HIV-Associated Tuberculosis in Children and Adolescents: Evolving Epidemiology, Screening, Prevention and Management Strategies
Abstract
:1. Introduction
2. Emerging Epidemiology on TB Risk and Outcomes in High-HIV/TB Burden Settings
3. TB Screening
4. Prevention Strategies in the Context of Anti-Retroviral Therapy
5. Diagnostic Strategies for HIV-Associated TB
6. Ongoing Challenges Navigating Co-Treatment and Drug-Drug Interactions in Children with HIV-Associated TB
- Does the child require TB treatment or prevention?
- What is the confirmed or most likely tuberculosis drug susceptibility pattern?
- Are there additional considerations for choosing drugs? For instance, meningitis requires that medication crosses the blood–brain barrier and potentially higher doses.
- Are there conditions that require consideration, for instance, severe anemia or electrolyte disorders that may complicate the use of some second line anti-tuberculosis drugs for instance linezolid and severe anemia.
- If you are treating meningitis and the child is not already on antiretrovirals then delay the initiation of antiretroviral therapy for 4 weeks; otherwise, start within 2 weeks [36]. In hospitalized children initiate TB therapy and if possible antiretroviral therapy prior to discharge. Ensure counselling is performed and that and they are referred to the appropriate outpatient service.
- Can the available antiretrovirals be adjusted to maximize viral suppression while treating or preventing tuberculosis?
- Are there any drug–drug interactions that should be considered? Remember to consider co-morbidities that the child may have and the therapy needed for those, for example, anticonvulsants.
- Will the selected TB and HIV regimen require additional monitoring needs? If yes, does the child have access to these monitoring tools?
- How will adherence be supported?
- How long has the child been on therapy? Children who recently initiated antiretroviral therapy may develop IRIS and possibly need steroid therapy.
- Is the child adherent, immune reconstituted and with a suppressed viral load? Children with severe immune suppression who are failing antiretroviral therapy are more likely to progress from TB infection to disease. Hence, switching antiretroviral therapy may be needed.
- For children failing antiretroviral therapy—what is the most appropriate time to switch the antiretroviral regimen and which regimen will be required? Will the preferred antiretroviral regimen be compatible with the anti-tuberculosis regimen?
7. Improving Care after Treatment for TB Disease
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- World Health Organization. Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery and Monitoring: Recommendations for a Public Health Approach; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Frigati, L.J.; Wilkinson, K.A.; le Roux, S.; Brown, K.; Ruzive, S.; Githinji, L.; Petersen, W.; Belard, S.; Cotton, M.F.; Myer, L.; et al. Tuberculosis infection and disease in South African adolescents with perinatally acquired HIV on antiretroviral therapy: A cohort study. J. Int. AIDS Soc. 2021, 24, e25671. [Google Scholar] [CrossRef] [PubMed]
- Tiruneh, F.; Deyas, Y. Effect of highly active antiretroviral treatment on TB incidence among HIV infected children and their clinical profile, retrospective cohort study, South West Ethiopia. Sci. Rep. 2020, 10, 21468. [Google Scholar] [CrossRef] [PubMed]
- Nduba, V.; Kaguthi, G.; Van’t Hoog, A.H.; Mitchell, E.M.H.; Borgdorff, M. The Incidence of Tuberculosis in Infants, Siaya District, Western Kenya. Pediatr. Infect. Dis. J. 2020, 39, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Mandalakas, A.M.; Kay, A.W.; Bacha, J.M.; Devezin, T.; Golin, R.; Simon, K.R.; Dhillon, D.; Dlamini, S.; DiNardo, A.; Matshaba, M.; et al. Tuberculosis among Children and Adolescents at HIV Treatment Centers in Sub-Saharan Africa. Emerg. Infect. Dis. 2020, 26, 2933–2943. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.; Cords, O.; Horsburgh, C.R.; Andrews, J.R. Pediatric TB Contact Studies Consortium The risk of tuberculosis in children after close exposure: A systematic review and individual-participant meta-analysis. Lancet 2020, 395, 973–984. [Google Scholar] [CrossRef] [Green Version]
- Osman, M.; du Preez, K.; Seddon, J.A.; Claassens, M.M.; Dunbar, R.; Dlamini, S.S.; Welte, A.; Naidoo, P.; Hesseling, A.C. Mortality in South African Children and Adolescents Routinely Treated for Tuberculosis. Pediatrics 2021, 147, e2020032490. [Google Scholar] [CrossRef]
- López-Medina, E.M.; Sainz, T.; de Ory, S.J.; Mellado-Peña, M.J.; González-Tomé, M.I.; Gil, E.C.; Cucurull, T.V.; Neyra, F.; Frick, M.A.; Martínez-Pérez, J.; et al. Tuberculosis in a Spanish cohort of children living with HIV: The CHOTIS study (Childhood HIV & TB study). Int. J. Tuberc. Lung Dis. 2020, 24, 303–309. [Google Scholar] [PubMed]
- Global HIV & AIDS Statistics—Fact Sheet. Available online: https://www.unaids.org/en/resources/fact-sheet (accessed on 15 November 2021).
- Boulle, A.; Davies, M.-A.; Hussey, H.; Ismail, M.; Morden, E.; Vundle, Z.; Zweigenthal, V.; Mahomed, H.; Paleker, M.; Pienaar, D.; et al. Risk factors for COVID-19 death in a population cohort study from the Western Cape Province, South Africa. Clin. Infect. Dis. 2020, 73, e2005–e2015. [Google Scholar] [CrossRef]
- National Institute of Communicable Diseases of South Africa. Monthly COVID-19 in Children. 2021. Available online: https://www.nicd.ac.za/diseases-a-z-index/disease-index-covid-19/surveillance-reports/monthly-covid-19-in-children/ (accessed on 11 November 2021).
- Dangor, Z.; Izu, A.; Moore, D.P.; Nunes, M.C.; Solomon, F.; Beylis, N.; von Gottberg, A.; McAnerney, J.M.; Madhi, S.A. Temporal association in hospitalizations for tuberculosis, invasive pneumococcal disease and influenza virus illness in South African children. PLoS ONE 2014, 9, e91464. [Google Scholar] [CrossRef] [PubMed]
- Lebina, L.; Dube, M.; Hlongwane, K.; Brahmbatt, H.; Lala, S.G.; Reubenson, G.; Martinson, N. Trends in paediatric tuberculosis diagnoses in two South African hospitals early in the COVID-19 pandemic. S. Afr. Med. J. 2020, 110, 1149–1150. [Google Scholar] [CrossRef] [PubMed]
- Onyango, D.O.; Yuen, C.M.; Masini, E.; Borgdorff, M.W. Epidemiology of Pediatric Tuberculosis in Kenya and Risk Factors for Mortality during Treatment: A National Retrospective Cohort Study. J. Pediatr. 2018, 201, 115–121. [Google Scholar] [CrossRef]
- World Health Organization. WHO Consolidated Guidelines on Tuberculosis Module 2: Screening Systematic Screening for Tuberculosis Disease; Web Annex A: Methods and Expert Panels; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Owiti, P.; Onyango, D.; Momanyi, R.; Harries, A.D. Screening and testing for tuberculosis among the HIV-infected: Outcomes from a large HIV programme in western Kenya. BMC Public Health 2019, 19, 29. [Google Scholar] [CrossRef]
- Vonasek, B.; Kay, A.; Devezin, T.; Bacha, J.M.; Kazembe, P.; Dhillon, D.; Dlamini, S.; Haq, H.; Thahane, L.; Simon, K.; et al. Tuberculosis symptom screening for children and adolescents living with HIV in six high HIV/TB burden countries in Africa. AIDS 2020, 35, 73–79. [Google Scholar] [CrossRef]
- Vonasek, B.; Ness, T.; Takwoingi, Y.; Kay, A.W.; Wyk, S.S.; Ouellette, L.; Marais, B.J.; Steingart, K.R.; Mandalakas, A.M. Screening tests for active pulmonary tuberculosis in children. Cochrane Database Syst. Rev. 2020. [Google Scholar] [CrossRef]
- Malik, A.A.; Farooq, S.; Jaswal, M.; Khan, H.; Nasir, K.; Fareed, U.; Shahbaz, S.; Amanullah, F.; Safdar, N.; Khan, A.J.; et al. Safety and feasibility of 1 month of daily rifapentine plus isoniazid to prevent tuberculosis in children and adolescents: A prospective cohort study. Lancet Child Adolesc Health 2021, 5, 350–356. [Google Scholar] [CrossRef]
- Swindells, S.; Ramchandani, R.; Gupta, A.; Benson, C.A.; Leon-Cruz, J.; Mwelase, N.; Juste, M.A.J.; Lama, J.R.; Valencia, J.; Omoz-Oarhe, A.; et al. One Month of Rifapentine plus Isoniazid to Prevent HIV-Related Tuberculosis. N. Engl. J. Med. 2019, 381, e23. [Google Scholar] [CrossRef] [PubMed]
- Hunter, O.F.; Kyesi, F.; Ahluwalia, A.K.; Daffé, Z.N.; Munseri, P.; von Reyn, C.F.; Adams, L.V. Successful implementation of isoniazid preventive therapy at a pediatric HIV clinic in Tanzania. BMC Infect. Dis. 2020, 20, 738. [Google Scholar]
- Ngugi, S.K.; Muiruri, P.; Odero, T.; Gachuno, O. Factors affecting uptake and completion of isoniazid preventive therapy among HIV-infected children at a national referral hospital, Kenya: A mixed quantitative and qualitative study. BMC Infect. Dis. 2020, 20, 294. [Google Scholar] [CrossRef]
- Kay, A.W.; Thivalapill, N.; Skinner, D.; Dube, G.S.; Dlamini, N.; Mzileni, B.; Fuentes, P.; Ustero, P.; Adams, L.V.; Mandalakas, A.M. Predictors of suboptimal adherence to isoniazid preventive therapy among adolescents and children living with HIV. PLoS ONE 2020, 15, e0243713. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Consolidated Guidelines on Tuberculosis: Module 3: Diagnosis: Rapid Diagnostics for Tuberculosis Detection; Web annex 4: Evidence synthesis and analysis; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Kay, A.W.; Gonzalez Fernandez, L.; Takwoingi, Y.; Eisenhut, M.; Detjen, A.K.; Steingart, K.R.; Mandalakas, A.M. Xpert MTB/RIF and Xpert MTB/RIF Ultra assays for active tuberculosis and rifampicin resistance in children. Cochrane Database Syst. Rev. 2020, 8, CD013359. [Google Scholar] [CrossRef] [PubMed]
- Nicol, M.P.; Schumacher, S.G.; Workman, L.; Broger, T.; Baard, C.; Prins, M.; Bateman, L.; du Toit, E.; van Heerden, J.; Szekely, R.; et al. Accuracy of a Novel Urine Test, Fujifilm SILVAMP Tuberculosis Lipoarabinomannan, for the Diagnosis of Pulmonary Tuberculosis in Children. Clin. Infect. Dis. 2021, 72, e280–e288. [Google Scholar] [CrossRef] [PubMed]
- Nkereuwem, E.; Togun, T.; Gomez, M.P.; Székely, R.; Macé, A.; Jobe, D.; Schumacher, S.G.; Kampmann, B.; Denkinger, C.M. Reach4KidsAfrica (R4KA) Consortium Comparing accuracy of lipoarabinomannan urine tests for diagnosis of pulmonary tuberculosis in children from four African countries: A cross-sectional study. Lancet Infect. Dis. 2021, 21, 376–384. [Google Scholar] [CrossRef]
- Marcy, O.; Borand, L.; Ung, V.; Msellati, P.; Tejiokem, M.; Huu, K.T.; Do Chau, V.; Ngoc Tran, D.; Ateba-Ndongo, F.; Tetang-Ndiang, S.; et al. A Treatment-Decision Score for HIV-Infected Children with Suspected Tuberculosis. Pediatrics 2019, 144, e20182065. [Google Scholar] [CrossRef]
- Berteloot, L.; Marcy, O.; Nguyen, B.; Ung, V.; Tejiokem, M.; Nacro, B.; Goyet, S.; Dim, B.; Blanche, S.; Borand, L.; et al. Value of chest X-ray in TB diagnosis in HIV-infected children living in resource-limited countries: The ANRS 12229-PAANTHER 01 study. Int. J. Tuberc. Lung Dis. 2018, 22, 844–850. [Google Scholar] [CrossRef]
- Choudhary, R.K.; Wall, K.M.; Njuguna, I.; Pavlinac, P.B.; LaCourse, S.M.; Otieno, V.; Gatimu, J.; Stern, J.; Maleche-Obimbo, E.; Wamalwa, D.; et al. Monocyte-to-Lymphocyte Ratio Is Associated With Tuberculosis Disease and Declines With Anti-TB Treatment in HIV-Infected Children. J. Acquir. Immune Defic. Syndr. 2019, 80, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, T.G.; Svensson, E.M.; Musiime, V.; Rojo, P.; Dooley, K.E.; McIlleron, H.; Aarnoutse, R.E.; Burger, D.M.; Turkova, A.; Colbers, A.; et al. Pharmacokinetics of antiretroviral and tuberculosis drugs in children with HIV/TB co-infection: A systematic review. J. Antimicrob. Chemother. 2020, 75, 3433–3457. [Google Scholar] [CrossRef] [PubMed]
- Rawizza, H.E.; Oladokun, R.; Ejeliogu, E.; Oguche, S.; Ogunbosi, B.O.; Agbaji, O.; Odaibo, G.; Imade, G.; Olaleye, D.; Wiesner, L.; et al. Rifabutin pharmacokinetics and safety among TB/HIV-coinfected children receiving lopinavir/ritonavir-containing second-line ART. J. Antimicrob. Chemother. 2021, 76, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Moultrie, H.; McIlleron, H.; Sawry, S.; Kellermann, T.; Wiesner, L.; Kindra, G.; Gous, H.; Van Rie, A. Pharmacokinetics and safety of rifabutin in young HIV-infected children receiving rifabutin and lopinavir/ritonavir. J. Antimicrob. Chemother. 2015, 70, 543–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waalewijn, H.; Mujuru, H.A.; Amuge, P.; Cotton, M.; Bollen, P.; Chan, M.; Ali, S.; Variava, E.; Makumbi, S.; Colbers, A.; et al. Adequate Dolutegravir Exposure Dosed BID with Rifampicin in Children 6 to <18 Years. Available online: https://2jg4quetidw2blbbq2ixwziw-wpengine.netdna-ssl.com/wp-content/uploads/sites/2/posters/2020/1430_9_Waalewijn_00847.pdf (accessed on 18 November 2021).
- World Health Organization. Guidelines: Updated Recommendations on HIV Prevention, Infant Diagnosis, Antiretroviral Initiation and Monitoring; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- SHINE Trial on Shorter Treatment for Children with Minimal TB. Available online: https://www.who.int/news/item/26-10-2020-shine-trial-on-shorter-treatment-for-children-with-minimal-tb (accessed on 22 November 2021).
- Dorman, S.E.; Nahid, P.; Kurbatova, E.V.; Phillips, P.P.J.; Bryant, K.; Dooley, K.E.; Engle, M.; Goldberg, S.V.; Phan, H.T.T.; Hakim, J.; et al. Four-Month Rifapentine Regimens with or without Moxifloxacin for Tuberculosis. N. Engl. J. Med. 2021, 384, 1705–1718. [Google Scholar] [CrossRef] [PubMed]
- Dodd, P.J.; Prendergast, A.J.; Beecroft, C.; Kampmann, B.; Seddon, J.A. The impact of HIV and antiretroviral therapy on TB risk in children: A systematic review and meta-analysis. Thorax 2017, 72, 559–575. [Google Scholar] [CrossRef] [Green Version]
- Attia, E.F.; Maleche-Obimbo, E.; West, T.E.; Ndukwe-Wambutsi, L.; Kiptinness, C.; Cagle, A.; McGrath, C.J.; Mugambi, C.K.; El Antouny, N.G.; Eskander, S.; et al. Adolescent age is an independent risk factor for abnormal spirometry among people living with HIV in Kenya. AIDS 2018, 32, 1353–1359. [Google Scholar] [CrossRef]
- Githinji, L.N.; Gray, D.M.; Hlengwa, S.; Machemedze, T.; Zar, H.J. Longitudinal Changes in Spirometry in South African Adolescents Perinatally Infected With Human Immunodeficiency Virus Who Are Receiving Antiretroviral Therapy. Clin. Infect. Dis. 2020, 70, 483–490. [Google Scholar] [CrossRef]
- Attia, E.F.; Miller, R.F.; Ferrand, R.A. Bronchiectasis and other chronic lung diseases in adolescents living with HIV. Curr. Opin. Infect. Dis. 2017, 30, 21–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allwood, B.W.; van der Zalm, M.M.; Amaral, A.F.S.; Byrne, A.; Datta, S.; Egere, U.; Evans, C.A.; Evans, D.; Gray, D.M.; Hoddinott, G.; et al. Post-tuberculosis lung health: Perspectives from the First International Symposium. Int. J. Tuberc. Lung Dis. 2020, 24, 820–828. [Google Scholar] [CrossRef]
- Stek, C.; Allwood, B.; Walker, N.F.; Wilkinson, R.J.; Lynen, L.; Meintjes, G. The Immune Mechanisms of Lung Parenchymal Damage in Tuberculosis and the Role of Host-Directed Therapy. Front. Microbiol. 2018, 9, 2603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attia, E.F.; Jacobson, D.; Yu, W.; Crowell, C.S.; Maleche-Obimbo, E.; Williams, P.L.; West, T.E.; Burchett, S.K.; Kattan, M.; Colin, A.A.; et al. Immune imbalance and activation are associated with lower lung function in youth with perinatally acquired HIV. J. Allergy Clin. Immunol. 2020, 145, 1473–1476. [Google Scholar] [CrossRef] [PubMed]
- du Plessis, A.-M.; Andronikou, S.; Machemedze, T.; Griffith-Richards, S.; Myer, L.; Mahtab, S.; Zar, H.J. High-resolution computed tomography features of lung disease in perinatally HIV-infected adolescents on combined antiretroviral therapy. Pediatr. Pulmonol. 2019, 54, 1765–1773. [Google Scholar] [CrossRef]
- Harries, A.D.; Dlodlo, R.A.; Brigden, G.; Mortimer, K.; Jensen, P.; Fujiwara, P.I.; Castro, J.L.; Chakaya, J.M. Should we consider a “fourth 90” for tuberculosis? Int. J. Tuberc. Lung Dis. 2019, 23, 1253–1256. [Google Scholar] [CrossRef]
- Ferrand, R.A.; McHugh, G.; Rehman, A.M.; Mujuru, H.; Simms, V.; Majonga, E.D.; Nicol, M.P.; Flaegstad, T.; Gutteberg, T.J.; Gonzalez-Martinez, C.; et al. Effect of Once-Weekly Azithromycin vs. Placebo in Children With HIV-Associated Chronic Lung Disease: The BREATHE Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2028484. [Google Scholar] [CrossRef]
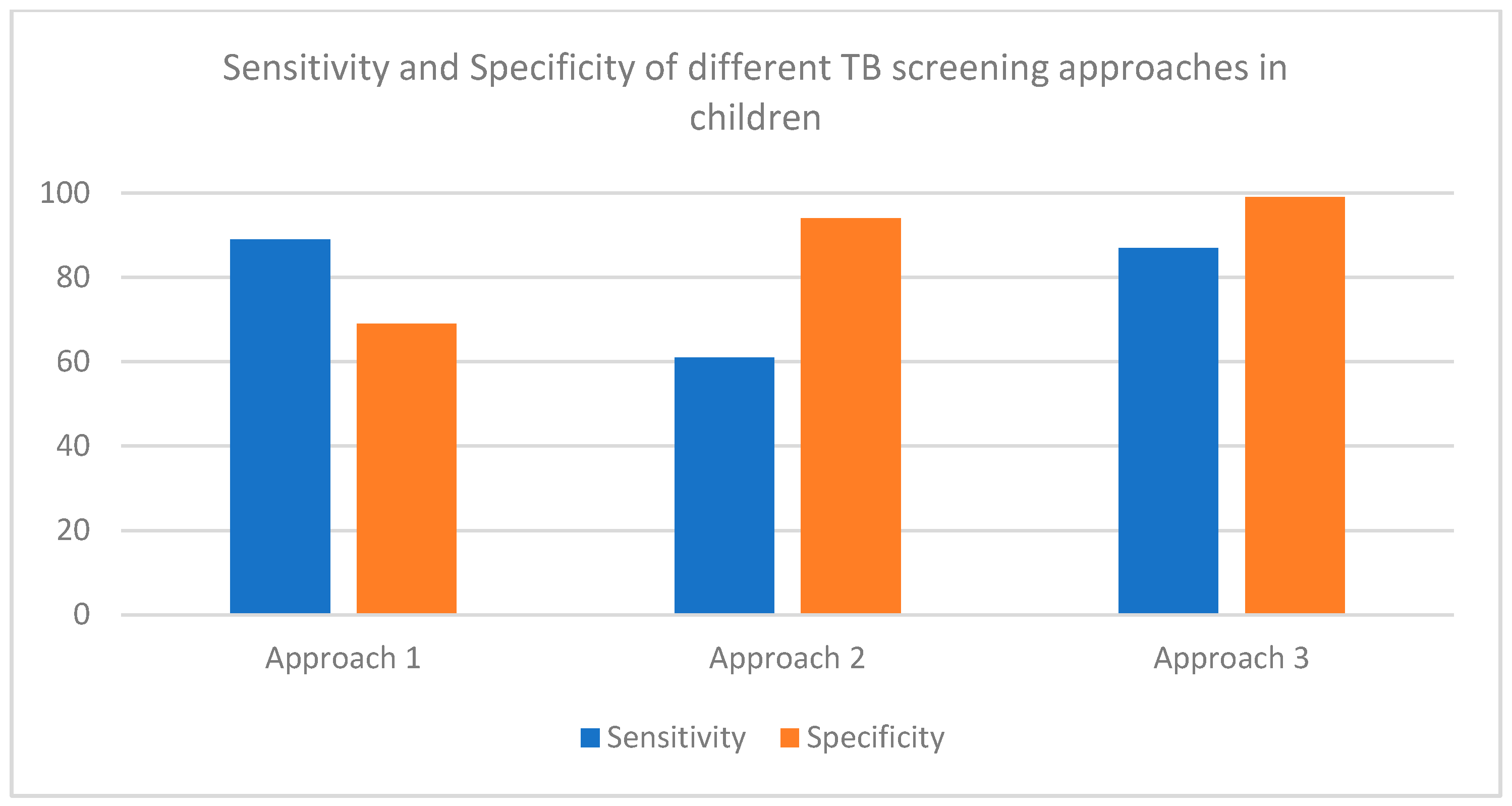
| TB Incidence in CALHIV | |||||
| Author | Setting | Study Design | Population | Sample Size | Results |
| Frigati, 2021 [3] | South Africa | Prospective cohort study | Perinatally infected adolescents living with HIV and HIV-uninfected adolescents | n = 599, 496 HIV positive | Adolescents with perinatally acquired HIV had a TB incidence of 2.2/100 person years. The IRR attributable to HIV was 7.4 (95% CI 1.01 to 53.6) |
| Tiruneh, 2020 [4] | Southwest Ethiopia | Retrospective observational study | CALHIV who were ART naïve and experienced | n = 800 | The incident rate was 7.7 per 100-years, (95% CI 6.3–9.2) in CALHIV on ART, similar to 8.2 per 100 person years (95% CI 6.8–9.8) in ART naïve CALHIV |
| Mandalakas, 2020 [6] | Lesotho, Eswatini, Botswana, Uganda, Tanzania | Retrospective observational study | CALHIV who were ART naïve and experienced | n = 1160 | The incident rate was 2 per 100-person years, which decreased significantly with increases in ART uptake |
| Martinez, 2020 [7] | 34 countries | Systematic review | Children with recent TB exposure with and without HIV | 137,647 TB-exposed children | HIV was associated with an incident aHR of 5.31, 95% CI 2.39–11.81 |
| Nduba, 2020 [5] | Kenya | Prospective observational cohort | Infants with and without HIV | n = 2900 | Infants with HIV had an adjusted HR of 4.71, 95% CI 2.13–10.4 |
| TB Outcomes in CALHIV | |||||
| Author | Setting | Study Design | Population | n | Results |
| Osman, 2021 [8] | South Africa | Retrospective review of programmatic TB outcomes | Children with TB, with and without HIV | n = 729,463, 102,643 HIV positive | HIV was associated with mortality: aHR = 5.11, 95% confidence interval 4.71–5.55 on ART and 7.99, 95% CI 7.02–9.09 off ART |
| Onyango, 2018 [15] | Kenya | Retrospective review of programmatic TB outcomes | Children with TB, with and without HIV | n = 24,216, 5991 HIV positive | HIV was associated with an aHR of death 3.69, 95% CI 3.14–4.35 on ART and an aHR of 4.84, 95% confidence interval 3.59–6.91 off ART |
| Drug | Induction | Effect | Suggested Adjustment | Comments |
|---|---|---|---|---|
| Integrase strand inhibitors | ||||
| Dolutegravir | UGT1A1 CYP3A | Reduced AUC and trough | Twice daily dose | Dose depends on the formulation used |
| Raltegravir | UGT1A1 | Reduced AUC and trough | Doubling each dose | Dose depends on the formulation used |
| Bictegravir | UGT1A1 CYP3A | Reduced AUC and trough | Avoid co-treatment fairliewangNo mitigating strategies proven to overcome interactions | Do not use |
| Elvitegravir/cobicistat | No data | No data | Do not use | |
| Non-nucleoside reverse transcriptase inhibitors | ||||
| Efavirenz | CYP 2B6fairliewangCYP 2A6fairliewangUGT2B7 | Reduced AUC and trough | No dose change | INH effects may contract some of the effects of induction |
| Nevirapine | CYP 3AfairliewangCYP 2B6 | Reduced AUC and trough | Increase the dose to 200 mg/m2/dose bd | No longer recommended |
| Doravirine | CYP 3A4 | Reduced AUC and trough | No mitigating strategies proven to overcome interactions | Do not use |
| Etravirine | CYP 3A4 | Reduced AUC and trough | No mitigating strategies proven to overcome interactions | Do not use |
| Rilpivirine | CYP 3A4 | Reduced AUC and trough | No mitigating strategies proven to overcome interactions | Do not use |
| Protease inhibitors | ||||
| Lopinavir/ritonavir 4:1 | CYP 3A | Reduced AUC and trough | Liquid formulation and solid granule formulations fairliewangAdd ritonavir to achieve a 1:1 ratio | Liquid and solid granule or pellet formulations should not be given 3 times a day or at double the dose |
| Solid tablet formulations: doubling the dose | Tablets should not be crushed | |||
| Atazanavir | CYP 3A4 | Reduced AUC and trough | No mitigating strategies proven to overcome interactions | Do not use |
| Darunavir | CYP 3A4 | Reduced AUC and trough | No mitigating strategies proven to overcome interactions | Do not use |
| Non-nucleoside reverse transcriptase inhibitors | ||||
| Abacavir | UGT | Reduction | No change in dose | |
| Zidovudine | UGT | Reduction | No change in dose | |
| Tenofovir disoproxil fumarate | P-gp | Reduction | No change in dose | |
| Tenofovir alafenamide | P-gp | Reduction | No change in dose | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kay, A.W.; Rabie, H.; Maleche-Obimbo, E.; Sekadde, M.P.; Cotton, M.F.; Mandalakas, A.M. HIV-Associated Tuberculosis in Children and Adolescents: Evolving Epidemiology, Screening, Prevention and Management Strategies. Pathogens 2022, 11, 33. https://doi.org/10.3390/pathogens11010033
Kay AW, Rabie H, Maleche-Obimbo E, Sekadde MP, Cotton MF, Mandalakas AM. HIV-Associated Tuberculosis in Children and Adolescents: Evolving Epidemiology, Screening, Prevention and Management Strategies. Pathogens. 2022; 11(1):33. https://doi.org/10.3390/pathogens11010033
Chicago/Turabian StyleKay, Alexander W., Helena Rabie, Elizabeth Maleche-Obimbo, Moorine Penninah Sekadde, Mark F. Cotton, and Anna M. Mandalakas. 2022. "HIV-Associated Tuberculosis in Children and Adolescents: Evolving Epidemiology, Screening, Prevention and Management Strategies" Pathogens 11, no. 1: 33. https://doi.org/10.3390/pathogens11010033





