.; Lopez, R.; Negro, P.; et al. Implementation of new tools in molecular epidemiology studies of Echinococcus granulosus sensu lato in South America. Parasitol. Int. 2017, 66, 250–257. [Google Scholar] [CrossRef]
- Mogoye, B.K.; Menezes, C.N.; Wong, M.L.; Stacey, S.; von Delft, D.; Wahlers, K.; Wassermann, M.; Romig, T.; Kern, P.; Grobusch, M.P.; et al. First insights into species and genotypes of Echinococcus in South Africa. Vet. Parasitol. 2013, 196, 427–432. [Google Scholar] [CrossRef] [PubMed]
- de la Rue, M.L.; Takano, K.; Brochado, J.F.; Costa, C.V.; Soares, A.G.; Yamano, K.; Yagi, K.; Katoh, Y.; Takahashi, K. Infection of humans and animals with Echinococcus granulosus (G1 and G3 strains) and E. ortleppi in Southern Brazil. Vet. Parasitol. 2011, 177, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Gawor, J.; Laskowski, Z.; Myczka, A.W.; Zwijacz-Kozica, T.; Sałamtin, R. Occurrence of Echinococcus spp. in red foxes and wolves in the protected area of the Tatra National Park in southern Poland—A threat to human health. Ann. Agric. Environ. Med. 2020. [Google Scholar] [CrossRef]
- Maksimov, P.; Bergmann, H.; Wassermann, M.; Romig, T.; Gottstein, B.; Casulli, A.; Conraths, F.J. Species Detection within the Echinococcus granulosus sensu lato Complex by Novel Probe-Based Real-Time PCRs. Pathogens 2020, 9, 791. [Google Scholar] [CrossRef]
- Shi, Y.L.; Wan, X.L.; Wang, Z.Y.; Li, J.; Jiang, Z.H.; Yang, Y.C. First description of Echinococcus ortleppi infection in China. Parasite Vector 2019, 12, 1–6. [Google Scholar] [CrossRef]
- Nakao, M.; McManus, D.P.; Schantz, P.M.; Craig, P.S.; Ito, A. A molecular phylogeny of the genus Echinococcus inferred from complete mitochondrial genomes. Parasitology 2007, 134, 713–722. [Google Scholar] [CrossRef] [Green Version]
- Casulli, A.; Manfredi, M.T.; La Rosa, G.; Di Cerbo, A.R.; Genchi, C.; Pozio, E. Echinococcus ortleppi and E. granulosus G1, G2 and G3 genotypes in Italian bovines. Vet. Parasitol. 2008, 155, 168–172. [Google Scholar] [CrossRef]
- Hofer, S.; Gloor, S.; Muller, U.; Mathis, A.; Hegglin, D.; Deplazes, P. High prevalence of Echinococcus multilocularis in urban red foxes (Vulpes vulpes) and voles (Arvicola terrestris) in the city of Zurich, Switzerland. Parasitology 2000, 120, 135–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- OIE. Echinococcosis/hydatidosis (infection with Echinococcus granulosus and E. multilocularis). In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 6th ed.; Office International des Epizooties: Paris, France, 2008; pp. 1–15. [Google Scholar]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Addy, F.; Wassermann, M.; Banda, F.; Mbaya, H.; Aschenborn, J.; Aschenborn, O.; Koskei, P.; Umhang, G.; De La Rue, M.; Elmahdi, I.E.; et al. Genetic polymorphism and population structure of Echinococcus ortleppi. Parasitology 2017, 144, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Kinkar, L.; Laurimae, T.; Sharbatkhori, M.; Mirhendi, H.; Kia, E.B.; Ponce-Gordo, F.; Andresiuk, V.; Simsek, S.; Lavikainen, A.; Irshadullah, M.; et al. New mitogenome and nuclear evidence on the phylogeny and taxonomy of the highly zoonotic tapeworm Echinococcus granulosus sensu stricto. Infect. Genet. Evol. 2017, 52, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Alvi, M.A.; Ohiolei, J.A.; Saqib, M.; Li, L.; Tayyab, M.H.; Alvi, A.A.; Wu, Y.T.; Fu, B.Q.; Yan, H.B.; Jia, W.Z. Echinococcus granulosus (sensu stricto) (G1, G3) and E. ortleppi (G5) in Pakistan: Phylogeny, genetic diversity and population structural analysis based on mitochondrial DNA. Parasite Vector 2020, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Moks, E.; Jogisalu, I.; Valdmann, H.; Saarma, U. First report of Echinococcus granulosus G8 in Eurasia and a reappraisal of the phylogenetic relationships of ‘genotypes’ G5-G10. Parasitology 2008, 135, 647–654. [Google Scholar] [CrossRef]
- Denk, D.; Boufana, B.; Masters, N.J.; Stidworthy, M.F. Fatal echinococcosis in three lemurs in the United Kingdom–A case series. Vet. Parasitol. 2016, 218, 10–14. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karamon, J.; Samorek-Pieróg, M.; Sroka, J.; Bilska-Zając, E.; Dąbrowska, J.; Kochanowski, M.; Różycki, M.; Zdybel, J.; Cencek, T. The First Record of Echinococcus ortleppi (G5) Tapeworms in Grey Wolf (Canis lupus). Pathogens 2021, 10, 853. https://doi.org/10.3390/pathogens10070853
Karamon J, Samorek-Pieróg M, Sroka J, Bilska-Zając E, Dąbrowska J, Kochanowski M, Różycki M, Zdybel J, Cencek T. The First Record of Echinococcus ortleppi (G5) Tapeworms in Grey Wolf (Canis lupus). Pathogens. 2021; 10(7):853. https://doi.org/10.3390/pathogens10070853
Chicago/Turabian StyleKaramon, Jacek, Małgorzata Samorek-Pieróg, Jacek Sroka, Ewa Bilska-Zając, Joanna Dąbrowska, Maciej Kochanowski, Mirosław Różycki, Jolanta Zdybel, and Tomasz Cencek. 2021. "The First Record of Echinococcus ortleppi (G5) Tapeworms in Grey Wolf (Canis lupus)" Pathogens 10, no. 7: 853. https://doi.org/10.3390/pathogens10070853