Habitat and Season Drive Chigger Mite Diversity and Abundance on Small Mammals in Peninsular Malaysia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Small-Mammal Trapping
2.2. Chigger Collection
2.3. Ecological Analyses
2.4. Multiple Regression Modelling
2.5. Network Analyses
2.6. Prevalence and Co-Occurrence Analysis between Chigger Species and with O. tsutsugamushi
3. Results
3.1. Small-Mammal Trapping
3.2. Chigger Fauna Identified
3.3. Chigger Intensity and Infestation Rates
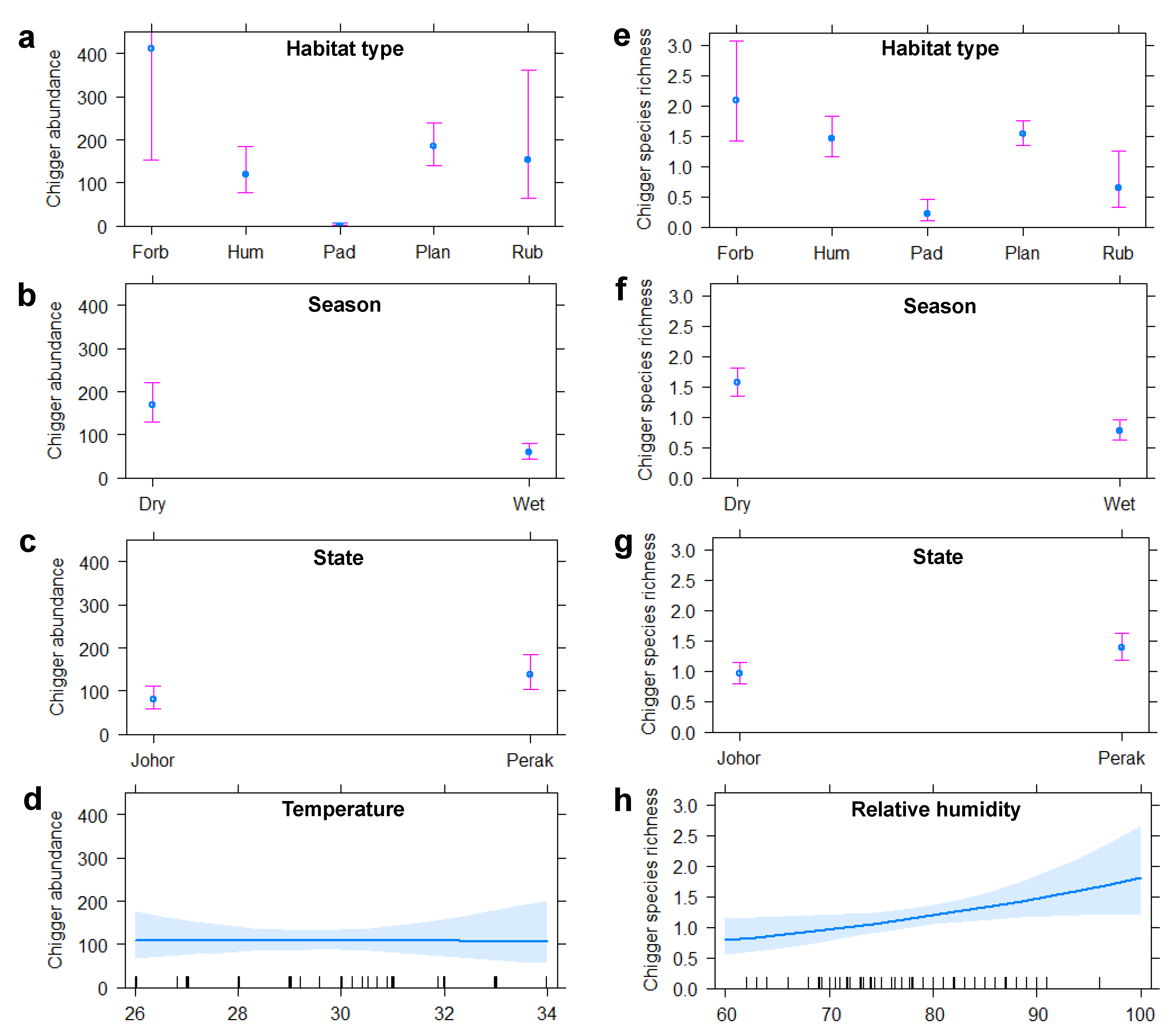
3.4. Influence of Host and Environmental Variables on Chigger Abundance and CSR
3.5. Chigger Habitat Preferences
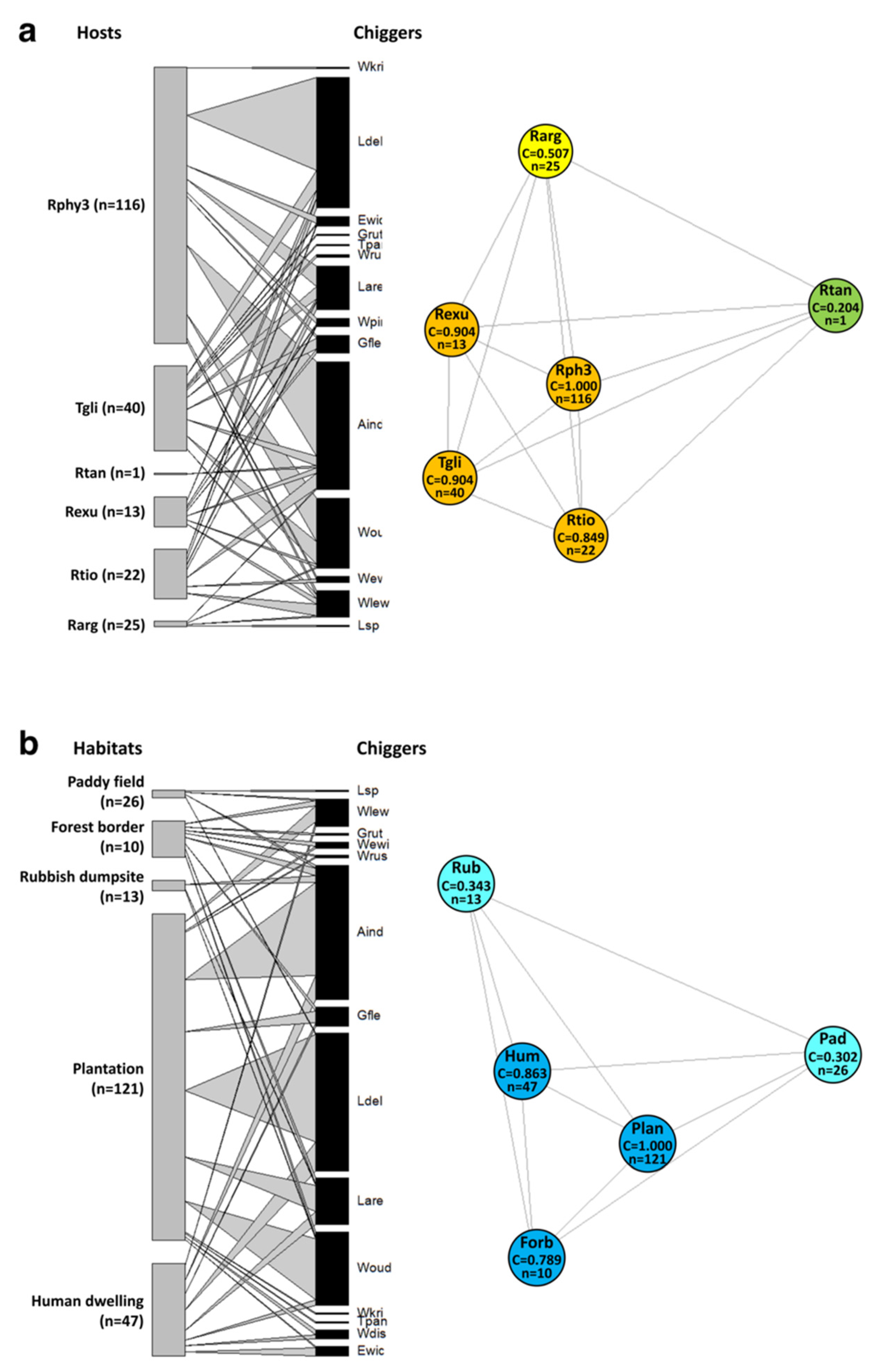
3.6. Network Analyses of Chigger–Host and Chigger–Habitat Association
3.7. O. tsutsugamushi Prevalence and Co-Occurrence with Chigger Species and between Chigger Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nielsen, D.H.; Robbins, R.G.; Rueda, L.M. Annotated world checklist of the Trombiculidae and Leeuwenhoekiidae (1758–2021) (Acari: Trombiculoidea), with notes on nomenclature, taxonomy, and distribution. Zootaxa 2021, 4967, 1–243. [Google Scholar] [CrossRef] [PubMed]
- Audy, J.E. Trombioulid mites infesting birds, reptiles, and arthropods in Malaya, with a taxonomic revision, and descriptions of a new genus, two new subgenera, and six new species. Bull. Raffles Mus. 1956, 28, 27–80. [Google Scholar]
- Heyne, H.; Coetzee, L. First report of a parasitic mite, Leptotrombidium (Hypotrombidium) subquadratum (Lawrence) (Acari: Trombiculidae: Trombiculinae), from dogs and children in the Bloemfontein area, South Africa. J. S. Afr. Vet. Assoc. 2001, 72, 105–106. [Google Scholar] [CrossRef] [PubMed]
- Bonell, A.; Lubell, Y.; Newton, P.N.; Crump, J.A.; Paris, D.H. Estimating the burden of scrub typhus: A systematic review. PLoS Negl. Trop. Dis. 2017, 11, e0005838. [Google Scholar] [CrossRef] [PubMed]
- Richards, A.L.; Jiang, J. Scrub typhus: Historic perspective and current status of the worldwide presence of Orientia species. Trop. Med. Infect. Dis. 2020, 5, 49. [Google Scholar] [CrossRef]
- Elliott, I.; Pearson, I.; Dahal, P.; Thomas, N.V.; Roberts, T.; Newton, P.N. Scrub typhus ecology: A systematic review of Orientia in vectors and hosts. Parasit. Vectors 2019, 12, 513. [Google Scholar] [CrossRef]
- Traub, R.; Wisseman, C.L., Jr.; Jones, M.R.; O’Keefe, J.J. The acquisition of Rickettsia tsutsugamushi by chiggers (trombiculid mites) during the feeding process. Ann. N. Y. Acad. Sci. 1975, 266, 91–114. [Google Scholar] [CrossRef]
- Peterson, R.K.D. The real enemy: Scrub typhus and the invasion of Sansapor. Am. Entomol. 2009, 55, 91–94. [Google Scholar] [CrossRef]
- Traub, R.; Wisseman, C.L., Jr. Review article*: The ecology of chigger-borne rickettsiosis (scrub typhus)1, 2. J. Med. Entomol. 1974, 11, 237–303. [Google Scholar] [CrossRef]
- Audy, J.R.; Harrison, J.L. A review of investigations of mite typhus in Burma and Malaya, 1945–1950. Trans. R. Soc. Trop. Med. Hyg. 1951, 44, 371–404. [Google Scholar] [CrossRef]
- Traub, R.; Wisseman, C.L., Jr. Ecological considerations in scrub typhus: 2. Vector species. Bull. World. Health. Organ. 1968, 39, 219. [Google Scholar] [PubMed]
- Weitzel, T.; Silva-de la Fuente, M.C.; Martínez-Valdebenito, C.; Stekolnikov, A.A.; Pérez, C.; Pérez, R.; Vial, C.; Abarca, K.; Acosta-Jamett, G. Novel vector of scrub typhus in sub-antarctic Chile: Evidence from human exposure. Clin. Infect. Dis. 2022, 74, 1862–1865. [Google Scholar] [CrossRef] [PubMed]
- Bhopdhornangkul, B.; Meeyai, A.C.; Wongwit, W.; Limpanont, Y.; Iamsirithaworn, S.; laosiritaworn, Y.; Tantrakarnapa, K. Non-linear effect of different humidity types on scrub typhus occurrence in endemic provinces, Thailand. Heliyon 2021, 7, e06095. [Google Scholar] [CrossRef] [PubMed]
- Wangrangsimakul, T.; Elliott, I.; Nedsuwan, S.; Kumlert, R.; Hinjoy, S.; Chaisiri, K.; Day, N.P.J.; Morand, S. The estimated burden of scrub typhus in Thailand from national surveillance data (2003–2018). PLoS Negl. Trop. Dis. 2020, 14, e0008233. [Google Scholar] [CrossRef]
- Chaisiri, K.; Gill, A.C.; Stekolnikov, A.A.; Hinjoy, S.; McGarry, J.W.; Darby, A.C.; Morand, S.; Makepeace, B.L. Ecological and microbiological diversity of chigger mites, including vectors of scrub typhus, on small mammals across stratified habitats in thailand. Anim. Microbiome 2019, 1, 18. [Google Scholar] [CrossRef]
- Elliott, I.; Thangnimitchok, N.; Chaisiri, K.; Wangrangsimakul, T.; Jaiboon, P.; Day, N.P.J.; Paris, D.H.; Newton, P.N.; Morand, S. Orientia tsutsugamushi dynamics in vectors and hosts: Ecology and risk factors for foci of scrub typhus transmission in northern Thailand. Parasit. Vectors 2021, 14, 540. [Google Scholar] [CrossRef]
- Chaisiri, K.; Cosson, J.-F.; Morand, S. Infection of rodents by Orientia tsutsugamushi, the agent of scrub typhus in relation to land use in Thailand. Trop. Med. Infect. Dis. 2017, 2, 53. [Google Scholar] [CrossRef]
- Linsuwanon, P.; Auysawasdi, N.; Wongwairot, S.; Leepitakrat, S.; Rodkhamtook, W.; Wanja, E.; Monkanna, T.; Wegner, M.; Davidson, S.; Poovorawan, Y.; et al. Assessing scrub typhus and rickettsioses transmission risks in the Chiang Rai province of northern Thailand. Travel Med. Infect. Dis. 2021, 42, 102086. [Google Scholar] [CrossRef]
- Fletcher, W.; Lesslar, J.E.; Lewthwaite, R. The Ætiology of the Tsutsugamushi disease and tropical typhus in the Federated Malay States. Part II. Trans. R. Soc. Trop. Med. Hyg. 1929, 23, 57–70. [Google Scholar] [CrossRef]
- Tay, S.T.; Zan, H.A.M.; Lim, Y.A.L.; Ngui, R. Antibody prevalence and factors associated with exposure to Orientia tsutsugamushi in different aboriginal subgroups in west Malaysia. PLoS Negl. Trop. Dis. 2013, 7, e2341. [Google Scholar] [CrossRef]
- Tee, T.S.; Kamalanathan, M.; Suan, K.A.; Chun, S.S.; Ming, H.T.; Yasin, R.M.; Devi, S. Seroepidemiologic survey of Orientia tsutsugamushi, Rickettsia typhi, and TT118 spotted fever group rickettsiae in rubber estate workers in Malaysia. Am. J. Trop. Med. Hyg. 1999, 61, 73–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dohany, A.L.; Shirai, A.; Lim, B.L.; Huxsoll, D.L. Variation in populations of chigger vectors of scrub typhus in developing oil palm areas of different ages. Jpn J. Med. Sci. Biol. 1980, 33, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Shirai, A.; Robinson, D.M.; Lim, B.L.; Dohany, A.L.; Huxsoll, D.L. Rickettsia tsutsugamushi infections in chiggers and small mammals on a mature oil palm estate. Southeast Asian J. Trop. Med. Public Health 1978, 9, 356–360. [Google Scholar] [PubMed]
- Cadigan, F.C., Jr.; Andre, R.G.; Bolton, M.; Gan, E.; Walker, J.S. The effect of habitat on the prevalence of human scrub typhus in Malaysia. Trans. R. Soc. Trop. Med. Hyg. 1972, 66, 582–587. [Google Scholar] [CrossRef]
- Francis, C.M. A Guide to The Mammals of Southeast Asia; Princeton University Press: Princeton, NJ, USA, 2008. [Google Scholar]
- Robins, J.H.; Hingston, M.; Matisoo-Smith, E.; Ross, H.A. Identifying Rattus species using mitochondrial DNA. Mol. Ecol. Notes 2007, 7, 717–729. [Google Scholar] [CrossRef]
- Lajos, R.; Jenö, R.; Gábor, M. Quantifying parasites in samples of hosts. J. Parasitol. 2000, 86, 228–232. [Google Scholar] [CrossRef]
- Chaisiri, K.; Stekolnikov, A.A.; Makepeace, B.L.; Morand, S. A revised checklist of chigger mites (Acari: Trombiculidae) from Thailand, with the description of three new species. J. Med. Entomol. 2016, 53, 321–342. [Google Scholar] [CrossRef]
- Kindt, R.; Coe, R. Tree Diversity Analysis. A Manual and Software for Common Statistical Methods for Ecological and Biodiversity Studies. Available online: http://www.worldagroforestry.org/output/tree-diversity-analysis (accessed on 8 August 2022).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Delignette-Muller, M.L.; Dutang, C. fitdistrplus: An R Package for fitting distributions. J. Stat. Softw. 2015, 64, 1–34. [Google Scholar] [CrossRef]
- Calcagno, V.; de Mazancourt, C. glmulti: An R Package for Easy Automated Model Selection with (Generalized) Linear Models. J. Stat. Softw. 2010, 34, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage publications: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Lüdecke, D. Sjstats: Statistical Functions for Regression Models (Version 0.18.1). Available online: https://zenodo.org/record/1400701#.YvGIBy8w3SU (accessed on 8 August 2022).
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 28 April 2018).
- Dormann, C.F.; Fründ, J.; Blüthgen, N.; Gruber, B. Indices, graphs and null models: Analyzing bipartite ecological networks. Open Ecol. J. 2009, 2, 7–24. [Google Scholar] [CrossRef]
- Opsahl, T. Tnet: Software for Analysis of Weighted, Two-Mode, and Longitudinal Networks. Available online: https://cran.r-project.org/package=tnet (accessed on 8 August 2022).
- Masakhwe, C.; Linsuwanon, P.; Kimita, G.; Mutai, B.; Leepitakrat, S.; Yalwala, S.; Abuom, D.; Auysawasi, N.; Gilbreath, T.; Wanja, E.; et al. Identification and characterization of Orientia chuto in trombiculid chigger mites collected from wild rodents in Kenya. J. Clin. Microbiol. 2018, 56, e01124-18. [Google Scholar] [CrossRef] [PubMed]
- Griffith, D.M.; Veech, J.A.; Marsh, C.J. cooccur: Probabilistic species co-occurrence analysis in R. J. Stat. Softw. 2016, 69, 1–17. [Google Scholar] [CrossRef]
- Veech, J.A. A probabilistic model for analysing species co-occurrence. Glob. Ecol. Biogeogr. 2013, 22, 252–260. [Google Scholar] [CrossRef]
- Grigg, M.; William, T.; Clemens, E.; Patel, K.; Chandna, A.; Wilkes, C.; Barber, B.; Anstey, N.; Dumler, J.S.; Yeo, T.; et al. Rickettsioses as major etiologies of unrecognized acute febrile illness, Sabah, East Malaysia. Emerg. Infect. Dis. 2020, 26, 1409. [Google Scholar] [CrossRef]
- Ernieenor, F.C.L.; NorJaiza, M.J.; Fadillah, A.; Canedy, J.; Mariana, A. Screening and genotyping of Orientia tsutsugamushi from field-collected on-host chiggers (Acari: Prostigmata) recovered from a positive scrub typhus locality in Kelantan, Malaysia. Exp. Appl. Acarol. 2021, 84, 171–182. [Google Scholar] [CrossRef]
- Yuhana, M.Y.; Hanboonkunupakarn, B.; Tanganuchitcharnchai, A.; Sujariyakul, P.; Sonthayanon, P.; Chotivanich, K.; Pukrittayakamee, S.; Blacksell, S.D.; Paris, D.H. Rickettsial infections are neglected causes of acute febrile illness in Teluk Intan, Peninsular Malaysia. Trop. Med. Infect. Dis. 2022, 7, 77. [Google Scholar] [CrossRef]
- Li, B.; Guo, X.-G.; Zhao, C.-F.; Zhang, Z.-W.; Fan, R.; Peng, P.-Y.; Song, W.-Y.; Ren, T.-G.; Zhang, L.; Qian, T.-J. Infestation of chigger mites on Chinese mole shrew, Anourosorex squamipes, in Southwest China and ecological analysis. Parasite 2022, 29, 39. [Google Scholar] [CrossRef]
- Stekolnikov, A.A. A checklist of chigger mites (Acariformes: Trombiculidae) of Southeast Asia. Zootaxa 2021, 4913, 1–163. [Google Scholar] [CrossRef]
- Wulandhari, S.A.; Paladsing, Y.; Saesim, W.; Charoennitiwat, V.; Sonthayanon, P.; Kumlert, R.; Morand, S.; Sumruayphol, S.; Chaisiri, K. High prevalence and low diversity of chigger infestation in small mammals found in Bangkok Metropolitan parks. Med. Vet. Entomol. 2021, 35, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Upham, R.W., Jr.; Hubert, A.A.; Phang, O.W.; Mat, Y.b.; Rapmund, G. Distribution of Leptotrombidium (Leptotrombidium) arenicola (Acarina: Trombiculidae) on the ground in West Malaysia. J. Med. Entomol. 1971, 8, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Rapmund, G.; Dohany, A.L.; Manikumaran, C.; Chan, T.C. Transovarial transmission of Rickettsia tsutsugamushi in Leptotrombidium (Leptotrombidium) arenicola Traub (Acarina: Trombiculidae)1. J. Med. Entomol. 1972, 9, 71–72. [Google Scholar] [CrossRef] [PubMed]
- Shirai, A.; Saunders, J.P.; Dohany, A.L.; Huxsoll, D.L.; Groves, M.G. Transmission of scrub typhus to human volunteers by laboratory-reared chiggers. Jpn J. Med. Sci. Biol. 1982, 35, 9–16. [Google Scholar] [CrossRef]
- Stekolnikov, A.A.; Shamsi, M.; Saboori, A.; Zahedi Golpayegani, A.; Hakimitabar, M. Distribution of chigger mites (Acari: Trombiculidae) over hosts, parasitopes, collection localities, and seasons in northern Iran. Exp. Appl. Acarol. 2022, 86, 21–47. [Google Scholar] [CrossRef]
- Wei, C.-Y.; Wang, J.-K.; Shih, H.-C.; Wang, H.-C.; Kuo, C.-C. Invasive plants facilitated by socioeconomic change harbor vectors of scrub typhus and spotted fever. PLoS Negl. Trop. Dis. 2020, 14, e0007519. [Google Scholar] [CrossRef]
- Clopton, R.E.; Gold, R.E. Distribution and Seasonal and Diurnal Activity Patterns of Eutrombicula alfreddugesi (Acari: Trombiculidae) in a Forest Edge Ecosystem. J. Med. Entomol. 1993, 30, 47–53. [Google Scholar] [CrossRef]
- André, V.R.; Javier, A.S. Ectoparasitism by Eutrombicula alfreddugesi larvae (Acari: Trombiculidae) on Liolaemus tenuis lizard in a Chilean fragmented temperate forest. J. Parasitol. 2009, 95, 244–245. [Google Scholar] [CrossRef]
- Kim, G.; Ha, N.-Y.; Min, C.-K.; Kim, H.-I.; Yen, N.T.H.; Lee, K.-H.; Oh, I.; Kang, J.-S.; Choi, M.-S.; Kim, I.-S.; et al. Diversification of Orientia tsutsugamushi genotypes by intragenic recombination and their potential expansion in endemic areas. PLoS Negl. Trop. Dis. 2017, 11, e0005408. [Google Scholar] [CrossRef]
- WMO: World Meteorological Organisation. World Weather Information Service-Weather Information for Ipoh. Available online: https://worldweather.wmo.int/en/city.html?cityId=77 (accessed on 8 August 2022).
- WMO: World Meteorological Organisation. World Weather Information Service-Weather Information for Johor Bahru. Available online: https://worldweather.wmo.int/en/city.html?cityId=78 (accessed on 8 August 2022).
- Chaisiri, K.; Tanganuchitcharnchai, A.; Kritiyakan, A.; Thinphovong, C.; Tanita, M.; Morand, S.; Blacksell, S.D. Risk factors analysis for neglected human rickettsioses in rural communities in Nan province, Thailand: A community-based observational study along a landscape gradient. PLoS Negl. Trop. Dis. 2022, 16, e0010256. [Google Scholar] [CrossRef]
- Morand, S.; Lajaunie, C. Outbreaks of vector-borne and zoonotic diseases are associated with changes in forest cover and oil palm expansion at global scale. Front. Vet. Sci. 2021, 8, 661063. [Google Scholar] [CrossRef] [PubMed]




| Species | Number Identified | Frequency (%) * | Prevalence of Infestation (%) ^ | Notes ~ |
|---|---|---|---|---|
| Ascoschoengastia indica | 691 | 35.4 | 42.9 | |
| Leptotrombidium deliense | 566 | 29.0 | 43.8 | |
| Walchiella oudemansi | 225 | 11.5 | 23.5 | |
| Leptotrombidium arenicola | 157 | 8.0 | 14.8 | New record on R. exulans, T. glis |
| Gahrliepia fletcheri | 128 | 6.6 | 6.0 | |
| Walchia lewthwaitei | 116 | 5.9 | 8.8 | New record on T. glis |
| Eutrombicula wichmanni | 22 | 1.1 | 3.2 | New record on R. tiomanicus |
| Walchia ewingi ewingi | 16 | 0.8 | 1.8 | New record on R. tiomanicus |
| Walchia disparunguis pingue | 14 | 0.7 | 2.8 | |
| Walchia rustica | 11 | 0.6 | 0.9 | |
| Gahrliepia rutila | 3 | 0.2 | 0.5 | |
| Trombiculindus paniculatum | 2 | 0.1 | 0.5 | New record for Malaysia |
| Walchia kritochaeta | 2 | 0.1 | 0.5 | New record for Malaysia |
| Leptotrombidium sp. | 1 | 0.1 | 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkathiry, H.; Al-Rofaai, A.; Ya’cob, Z.; Cutmore, T.S.; Mohd-Azami, S.N.I.; Husin, N.A.; Lim, F.S.; Koosakulnirand, S.; Mahfodz, N.H.; Ishak, S.N.; et al. Habitat and Season Drive Chigger Mite Diversity and Abundance on Small Mammals in Peninsular Malaysia. Pathogens 2022, 11, 1087. https://doi.org/10.3390/pathogens11101087
Alkathiry H, Al-Rofaai A, Ya’cob Z, Cutmore TS, Mohd-Azami SNI, Husin NA, Lim FS, Koosakulnirand S, Mahfodz NH, Ishak SN, et al. Habitat and Season Drive Chigger Mite Diversity and Abundance on Small Mammals in Peninsular Malaysia. Pathogens. 2022; 11(10):1087. https://doi.org/10.3390/pathogens11101087
Chicago/Turabian StyleAlkathiry, Hadil, Ahmed Al-Rofaai, Zubaidah Ya’cob, Tamsin S. Cutmore, Siti Nurul Izzah Mohd-Azami, Nurul Aini Husin, Fang Shiang Lim, Sirikamon Koosakulnirand, Nor Hidayana Mahfodz, Siti Nabilah Ishak, and et al. 2022. "Habitat and Season Drive Chigger Mite Diversity and Abundance on Small Mammals in Peninsular Malaysia" Pathogens 11, no. 10: 1087. https://doi.org/10.3390/pathogens11101087






