The Antimicrobial Properties of Poplar and Aspen–Poplar Propolises and Their Active Components against Selected Microorganisms, including Helicobacter pylori
Abstract
:1. Introduction
- Evaluate the anti-H. pylori activity of tested propolis samples of different origins;
- Assess the relation between the polyphenolic profile of propolis, their plant origins, and their antimicrobial activity;
- Evaluate the general antimicrobial profile of the tested propolis as a way to better understand the activity of the tested propolis extracts against H. pylori. For this purpose, the antibacterial activities of propolis extracts against six Gram+ and five Gram− (not including H. pylori) bacterial strains, as well as three species of yeasts, were evaluated.
2. Results and Discussion
2.1. The Chemical Composition and the Classification of Tested Propolis Samples
2.2. The Antimicrobial Activity of Tested Propolis Samples
2.2.1. The Antibacterial Activity of Tested Propolis Extracts against H. pylori
2.2.2. The Antimicrobial Activity of Tested Propolis Extracts
2.3. Impact of Components on Antimicrobial Activity
2.4. Principal Component Analysis and Hierarchical Fuzzy Clustering
3. Materials and Methods
3.1. Sample Preparation
3.2. UPLC-DAD-MS Analysis of Propolis Extracts
3.3. The Determination of Antimicrobial Activity
3.4. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Baltas, N.; Karaoglu, S.A.; Tarakci, C.; Kolayli, S. Effect of propolis in gastric disorders: Inhibition studies on the growth of helicobacter pylori and production of its urease. J. Enzyme Inhib. Med. Chem. 2016, 31, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Lee, D.; Kim, E. Anti-inflammatory and anti-oxidative effect of Korean propolis on Helicobacter pylori-induced gastric damage in vitro. J. Microbiol. 2020, 58, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Makola, D.; Peura, D.A.; Crowe, S.E. Helicobacter pylori infection and related gastrointestinal diseases. J. Clin. Gastroenterol. 2007, 41, 548–558. [Google Scholar] [CrossRef]
- Robinson, K.; Atherton, J.C. The spectrum of Helicobacter-mediated diseases. Annu. Rev. Pathol. 2021, 16, 123–144. [Google Scholar] [CrossRef]
- Krzyżek, P.; Junka, A.; Słupski, W.; Dołowacka-Jóźwiak, A.; Płachno, B.J.; Sobiecka, A.; Zielińska, S. Antibiofilm and antimicrobial-enhancing activity of chelidonium majus and corydalis cheilanthifolia extracts against multidrug-resistant helicobacter pylori. Pathogens 2021, 10, 1033. [Google Scholar] [CrossRef]
- Romero, M.; Freire, J.; Pastene, E.; García, A.; Aranda, M.; González, C. Propolis polyphenolic compounds affect the viability and structure of helicobacter pylori in vitro. Rev. Bras. Pharmacogn. 2019, 29, 325–332. [Google Scholar] [CrossRef]
- Camargo, M.C.; García, A.; Riquelme, A.; Otero, W.; Camargo, C.A.; Hernandez-García, T.; Candia, R.; Bruce, M.G.; Rabkin, C.S. The problem of helicobacter pylori resistance to antibiotics: A systematic review in Latin America. Am. J. Gastroenterol. 2014, 109, 485–495. [Google Scholar] [CrossRef]
- Megraud, F.; Bruyndonckx, R.; Coenen, S.; Wittkop, L.; Huang, T.; Hoebeke, M.; Benejat, L.; Lehours, P.; Goossens, H.; Glupczynski, Y. Helicobacter pylori resistance to antibiotics in Europe in 2018 and its relationship to antibiotic consumption in the community. Gut 2021, 70, 1815–1822. [Google Scholar] [CrossRef]
- Tacconelli, E.; Magrini, N. Global Priority List of Antibiotic-Resistant Bacteria; World Health Organisation: Geneve, Switzerland, 2017; Volume 7. [Google Scholar]
- Baj, T.; Korona-Głowniak, I.; Kowalski, R.; Malm, A. Chemical composition and microbiological evaluation of essential oil from Hyssopus officinalis L. with white and pink flowers. Open Chem. 2018, 16, 317–323. [Google Scholar] [CrossRef]
- Popova, M.; Giannopoulou, E.; Skalicka-Wózniak, K.; Graikou, K.; Widelski, J.; Bankova, V.; Kalofonos, H.; Sivolapenko, G.; Gaweł-Bęben, K.; Antosiewicz, B.; et al. Characterization and biological evaluation of propolis from Poland. Molecules 2017, 22, 1159. [Google Scholar] [CrossRef]
- Ratnasari, N.; Rezkitha, Y.A.; Adnyana, I.K.; Alfaray, R.I.; Fauzia, K.A.; Doohan, D.; Panjaitan, A.; Priskila, Y.; Yulinah, E.; Khomsan, A.; et al. Anti-helicobacter pylori effects of propolis ethanol extract on clarithromycin and metronidazole resistant strains. Syst. Rev. Pharm. 2020, 11, 429–434. [Google Scholar] [CrossRef]
- Okińczyc, P.; Widelski, J.; Szperlik, J.; Żuk, M.; Mroczek, T.; Skalicka-Woźniak, K.; Sakipova, Z.; Widelska, G.; Kuś, P.M. Article impact of plant origin on euroasian propolis on phenolic profile and classical antioxidant activity. Biomolecules 2021, 11, 68. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.; Chiu, H.; Wu, C.; Lu, Y.; Han, Y.; Shen, Y.; Venkatakrishnan, K.; Wang, C. Beneficial efficacy of various propolis extracts and their digestive products by in vitro simulated gastrointestinal digestion. LWT 2017, 84, 281–289. [Google Scholar] [CrossRef]
- Okinczyc, P.; Szumny, A.; Szperlik, J.; Kulma, A.; Franiczek, R.; Zbikowska, B.; Krzyzanowska, B.; Sroka, Z. Profile of polyphenolic and essential oil composition of polish propolis, black poplar and aspens buds. Molecules 2018, 23, 1262. [Google Scholar] [CrossRef] [PubMed]
- Graikou, K.; Popova, M.; Gortzi, O.; Bankova, V.; Chinou, I. Characterization and biological evaluation of selected Mediterranean propolis samples. Is it a new type? LWT 2016, 65, 261–267. [Google Scholar] [CrossRef]
- Popova, M.P.; Chinou, I.B.; Markov, I.N.; Bankova, V.S. Terpenes with antimicrobial activity from Cretan propolis. Phytochemistry 2009, 70, 1262–1271. [Google Scholar] [CrossRef]
- Bankova, V.S.; De Castro, S.L.; Marcucci, M.C. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- O’Donnell, F.; Smyth, T.J.P.; Ramachandran, V.N.; Smyth, W.F. A study of the antimicrobial activity of selected synthetic and naturally occurring quinolines. Int. J. Antimicrob. Agents 2010, 35, 30–38. [Google Scholar] [CrossRef]
- Bonvehí, J.S.; Gutiérrez, A.L. The antimicrobial effects of propolis were collected in different regions in the basque country (northern Spain). World J. Microbiol. Biotechnol. 2012, 28, 1351–1358. [Google Scholar] [CrossRef]
- Tamokou, J.; Mbaveng, A.T.; Kuete, V. Antimicrobial activities of African medicinal spices and vegetables. In Medicinal Spices and Vegetables from Africa: Therapeutic Potential Against Metabolic, Inflammatory, Infectious and Systemic Diseases; Academic Press: Cambridge, MA, USA, 2017; pp. 207–237. [Google Scholar] [CrossRef]
- Wang, Y. Medicinal plant activity on helicobacter pylori related diseases. World J. Gastroenterol. 2014, 20, 10368–10382. [Google Scholar] [CrossRef]
- Alaribe, C.S.; Esposito, T.; Sansone, F.; Sunday, A.; Pagano, I.; Piccinelli, A.L.; Picerno, P. Nigerian propolis: Chemical composition, antioxidant activity and α-amylase and α-glucosidase inhibition. Nat. Prod. Res. 2021, 35, 3095–3099. [Google Scholar] [CrossRef]
- Liu, Q.; Meng, X.; Li, Y.; Zhao, C.; Tang, G.; Li, S.; Gan, R.; Li, S.; Li, H. Natural products for the prevention and management of helicobacter pylori infection. Compr. Rev. Food Sci. Food Saf. 2018, 17, 937–952. [Google Scholar] [CrossRef] [PubMed]
- Okińczyc, P. Phytochemical Characteristics and Biological Activity of Different Kinds of Propolis and Their Plant Sources; [title in Polish: Charakterystyka Fitochemiczna Oraz Aktywność Biologiczna Różnych Rodzajów Propolisów Oraz ich Źródeł Roślinnych]; Uniwersytet Medyczny we Wrocławiu: Wrocław, Poland, 2017. [Google Scholar]
- Hagag, A.A.; Amin, S.M.; Emara, M.H.; Abo-Resha, S.E. Gastric mucosal oxidative stress markers in children with Helicobacter pylori infection. Infect. Disord. Drug Targets 2018, 18, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Grecka, K.; Kuś, P.M.; Okińczyc, P.; Worobo, R.W.; Walkusz, J.; Szweda, P. The anti-staphylococcal potential of ethanolic Polish propolis extracts. Molecules 2019, 24, 1732. [Google Scholar] [CrossRef]
- Neto, M.R.; Tintino, S.R.; da Silva, A.R.; do Socorro Costa, M.; Boligon, A.A.; Matias, E.F.; de Queiroz Balbino, V.; Menezes, I.R.; Coutinho, H.D. Seasonal variation of Brazilian red propolis: Antibacterial activity, synergistic effect, and phytochemical screening. Food Chem. Toxicol. 2017, 107, 572–580. [Google Scholar] [CrossRef]
- Machado, B.A.; Silva, R.P.; Barreto, G.D.; Costa, S.S.; Silva, D.F.; Brandao, H.N.; Rocha, J.L.; Dellagostin, O.A.; Henriques, J.A.; Umsza-Guez, M.A.; et al. Chemical composition and biological activity of extracts obtained by supercritical extraction and ethanolic extraction of brown, green, and red propolis derived from different geographic regions in Brazil. PLoS ONE 2016, 11, e0145954. [Google Scholar] [CrossRef]
- Suleman, T.; van Vuuren, S.; Sandasi, M.; Viljoen, A.M. Antimicrobial activity and chemometric modelling of south african propolis. J. Appl. Microbiol. 2015, 119, 981–990. [Google Scholar] [CrossRef]
- AL-Ani, I.; Zimmermann, S.; Reichling, J.; Wink, M. Antimicrobial Activities of European Propolis Collected from Various Geographic Origins Alone and in Combination with Antibiotics. Medicines 2018, 5, 2. [Google Scholar] [CrossRef]
- Okińczyc, P.; Paluch, E.; Franiczek, R.; Widelski, J.; Wojtanowski, K.K.; Mroczek, T.; Krzyżankowska, B.; Skalicka-Woźniak, K.; Sroka, Z. Antimicrobial activity of Apis mellifera L. and trigona sp. propolis from nepal and its phytochemical analysis. Biomed. Pharmacother. 2020, 129, 110435. [Google Scholar] [CrossRef]
- Ristivojević, P.; Dimkić, I.; Trifković, J.; Berić, T.; Vovk, I.; Milojkovič-Opsenica, D.; Stanković, S. Antimicrobial activity of Serbian propolis evaluated using MIC, HPTLC, bioautography, and chemometrics. PLoS ONE 2016, 11, e0157097. [Google Scholar] [CrossRef]
- Uzel, A.; Sorkun, K.; Önçaǧ, O.; Çoǧulu, D.; Gençay, O.; Salih, B. Chemical compositions and antimicrobial activities of four different Anatolian propolis samples. Microbiol. Res. 2005, 160, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.M.; Stock, D.; Chada, F.J.G.; Finger, D.; Sawaya, A.C.; Eberlin, M.N.; Torres, Y.R. A comparison between characterization and biological properties of Brazilian fresh and aged propolis. BioMed Res. Int. 2014, 2014, 257617. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Chung, H. The effects of Korean propolis against foodborne pathogens and transmission electron microscopic examination. New Biotechnol. 2011, 28, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Wojtyczka, R.D.; Dziedzic, A.; Idzik, D.; Kepa, M.; Kubina, R.; Kabała-Dzik, A.; Soleń-Dzirba, J.; Stojko, J.; Sajewicz, M.; Wasik, T.J. Susceptibility of staphylococcus aureus clinical isolates to propolis extract alone or in combination with antimicrobial drugs. Molecules 2013, 18, 9623–9640. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V. Recent trends and important developments in propolis research. Evid. Based Complementary Altern. Med. 2005, 2, 29–32. [Google Scholar] [CrossRef]
- Bankova, V. Chemical diversity of propolis and the problem of standardization. J. Ethnopharmacol. 2005, 100, 114–117. [Google Scholar] [CrossRef]
- Kaleta, J. The Physicochemical Analysis of Propolis and the Possibility of its Standardization [in Polish: Analiza fizykochemiczna propolisu i możliwości jego standaryzacji]. Ph.D. Thesis, Jagiellonian University in Kraków, Kraków, Poland, 2007. [Google Scholar]
- Przybyłek, I.; Karpiński, T.M. Antibacterial properties of propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef]
- Oliveira, A.V.; Ferreira, A.L.; Nunes, S.; Dandlen, S.A.; Miguel, M.D.G.; Faleiro, M.L. Antibacterial activity of propolis extracts from the south of Portugal. Pak. J. Pharm. Sci. 2017, 30, 1–9. [Google Scholar]
- Isidorov, V.A.; Baker, S.; Pirożnikow, E.; Zambrzycka, M.; Swiecicka, I. Selective Behaviour of Honeybees in Acquiring European Propolis Plant Precursors. J. Chem. Ecol. 2016, 42, 475–485. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Szczepaniak, L.; Baker, S. Rapid GC/ms determination of botanical precursors of Eurasnnn propolis. Food Chem. 2014, 142, 101–106. [Google Scholar] [CrossRef]
- Ristivojević, P.; Trifković, J.; Andrić, F.; Milojković-Opsenica, D. Poplar-type propolis: Chemical composition, botanical origin, and biological activity. Nat. Prod. Commun. 2015, 10, 1869–1875. [Google Scholar] [CrossRef]
- Ristivojević, P.; Trifković, J.; Gašić, U.; Andrić, F.; Nedić, N.; Tešić, Ž.; Milojković-Opsenica, D. Ultrahigh-performance Liquid Chromatography and Mass Spectrometry (UHPLC-LTQ/Orbitrap/MS/MS) study of the phenolic profile of Serbian poplar type propolis. Phytochem. Anal. 2015, 26, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Bertrams, J.; Müller, M.B.; Kunz, N.; Kammerer, D.R.; Stintzing, F.C. Phenolic compounds as marker compounds for botanical origin determination of German propolis samples based on TLC and TLC-MS. J. Appl. Bot. Food Qual. 2013, 86, 143–153. [Google Scholar] [CrossRef]
- Wu, Y.W.; Sun, S.Q.; Zhao, J.; Li, Y.; Zhou, Q. Rapid discrimination of extracts of Chinese propolis and poplar buds by FT-IR and 2D IR correlation spectroscopy. J. Mol. Struct. 2008, 883, 48–54. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing—EUCAST. Available online: www.eucast.org (accessed on 1 March 2021).
- Korona-Glowniak, I.; Glowniak-Lipa, A.; Ludwiczuk, A.; Baj, T.; Malm, A. The In Vitro Activity of Essential Oils against Helicobacter Pylori Growth and Urease Activity. Molecules 2020, 25, 586. [Google Scholar] [CrossRef] [PubMed]
- Pobiega, K.; Kraśniewska, K.; Przybył, J.L.; Bączek, K.; Żubernik, J.; Witrowa-Rajchert, D.; Gniewosz, M. Growth biocontrol of foodborne pathogens and spoilage microorganisms of food by polish propolis extracts. Molecules 2019, 24, 2965. [Google Scholar] [CrossRef]
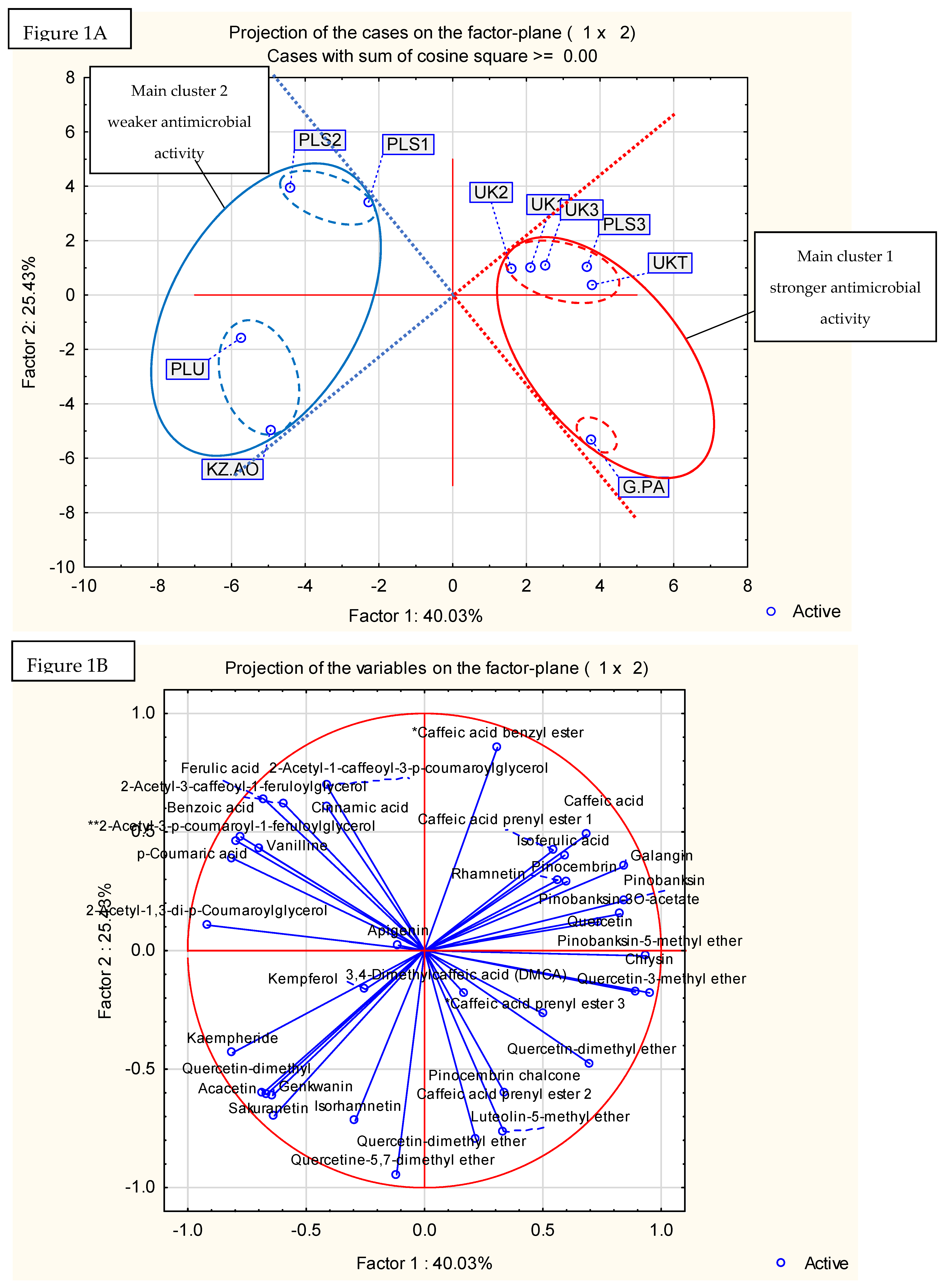
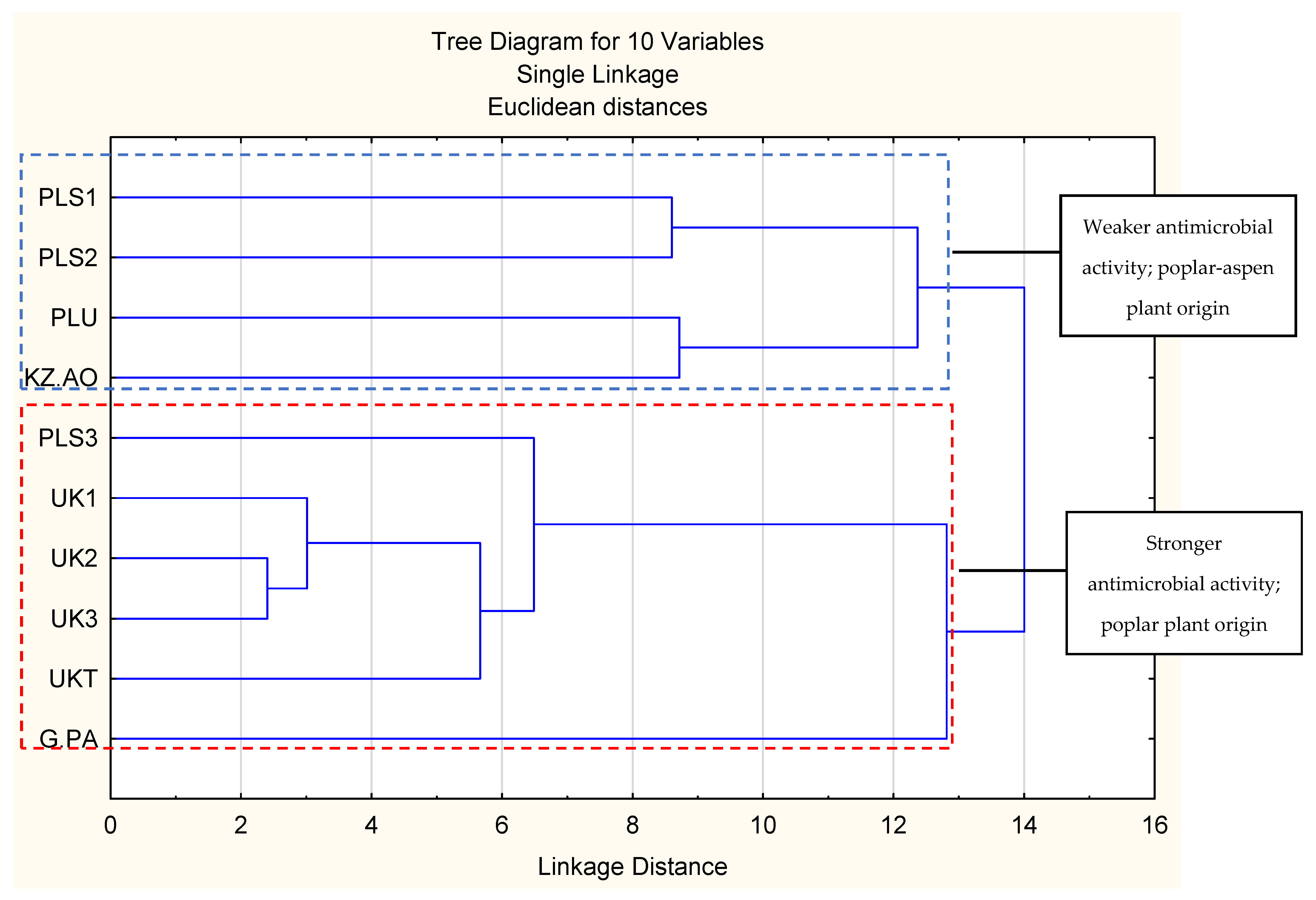
| Component | RT | UV λ Max (nm) | [M-H]- | PLS1 | PLS2 | PLS3 | PLU | UK1 | UK2 | UK3 | UT | KZ | GP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| * Caffeoyl-glycerol | 1.88 | 324, 298sh, 242 | 253 | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr |
| p-Hydroxy benzoic acid | 2.13 | 256 | 137 | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr |
| Caffeic acid | 2.17 | 324, 298sh, 242 | 179 | + | + | + | tr | + | + | + | + | + | + |
| * p-Coumaroylglycerol | 2.63 | 310, 300sh, 229 | 237 | tr | tr | tr | tr | - | - | - | - | tr | - |
| p-Coumaric acid | 3.25 | 310, 300sh, 229 | 163 | ++ | +++ | ++ | ++ | + | + | + | + | + | tr |
| Metoxybenzaldehyd | 3.31 | 276 | 135 | tr | tr | tr | - | - | - | - | - | - | - |
| Vanillin | 3.42 | 310,280, 231 | 151 | tr | + | tr | + | tr | tr | tr | - | tr | - |
| Ferulic acid | 3.70 | 324, 298sh, 236 | 193 | ++ | ++ | + | + | + | + | + | tr | + | tr |
| Isoferulic acid | 4.11 | 323, 295sh, 221 | 193 | + | tr | + | tr | + | tr | + | tr | tr | tr |
| Benzoic acid | 5.97 | 281sh, 274sh, 236 | 121 | + | + | tr | + | + | + | + | tr | + | tr |
| * Ferulic acid derivate | 6.82 | 326, 298sh, 236 | 389 | - | - | - | tr | tr | tr | tr | tr | tr | - |
| Acetyl-p-coumraoylglycerol | 7.40 | 311 | 279 | tr | tr | tr | tr | tr | tr | tr | - | tr | - |
| 3,4-Dimethylcaffeic acid (DMCA) | 8.07 | 322, 294sh, 236 | 207 | + | + | + | tr | tr | tr | tr | tr | tr | + |
| * Apigenin-O-glucoside | 8.52 | 315sh, 265 | 431 | tr | tr | tr | - | tr | tr | tr | tr | tr | tr |
| Luteolin | 11.30 | 345, 254 | 285 | tr | tr | tr | - | tr | tr | tr | tr | tr | tr |
| Quercetin | 11.43 | 368, 270sh, 256 | 301 | tr | tr | tr | tr | tr | tr | tr | + | tr | tr |
| Pinobanksin-5-methyl ether | 12.32 | 322sh, 288, 228 | 285 | tr | tr | + | tr | + | + | + | + | tr | + |
| Cinnamic acid | 12.94 | 277 | 147 | tr | + | tr | tr | tr | tr | tr | - | - | - |
| Quercetin-3-methyl ether | 13.68 | 355, 293sh, 255 | 315 | tr | tr | tr | tr | tr | tr | tr | tr | tr | + |
| * 1-Caffeoyl-3-p-coumaroyl glycerol | 15.23 | 315, 298sh, 235 | 399 | tr | tr | - | tr | - | - | - | - | tr | - |
| Naringenine | 15.83 | 330sh, 290, 230 | 271 | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr |
| Pinobanksin | 16.30 | 332sh, 292, 229 | 271 | + | tr | + | tr | + | + | + | + | + | + |
| Apigenin | 16.40 | 338, 290sh, 268 | 269 | + | tr | + | + | + | + | + | tr | + | tr |
| Caffeoyl-feruloylglycerol | 16.12 | 326, 298sh, 240 | 429 | - | - | - | - | - | - | - | - | tr | - |
| Chrysin-5-met ether | 16.80 | 314sh, 264, 247sh | 267 | - | - | tr | tr | tr | tr | tr | tr | tr | tr |
| Kempferolhyl | 17.48 | 366, 322sh, 295sh, 266 | 285 | tr | + | + | + | + | + | + | + | + | tr |
| Isorhamnetin | 19.11 | 371, 298sh, 268sh, 255 | 315 | tr | tr | tr | tr | tr | tr | tr | tr | + | tr |
| Quercetin-methyl ether | 19.57 | 371, 298sh, 268sh, 255 | 315 | - | - | - | - | tr | tr | tr | - | tr | tr |
| Luteolin-5-methyl ether | 20.15 | 350, 298sh, 266, 232sh | 299 | tr | tr | + | tr | + | + | + | tr | + | + |
| ** 1,3-Di-p-coumaroylglycerol | 20.25 | 310, 300sh, 233 | 383 | tr | tr | tr | tr | tr | tr | tr | tr | + | - |
| Quer-5,7-dimethyl ether | 20.71 | 356, 296sh, 269sh, 255 | 329 | tr | tr | tr | tr | tr | tr | tr | tr | + | + |
| p-Coumaroyl-feruloylglycerol | 21.37 | 316, 298sh, 233 | 413 | tr | tr | - | - | - | - | - | tr | tr | - |
| Di-feruloiloglicerol | 21.95 | 323, 298sh | 443 | tr | tr | - | - | tr | tr | tr | - | tr | - |
| 2-Acetyl-1,3-di-caffeoylglycerol | 22.57 | 328, 298sh, 244 | 457 | tr | tr | - | tr | tr | tr | tr | tr | tr | - |
| β-styrylacrilic acid | 23.82 | 311, 240sh | 173 | tr | tr | tr | tr | tr | tr | tr | tr | - | tr |
| Galangin-5-methyl ether | 25.42 | 352, 300sh, 261, 240sh | 283 | tr | tr | tr | - | tr | tr | tr | tr | tr | tr |
| Pinobanksin-5-methyl-ether-3-O-acetate | 25.60 | 289 | 327 | tr | tr | tr | - | tr | tr | tr | tr | tr | tr |
| * Caffeic acid buteniccetin or isobutenic ester | 24.73 | 326, 298sh, 248 | 233 | - | - | - | - | - | - | - | - | tr | tr |
| Rhamnetin | 25.92 | 354, 298sh, 268sh, 255 | 315 | tr | tr | tr | tr | tr | tr | tr | + | tr | - |
| Quercetin-dimethyl ether | 27.22 | 356, 292sh, 268sh, 256 | 329 | tr | tr | tr | tr | - | - | - | tr | tr | + |
| 2-Acetyl-1-caffeoyl-3-p-coumaroylglycerol | 29.23 | 316, 299sh 235 | 441 | + | + | tr | tr | tr | tr | tr | tr | tr | - |
| Quercetin-dimethyl ether | 29.46 | 356, 268sh, 256 | 329 | tr | tr | tr | tr | tr | tr | tr | tr | tr | + |
| Caffeic acid butyl or isobutyl ester | 30.20 | 326, 298sh, 242 | 235 | - | - | - | - | - | - | - | - | tr | tr |
| 2-Acetyl-3-caffeoyl-1-feruloylglycerol | 30.28 | 328, 300sh, 244 | 471 | + | tr | tr | tr | tr | tr | tr | - | tr | - |
| Quercetin-trimethyl ether | 30.63 | 353, 266sh, 254 | 343 | - | - | - | - | - | - | - | tr | - | tr |
| Caffeic acid prenyl ester 1 | 31.92 | 326, 298sh, 246 | 247 | + | tr | + | tr | tr | + | + | + | tr | tr |
| Chrysin | 32.11 | 314sh, 268, | 253 | + | + | +++ | + | +++ | +++ | +++ | +++ | + | +++ |
| Pinobanksin-7-methyl ether | 33.62 | 291, 247sh | 285 | - | - | re | - | tr | tr | tr | - | - | tr |
| Pinocembrin | 33.68 | 330sh, 290, | 255 | ++ | ++ | ++ | ++ | +++ | +++ | +++ | +++ | ++ | + |
| Acacetin | 34.08 | 335, 299sh, 268 | 283 | tr | tr | tr | + | - | - | - | - | +++ | - |
| Caffeic acid prenyl ester 2 | 34.10 | 326, 298sh, 246 | 247 | - | - | - | - | tr | tr | tr | - | tr | + |
| Pinocembrin chalcone | 34.14 | 345 | 255 | - | - | - | - | - | - | - | - | - | + |
| * Caffeic acid prenyl ester 3 | 34.21 | 326, 298sh, 246 | 247 | + | + | + | - | tr | tr | tr | + | tr | ++ |
| * Caffeic acid benzyl ester | 34.62 | 328, 298sh, 244 | 269 | ++ | + | ++ | tr | + | + | + | ++ | tr | tr |
| Caffeic acid prenyl ester 4 | 34.58 | 326, 298sh, 246 | 247 | tr | tr | tr | tr | tr | tr | tr | - | tr | tr |
| Sakuranetin | 35.16 | 290 | 285 | tr | tr | tr | ++ | + | + | + | + | +++ | + |
| Genkwanin | 35.64 | 337, 267, 242sh | 283 | tr | tr | tr | + | - | - | - | tr | + | tr |
| Galangin | 36.07 | 360, 290sh, 266, | 269 | ++ | + | +++ | + | +++ | +++ | +++ | +++ | + | ++ |
| Kaempheride | 37.31 | 366, 292sh, 266, | 299 | + | + | tr | ++ | tr | tr | tr | tr | ++ | tr |
| 2-Acetyl-1,3-di-p-coumaroylglycerol (lasiocarpin A) | 37.55 | 360sh, 312, 232 | 425 | + | ++ | tr | + | tr | tr | tr | tr | ++ | - |
| Pinobanksin-3O-acetate | 37.82 | 332sh, 294, 238 | 313 | + | + | ++ | + | +++ | +++ | +++ | ++ | + | ++ |
| Quercetin-dimethyl | 38.33 | 370, 268sh, 255 | 329 | - | - | - | + | - | - | - | - | + | tr |
| ** 2-Acetyl-3-p-coumaroyl-1-feruloylglycerol | 38.96 | 318, 299sh 235 | 455 | + | + | - | + | tr | tr | tr | tr | + | - |
| Metoxychrysin | 39.27 | 340sh, 310sh, 266 | 283 | tr | - | + | - | + | + | + | + | tr | + |
| 3-Acetyl-1,2-di-p-coumaroylglycerol | 40.09 | 312, 300sh, 238 | 425 | tr | + | - | tr | tr | tr | tr | - | tr | + |
| Caffeic acid phenethyl ester (CAPE) | 40.15 | 326, 298sh, 264 | 283 | + | tr | + | - | + | + | + | + | + | + |
| 2-Acetyl-1,3-di-feruloylglycerol | 40.37 | 328, 298sh, 243 | 485 | + | + | tr | tr | tr | + | tr | - | tr | - |
| Dimethyl luteolin | 41.05 | 348, 267, 246sh | 313 | - | - | - | + | tr | tr | tr | tr | ++ | - |
| Caffeic acid pentyl or isopentyl ester | 41.90 | 326, 298sh, 247 | 249 | - | - | - | - | - | - | - | - | - | - |
| Flavonoid dimethyl ether | 42.53 | 343, 271, 248sh | 343 | - | - | - | - | tr | tr | tr | - | + | - |
| p-Coumaric acid prenyl ester 1 | 45.42 | 311, 299sh, 245sh | 231 | - | - | tr | - | tr | tr | tr | tr | - | - |
| p-Coumaric acid prenyl ester 2 | 45.42 | 311, 299sh, 245sh | 231 | + | + | tr | + | tr | tr | tr | tr | - | tr |
| p-Coumaric acid benzyl ester | 45.80 | 312, 298sh, 244sh | 253 | + | ++ | + | ++ | + | + | + | + | + | tr |
| * Ferulic or isoferulic acid benzyl ester | 47.13 | 326, 298sh | 283 | + | + | + | + | + | + | + | + | + | + |
| Caffeic acid cinnamic ester | 48.04 | 326, 300sh, 243 | 295 | tr | tr | tr | tr | + | + | tr | tr | tr | tr |
| Pinobanksin-3-O-propanoate | 48.58 | 329sh, 294, 234 | 327 | tr | tr | tr | - | tr | tr | tr | tr | tr | + |
| p-Coumaric acid phenethyl ester | 49.21 | 312, 300sh, | 267 | + | + | tr | tr | - | tr | tr | - | - | tr |
| Pinostrobin chalcone | 49.67 | 345, 309sh, 267 | 269 | - | - | - | + | - | tr | tr | tr | tr | + |
| Tectochrysin (chrysin-7-methyl ether) | 51.23 | 310sh, 268 | 267 | tr | - | + | - | + | + | tr | + | - | + |
| Pinostrobin (pinocembrin-5-methyl ether) | 51.40 | 328sh, 290, | 269 | tr | - | tr | + | tr | + | tr | tr | + | + |
| Pinobanksin-3-O-butanoate or isonutanoate | 51.65 | 329sh, 294, 234 | 341 | tr | tr | + | tr | + | tr | + | + | + | + |
| Galangin-7-methyl ether | 52.21 | 353, 268 | 283 | tr | - | tr | tr | + | + | tr | tr | + | + |
| Pinobanksin-3-O-pentanoate or isopentanoate | 53.07 | 332sh, 293, 242 | 355 | tr | - | tr | - | tr | tr | tr | + | tr | + |
| Pinobanksin-3-O-pentenoate or isopentenoate | 53.24 | 332sh, 293, 242 | 353 | - | - | tr | - | tr | tr | tr | tr | ++ | + |
| Pinobanksin-3-O-hexanoate or isohexanoate | 54.09 | 282 | 369 | - | - | - | - | tr | tr | tr | tr | tr | - |
| Metoxycinnamic acid cinnamyl ester | 54.26 | 280 | 293 | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr |
| Propolis Extract/ Microorganism | PLS1 | PLS2 | PLS3 | PLU | UK1 | UK2 | UK3 | UKT | KZ | GP | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gram− bacteria | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MIC | MBC | MIC | MBC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC |
| H. pylori | 0.03 | 0.03 | 0.06 | 0.06 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.015 | 0.03 | 0.03 | 0.03 | 0.02 | 0.03 | 0.03 | 0.03 |
| S. typhimurium | 1.25 | >10 | >10 | >10 | >10 | >10 | >10 | 5.00 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 |
| E. coli | 2.50 | >10 | >10 | >10 | >10 | >10 | >10 | 5.00 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 |
| P. mirabilis | 2.50 | 10.0 | >10 | >10 | >10 | >10 | >10 | 5.00 | 10.0 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 |
| K. pneumoniae | 5.00 | >10 | >10 | >10 | >10 | >10 | >10 | 5.00 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 |
| P. aeruginosa | 1.25 | 10.0 | >10 | >10 | >10 | >10 | >10 | 1.25 | 10.0 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 | >10 |
| Gram+ bacteria | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MIC | MBC | MIC | MBC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC |
| S. aureus S1 | 0.16 | 0.31 | 0.63 | 2.50 | 0.08 | 0.16 | 0.16 | 0.16 | 0.63 | 19.50 | 78.0 | 0.63 | 0.04 | 0.16 | 0.01 | 0.04 | 0.08 | 0.16 | 0.01 | 0.04 |
| S. aureus S2 | 0.31 | 1.25 | 2.50 | 10.0 | 0.08 | 0.16 | 0.31 | 0.31 | 0.63 | 0.08 | 0.16 | 1.25 | 0.16 | 0.31 | 0.01 | 0.04 | 0.16 | 0.16 | 0.01 | 0.04 |
| S. epidermidis | 0.08 | 0.31 | 0.31 | 2.50 | 0.04 | 0.08 | 0.16 | 0.08 | 0.31 | 0.0195 | 0.08 | 0.31 | 0.04 | 0.16 | 0.01 | 0.04 | 0.08 | 0.16 | 0.01 | 0.04 |
| E. faecalis | 0.31 | 2.50 | 2.50 | 10.0 | 0.16 | 0.63 | 0.63 | 2.50 | 2.50 | 0.08 | 0.31 | 10.0 | 0.16 | 0.63 | 0.08 | 0.31 | 0.16 | 0.63 | 0.08 | 0.31 |
| M. luteus | 0.08 | 0.16 | 0.31 | 2.5 | 0.04 | 0.08 | 0.08 | 0.08 | 0.16 | 0.02 | 0.08 | 0.31 | 0.04 | 156 | 0.01 | 0.02 | 0.04 | 0.08 | 0.01 | 0.02 |
| B. cereus | 0.16 | 0.16 | 0.63 | 0.63 | 0.04 | 0.08 | 0.31 | 0.31 | 0.31 | 0.02 | 0.08 | 1.25 | 0.16 | 0.16 | 0.02 | 0.04 | 0.16 | 0.16 | 0.02 | 0.04 |
| Yeasts | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MIC | MFC | MIC | MFC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC |
| C. albicans | 0.31 | 1.25 | 1.25 | 5.00 | 0.08 | 0.16 | 0.31 | 0.31 | 1.25 | 0.16 | 0.16 | 1.25 | 0.16 | 0.31 | 0.16 | 0.31 | 0.16 | 0.31 | 0.08 | 0.16 |
| C. parapsilosis | 0.31 | 2.50 | 1.25 | 10.0 | 0.04 | 0.31 | 0.31 | 0.31 | 5.00 | 0.08 | 0.31 | 5.00 | 0.08 | 0.63 | 0.16 | 0.63 | 0.08 | 0.63 | 0.02 | 0.16 |
| C. glabrata | 0.31 | 0.63 | 0.63 | 2.5 | 0.08 | 0.16 | 0.63 | 0.31 | 0.63 | 0.08 | 0.16 | 2.5 | 0.16 | 0.31 | 0.16 | 0.31 | 0.16 | 0.31 | 0.08 | 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Widelski, J.; Okińczyc, P.; Paluch, E.; Mroczek, T.; Szperlik, J.; Żuk, M.; Sroka, Z.; Sakipova, Z.; Chinou, I.; Skalicka-Woźniak, K.; et al. The Antimicrobial Properties of Poplar and Aspen–Poplar Propolises and Their Active Components against Selected Microorganisms, including Helicobacter pylori. Pathogens 2022, 11, 191. https://doi.org/10.3390/pathogens11020191
Widelski J, Okińczyc P, Paluch E, Mroczek T, Szperlik J, Żuk M, Sroka Z, Sakipova Z, Chinou I, Skalicka-Woźniak K, et al. The Antimicrobial Properties of Poplar and Aspen–Poplar Propolises and Their Active Components against Selected Microorganisms, including Helicobacter pylori. Pathogens. 2022; 11(2):191. https://doi.org/10.3390/pathogens11020191
Chicago/Turabian StyleWidelski, Jarosław, Piotr Okińczyc, Emil Paluch, Tomasz Mroczek, Jakub Szperlik, Magdalena Żuk, Zbigniew Sroka, Zuriyadda Sakipova, Ioanna Chinou, Krystyna Skalicka-Woźniak, and et al. 2022. "The Antimicrobial Properties of Poplar and Aspen–Poplar Propolises and Their Active Components against Selected Microorganisms, including Helicobacter pylori" Pathogens 11, no. 2: 191. https://doi.org/10.3390/pathogens11020191







