Methods Combining Genomic and Epidemiological Data in the Reconstruction of Transmission Trees: A Systematic Review
Abstract
:1. Introduction
- Mutation: includes nucleotide “indel” (either a deletion or an insertion, i.e., a nucleotide disappears from or is added to the sequence) and substitution (a nucleotide in the sequence changes into another).
- Within-host evolution: represents how the pathogen genome changes within an individual or a group of individuals, which leads to genome diversification.
- Transmission: passage of a pathogen from an infected host to a susceptible host and the subsequent infection in the newly infected host. In transmission models, assumptions are thus made regarding how the disease was introduced in the host population then spread from host to host, as well as regarding the natural history of the disease.
- Case observation: process of identifying and sampling infected hosts in the host population.
2. Results
2.1. Non-Phylogenetic Family
2.1.1. Methods That Consider Mutations to Occur at Transmission
| Family | Method (Name) [Reference] | Start of Exposure | Onset of Infectiousness | Sampling Time | Removal Time | Contact Data | Geographical Data | Intrinsic Characteristics | Phylogenetic Tree or Sequences | |
|---|---|---|---|---|---|---|---|---|---|---|
| Non-phylogenetic | Aldrin et al., 2011 [28] | X | X | X | X | S | ||||
| Jombart et al., 2011 (Seqtrack) [16] | X | S | ||||||||
| Ypma et al., 2012 [32] | X | X | X | S | ||||||
| Jombart et al., 2014 (outbreaker) [24] | X | S | ||||||||
| Worby et al., 2014 [37] | X | S | ||||||||
| Famulare et al. 2015 [38] | X | S | ||||||||
| Worby et al., 2016 (bitrugs) [6] | X | X | X | S | ||||||
| Campbell et al., 2019 (outbreaker2) [30] | X | X | S | |||||||
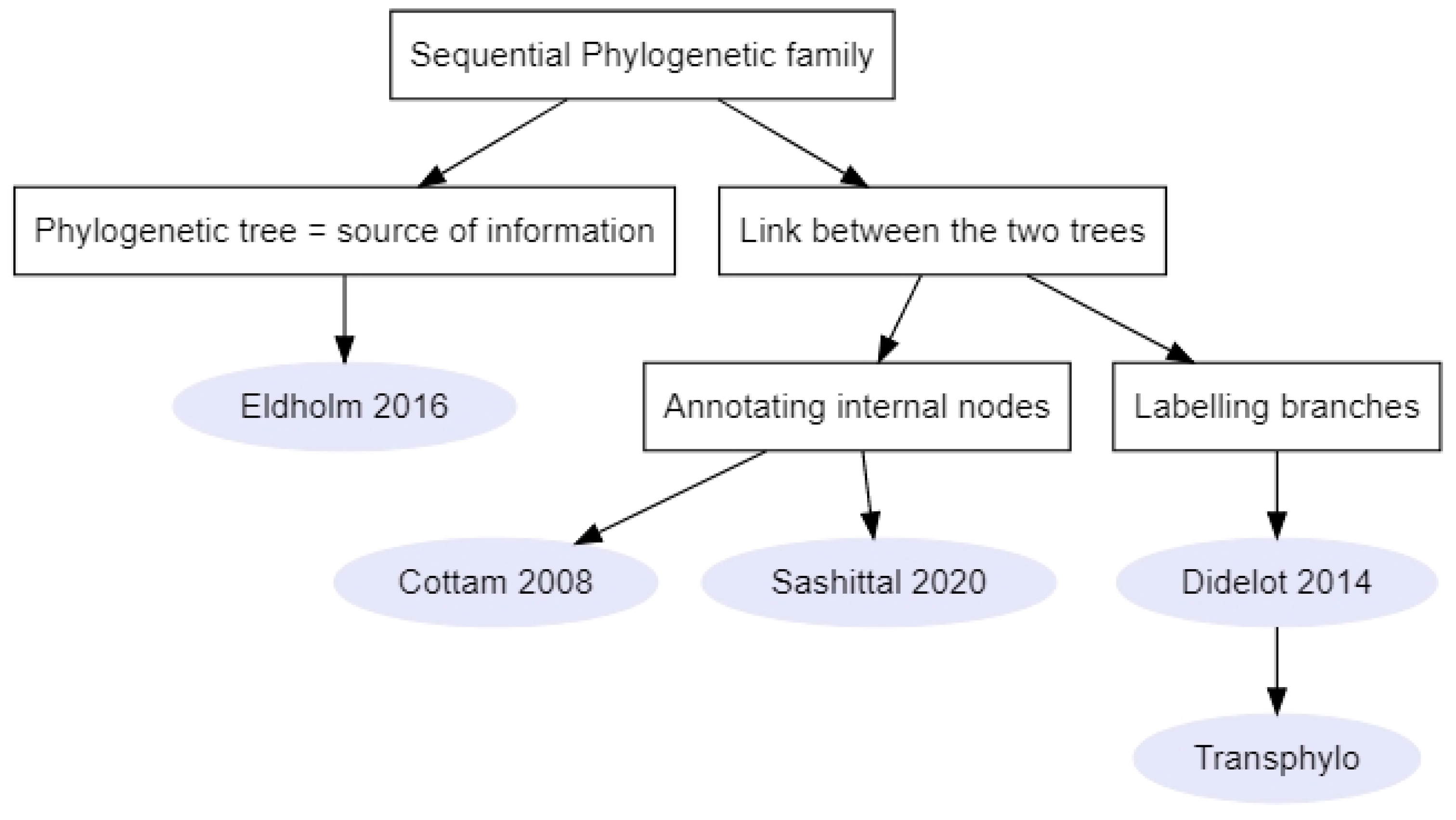
| Sequential phylogenetic | Cottam et al., 2008 [2] | X | X | X | P | |||||
| Didelot et al., 2014 [17] | X | (X) | P | |||||||
| Eldholm et al., 2016 [39] | X | P | ||||||||
| Didelot et al., 2017 (Transphylo) [40] | X | P | ||||||||
| Sashittal et al., 2020 (TiTUS) [31] | X | X | X | X | P | |||||
| Simultaneous phylogenetic | Explicitly phylogenetic | Ypma et al., 2013 [5] | X | X | X | X | S | |||
| Hall et al., 2015 (beastlier) [18] | X | X | (X) | X | S | |||||
| De Maio et al., 2016 (SCOTTI) [41] | X | X | X | S | ||||||
| Klinkenberg et al., 2017 (phybreak) [26] | X | S | ||||||||
| Implicitly phylogenetic | Morelli et al., 2012 [23] | X | X | X | X | S | ||||
| Mollentze et al., 2014 [1] | X | X | S | |||||||
| Lau et al., 2015 [42] | X | X | X | X | S | |||||
| Firestone et al., 2020 (BORIS) [29] | X | X | X | X | X | S | ||||
| Montazeri et al., 2020 [43] | X | S | ||||||||
| Method (Name) [Reference] | Sequence Mutation | Within-Host Evolution | Transmission | Case Observation | Inference Method |
|---|---|---|---|---|---|
| Aldrin et al., 2011 [28] | Kimura model | No explicit model | SIR (infectious period) | All cases are observed but not always sampled | Partial Maximum Likelihood |
| Complete | Distance kernel | ||||
| Multiple | |||||
| Jombart et al., 2011 (Seqtrack) [16] | User’s choice | No explicit model | No explicit model | All cases are observed and sampled | Edmonds algorithm |
| Complete | |||||
| Ypma et al., 2012 [32] | Deletion + Transition + Transversion | No explicit model | SEIR (latency/infectious period) | All cases are observed but not always sampled | Bayesian |
| Complete | Spatial kernel | ||||
| Single | |||||
| Jombart et al., 2014 (outbreaker) [24] | Mutation rate | No explicit model | SI (generation times) | Proportion of sampled cases | Bayesian |
| Complete | Random mixing | ||||
| Multiple | |||||
| Worby et al., 2014 [37] | Mutation rate | Pathogen population size | No explicit model | All cases are observed and sampled | Observed genetic distance vs. theoretical distribution |
| Weak | |||||
| Famulare et al., 2015 [38] | Mutation rate | No explicit model | No explicit model | No assumption | Likelihood ratio test + Pruning algorithm |
| Worby et al., 2016 (bitrugs) [6] | No explicit model | No explicit model | SEIR (latency/infectious period) | Test sensitivity < 1 | Bayesian |
| No assumption | Random mixing | ||||
| Multiple | |||||
| Campbell et al., 2019 (outbreaker2) [30] | Mutation rate | No explicit model | SI (generation times) | Proportion of sampled cases | Bayesian |
| Complete | Contact data | ||||
| Multiple |
| Method (Name) [Reference] | Sequence Mutation | Within-Host Evolution | Transmission | Case Observation | Inference Method |
|---|---|---|---|---|---|
| Cottam et al., 2008 [2] | NA | No explicit model | SEIR (latency/infectious period) | All cases are observed and sampled | Label internal nodes |
| Complete | Random mixing | Maximum Likelihood | |||
| Single | |||||
| Didelot et al., 2014 [17] | NA | Coalescent process | SIR (infectious period) | All cases are observed and sampled | Label branches |
| Complete | Random mixing | Bayesian | |||
| Single | |||||
| Eldholm et al., 2016 [39] | NA | Coalescent process | SEIR (latency/infectious period) | Probability threshold | Information source |
| Complete | Random mixing | Edmonds’ algorithm | |||
| Single | |||||
| Didelot et al., 2017 (Transphylo) [40] | NA | Coalescent process | SI (generation times) | Proportion of sampled cases | Label branches |
| Complete | Random mixing | Bayesian | |||
| Single | |||||
| Sashittal et al., 2020 (TiTUS) [31] | NA | No explicit model | No explicit model | All cases are observed and sampled | Label internal nodes |
| Weak * | Logical problem |
| Method (Name) [Reference] | Sequence Mutation | Within-Host Evolution | Transmission | Case Observation | Inference Method |
|---|---|---|---|---|---|
| Ypma et al., 2013 [5] | Mutation rate | Coalescent process | SEIR (latency/infectious period) | All cases are observed and sampled | Bayesian |
| Complete | Spatial kernel | ||||
| Single | |||||
| Hall et al., 2015 (beastlier) [18] | User’s choice | Coalescent process | SEIR (latency/infectious period) | All cases are observed but not always sampled | Bayesian |
| Complete * | Spatial kernel | ||||
| Single | |||||
| De Maio et al., 2016 (SCOTTI) [41] | User’s choice | Coalescent process | Migration model | Maximum number of hosts | Bayesian |
| Weak * | |||||
| Klinkenberg et al., 2017 (phybreak) [26] | Mutation rate | Coalescent process | SI (generation times) | All cases are observed but not always sampled | Bayesian |
| Complete | Random mixing | ||||
| Single | |||||
| Morelli et al., 2012 [23] | Jukes Cantor model | No explicit model | SEIR (latency/infectious period) | All cases are observed and sampled | Bayesian |
| Complete | Spatial kernel | ||||
| Single | |||||
| Mollentze et al., 2014 [1] | Kimura model | No explicit model | SEIR (latency/infectious period) | Observed cases contribute to transmission after removal time | Bayesian |
| Complete | Spatial kernel | ||||
| Multiple | |||||
| Lau et al., 2015 [42] | Kimura model | No explicit model | SEIR (latency/infectious period) | All cases are observed but not always sampled | Bayesian |
| Complete | Spatial kernel | ||||
| Multiple | |||||
| Firestone et al., 2020 (BORIS) [29] | Kimura model | No explicit model | SEIR (latency/infectious period) | All cases are observed but not always sampled | Bayesian |
| Complete | Spatial kernel | ||||
| Multiple | |||||
| Montazeri et al., 2020 [43] | Jukes Cantor model | No explicit model | No explicit model | All cases are observed and sampled | Bayesian |
| Complete |
2.1.2. Methods That Allow Within-Host Diversity
2.1.3. Other Methods
2.2. Phylogenetic Families
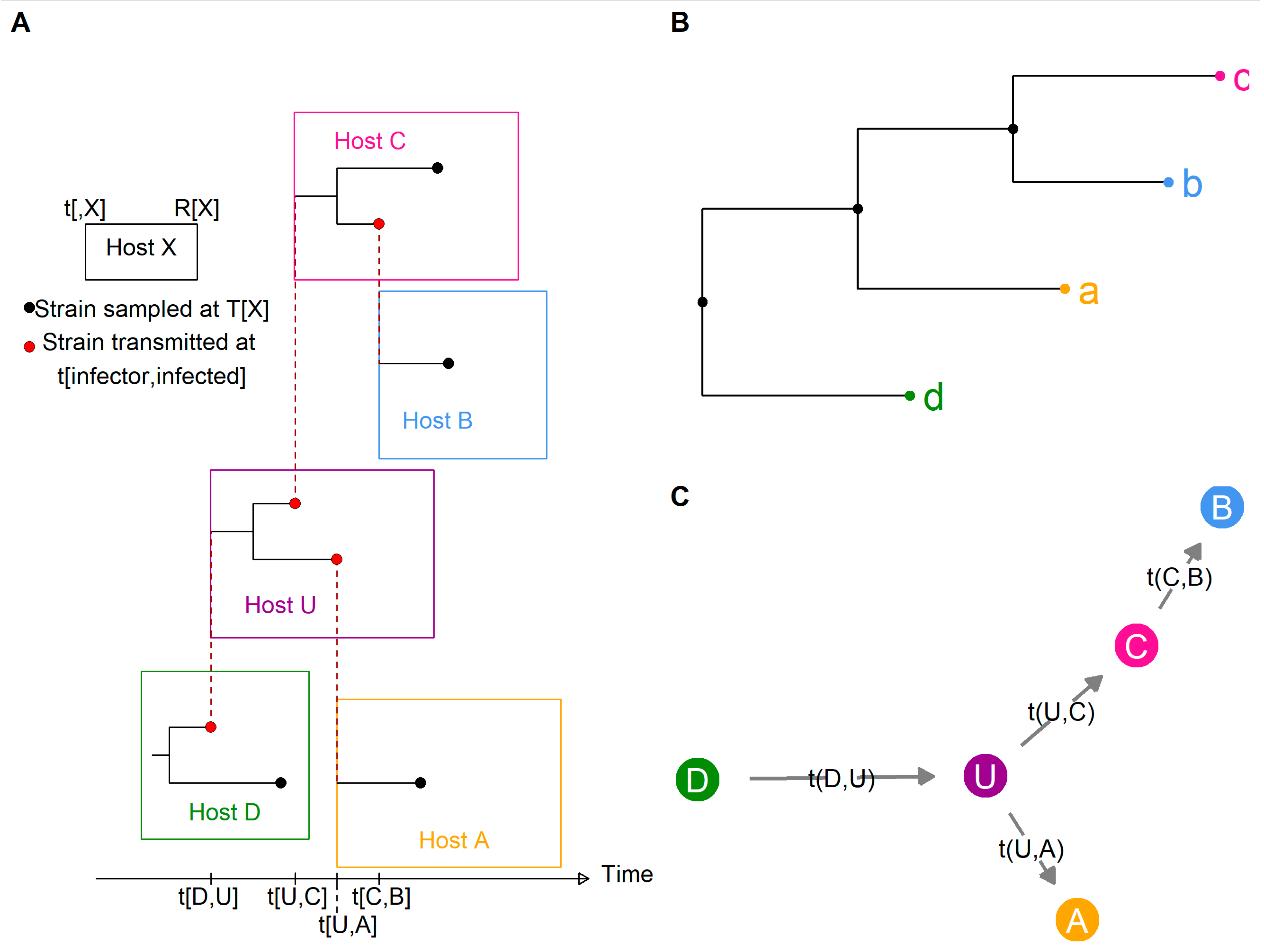
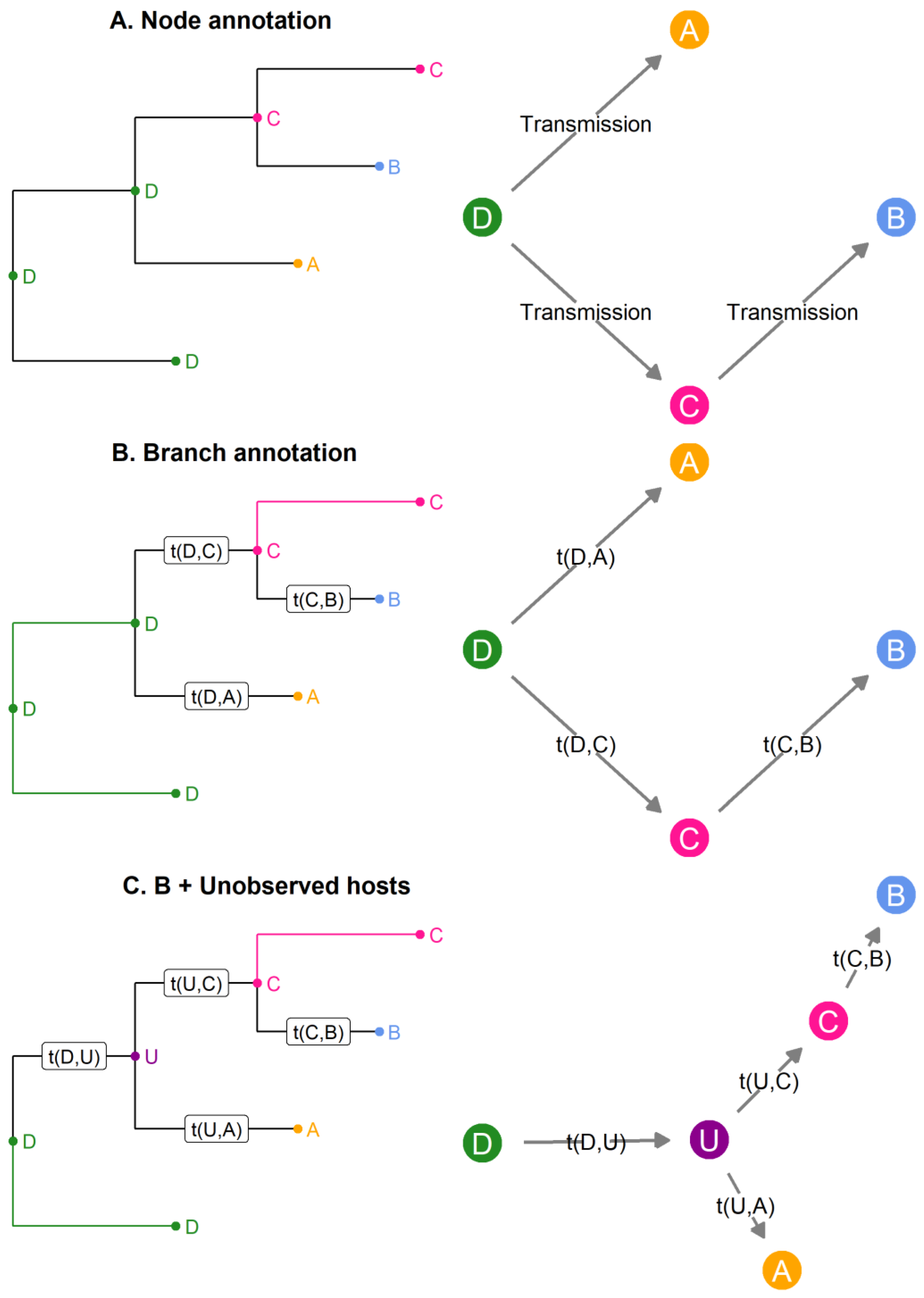
2.2.1. Sequential Phylogenetic Family
2.2.2. Simultaneous Phylogenetic Family
2.3. Application to M. tuberculosis, FMDV, and MRSA Outbreaks
3. Discussion
4. Materials and Methods
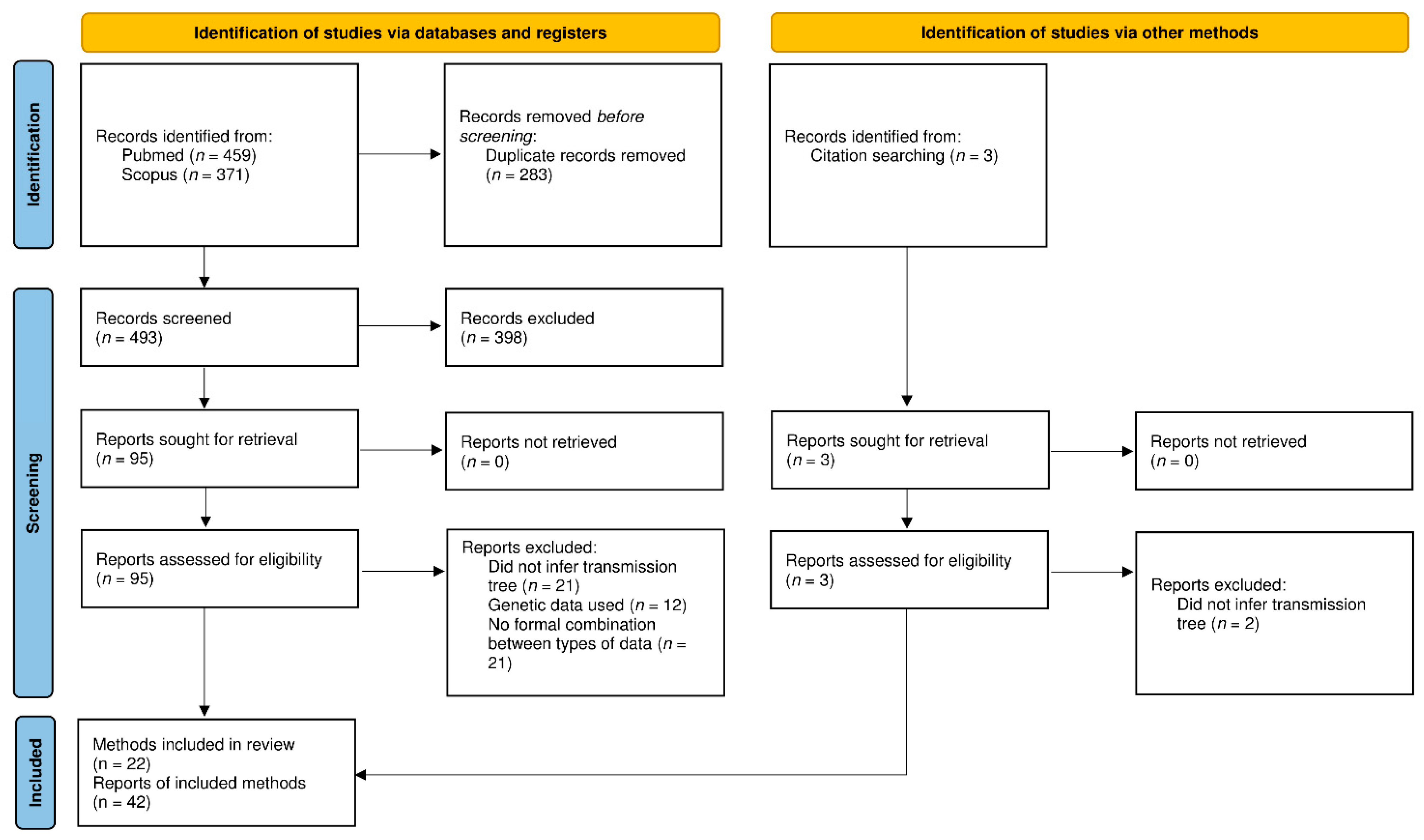
4.1. Search Strategy
4.2. Eligibility Criteria
4.3. Data Management
4.4. Data Collection Process
- Within-host evolution can be modeled by population models (e.g., the coalescent [65]) that are commonly used in phylogenetic tree reconstruction to describe the ancestry between sampled pathogens. When possible, we recorded the population model describing the within-host evolution.
- Three sub-categories were considered to describe the transmission model. Since an individual’s infectiousness varies over time depending on pathogen shedding [66], transmission models consider different stages of an infectious disease according to transmission potential. Parameters such as latency period and generation time can be fixed beforehand or estimated in the inference. The latency period corresponds to the time from infection by a pathogen to onset of infectiousness and is followed by an infectious period during which the individual can transmit the pathogen to others [67]. Generation times (Tg) represent the time interval between the infection of an index case and the time of transmission from that index case to secondary cases; Tg are related to the latency and infectious periods but also to the variation of an individual’s infectiousness over time [68]. Thus, we identified the different states considered for a host (for instance, S: susceptible, E: exposed, I: infectious, R: removed) and whether latency and infectious periods or generation times were considered to model the natural history of the disease. Moreover, since a transmission event is the result of direct or indirect contact between an infectious individual and a susceptible individual, this contact can be modeled by assuming a random mixing of individuals, considering transmission probability as a function of geographical distances (i.e., a spatial transmission kernel) or taking into account explicit contact data. In our second subcategory, we were interested in how contacts between hosts were modeled (random mixing, spatial kernel, or contact data). Finally, we recorded whether the method assumed that a single introduction of the disease was responsible for the outbreak or if multiple introductions into the host population were possible.
- For case observation, we were interested in how the methods accounted for imperfect case detection and whether all observed cases were sampled or if the method had a way to handle missing genomic data.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mollentze, N.; Nel, L.; Townsend, S.; le Roux, K.; Hampson, K.; Haydon, D.T.; Soubeyrand, S. A Bayesian approach for inferring the dynamics of partially observed endemic infectious diseases from space-time-genetic data. Proc. R. Soc. B Boil. Sci. 2014, 281, 20133251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cottam, E.M.; Thébaud, G.; Wadsworth, J.; Gloster, J.; Mansley, L.; Paton, D.J.; King, D.P.; Haydon, D.T. Integrating genetic and epidemiological data to determine transmission pathways of foot-and-mouth disease virus. Proc. R. Soc. B Boil. Sci. 2008, 275, 887–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ypma, R.J.; Jonges, M.; Bataille, A.; Stegeman, A.; Koch, G.; van Boven, M.; Koopmans, M.; van Ballegooijen, W.M.; Wallinga, J. Genetic data provide evidence for wind-mediated transmission of highly pathogenic avian influenza. J. Infect. Dis. 2012, 207, 730–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faye, O.; Boëlle, P.-Y.; Heleze, E.; Faye, O.; Loucoubar, C.; Magassouba, N.; Soropogui, B.; Keita, S.; Gakou, T.; Bah, E.H.I.; et al. Chains of transmission and control of Ebola virus disease in Conakry, Guinea, in 2014: An observational study. Lancet Infect. Dis. 2015, 15, 320–326. [Google Scholar] [CrossRef] [Green Version]
- Ypma, R.J.F.; van Ballegooijen, W.M.; Wallinga, J. Relating phylogenetic trees to transmission trees of infectious disease outbreaks. Genetics 2013, 195, 1055–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Worby, C.; O’Neill, P.D.; Kypraios, T.; Robotham, J.; de Angelis, D.; Cartwright, E.J.P.; Peacock, S.J.; Cooper, B. Reconstructing transmission trees for communicable diseases using densely sampled genetic data. Ann. Appl. Stat. 2016, 10, 395–417. [Google Scholar] [CrossRef] [Green Version]
- Varia, M.; Wilson, S.; Sarwal, S.; McGeer, A.; Gournis, E.; Galanis, E.; Henry, B.; Team, H.O.I. Investigation of a nosocomial outbreak of severe acute respiratory syndrome (SARS) in Toronto, Canada. Can. Med. Assoc. J. 2003, 169, 285–292. [Google Scholar]
- Garry, M.; Hope, L.; Zajac, R.; Verrall, A.J.; Robertson, J.M. Contact tracing: A memory task with consequences for public health. Perspect. Psychol. Sci. 2021, 16, 175–187. [Google Scholar] [CrossRef]
- Crozet, G.; Dufour, B.; Rivière, J. Investigation of field intradermal tuberculosis test practices performed by veterinarians in France and factors that influence testing. Res. Veter. Sci. 2019, 124, 406–416. [Google Scholar] [CrossRef]
- Podsiadło, Ł.; Polz-Dacewicz, M. Molecular evolution and phylogenetic implications in clinical research. Ann. Agric. Environ. Med. 2013, 20, 455–459. [Google Scholar]
- Vaz, C.; Nascimento, M.; Carriço, J.A.; Rocher, T.; Francisco, A.P. Distance-based phylogenetic inference from typing data: A unifying view. Brief. Bioinform. 2021, 22, 147. [Google Scholar] [CrossRef] [PubMed]
- Francisco, A.P.; Vaz, C.; Monteiro, P.T.; Melo-Cristino, J.; Ramirez, M.; Carriço, J.A. PHYLOViZ: Phylogenetic inference and data visualization for sequence based typing methods. BMC Bioinform. 2012, 13, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suchard, M.A.; Lemey, P.; Baele, G.; Ayres, D.L.; Drummond, A.J.; Rambaut, A. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol. 2018, 4, vey016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouckaert, R.; Vaughan, T.G.; Barido-Sottani, J.; Duchêne, S.; Fourment, M.; Gavryushkina, A.; Heled, J.; Jones, G.; Kühnert, D.; de Maio, N.; et al. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2019, 15, e1006650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volz, E.M.; Pond, S.; Ward, M.J.; Brown, A.L.; Frost, S. Phylodynamics of infectious disease epidemics. Genetics 2009, 183, 1421–1430. [Google Scholar] [CrossRef] [Green Version]
- Jombart, T.; Eggo, R.M.; Dodd, P.; Balloux, F. Reconstructing disease outbreaks from genetic data: A graph approach. Heredity 2010, 106, 383–390. [Google Scholar] [CrossRef] [Green Version]
- Didelot, X.; Gardy, J.; Colijn, C. Bayesian inference of infectious disease transmission from whole-genome sequence data. Mol. Biol. Evol. 2014, 31, 1869–1879. [Google Scholar] [CrossRef] [Green Version]
- Hall, M.; Woolhouse, M.; Rambaut, A. Epidemic reconstruction in a phylogenetics framework: Transmission trees as partitions of the node set. PLoS Comput. Biol. 2015, 11, e1004613. [Google Scholar] [CrossRef] [Green Version]
- Hassan, A.S.; Pybus, O.; Sanders, E.J.; Albert, J.; Esbjörnsson, J. Defining HIV-1 transmission clusters based on sequence data. AIDS 2017, 31, 1211–1222. [Google Scholar] [CrossRef]
- Campbell, F.; Strang, C.; Ferguson, N.; Cori, A.; Jombart, T. When are pathogen genome sequences informative of transmission events? PLoS Pathog. 2018, 14, e1006885. [Google Scholar] [CrossRef]
- Walker, T.M.; Ip, C.L.; Harrell, R.H.; Evans, J.T.; Kapatai, G.; Dedicoat, M.J.; Eyre, D.; Wilson, D.; Hawkey, P.M.; Crook, D.W.; et al. Whole-genome sequencing to delineate Mycobacterium tuberculosis outbreaks: A retrospective observational study. Lancet Infect. Dis. 2013, 13, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Worby, C.J.; Lipsitch, M.; Hanage, W.P. Within-host bacterial diversity hinders accurate reconstruction of transmission networks from genomic distance data. PLoS Comput. Biol. 2014, 10, e1003549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morelli, M.J.; Thébaud, G.; Chadœuf, J.; King, D.P.; Haydon, D.T.; Soubeyrand, S. A Bayesian Inference framework to reconstruct transmission trees using epidemiological and genetic data. PLoS Comput. Biol. 2012, 8, e1002768. [Google Scholar] [CrossRef] [Green Version]
- Jombart, T.; Cori, A.; Didelot, X.; Cauchemez, S.; Fraser, C.; Ferguson, N. Bayesian reconstruction of disease outbreaks by combining epidemiologic and genomic data. PLoS Comput. Biol. 2014, 10, e1003457. [Google Scholar] [CrossRef] [PubMed]
- Cayley, A. A theorem on trees. Collect. Math. Pap. 2011, 23, 26–28. [Google Scholar] [CrossRef]
- Klinkenberg, D.; Backer, J.A.; Didelot, X.; Colijn, C.; Wallinga, J. Simultaneous inference of phylogenetic and transmission trees in infectious disease outbreaks. PLoS Comput. Biol. 2017, 13, e1005495. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Aldrin, M.; Lyngstad, T.M.; Kristoffersen, A.B.; Storvik, B.; Borgan, Ø.; Jansen, P.A. Modelling the spread of infectious salmon anaemia among salmon farms based on seaway distances between farms and genetic relationships between infectious salmon anaemia virus isolates. J. R. Soc. Interface 2011, 8, 1346–1356. [Google Scholar] [CrossRef]
- Firestone, S.M.; Hayama, Y.; Lau, M.S.Y.; Yamamoto, T.; Nishi, T.; Bradhurst, R.A.; Demirhan, H.; Stevenson, M.A.; Tsutsui, T. Transmission network reconstruction for foot-and-mouth disease outbreaks incorporating farm-level covariates. PLoS ONE 2020, 15, e0235660. [Google Scholar] [CrossRef]
- Campbell, F.; Cori, A.; Ferguson, N.; Jombart, T. Bayesian inference of transmission chains using timing of symptoms, pathogen genomes and contact data. PLoS Comput. Biol. 2019, 15, e1006930. [Google Scholar] [CrossRef]
- Sashittal, P.; El-Kebir, M. Sampling and summarizing transmission trees with multi-strain infections. Bioinformatics 2020, 36 (Suppl. S1), i362–i370. [Google Scholar] [CrossRef] [PubMed]
- Ypma, R.J.F.; Bataille, A.; Stegeman, A.; Koch, G.; Wallinga, J.; van Ballegooijen, W.M. Unravelling transmission trees of infectious diseases by combining genetic and epidemiological data. Proc. R. Soc. B Boil. Sci. 2011, 279, 444–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerutti, F.; Luzzago, C.; Lauzi, S.; Ebranati, E.; Caruso, C.; Masoero, L.; Moreno, A.; Acutis, P.L.; Zehender, G.; Peletto, S. Phylogeography, phylodynamics and transmission chains of bovine viral diarrhea virus subtype 1f in Northern Italy. Infect. Genet. Evol. 2016, 45, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Stoesser, N.; Giess, A.; Batty, L.; Sheppard, A.; Walker, A.S.; Wilson, D.; Didelot, X.; Bashir, A.; Sebra, R.; Kasarskis, A.; et al. Genome sequencing of an extended series of NDM-producing Klebsiella pneumoniae isolates from neonatal infections in a Nepali hospital characterizes the extent of community- versus hospital-associated transmission in an endemic setting. Antimicrob. Agents Chemother. 2014, 58, 7347–7357. [Google Scholar] [CrossRef] [Green Version]
- Kanamori, H.; Parobek, C.; Weber, D.J.; van Duin, D.; Rutala, W.A.; Cairns, B.A.; Juliano, J.J. Next-generation sequencing and comparative analysis of sequential outbreaks caused by multidrug-resistant Acinetobacter baumannii at a large academic burn center. Antimicrob. Agents Chemother. 2016, 60, 1249–1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makke, G.; Bitar, I.; Salloum, T.; Panossian, B.; Alousi, S.; Arabaghian, H.; Medvecky, M.; Hrabak, J.; Merheb-Ghoussoub, S.; Tokajian, S. Whole-genome-sequence-based characterization of extensively drug-resistant Acinetobacter baumannii hospital outbreak. mSphere 2020, 5, e00934-19. [Google Scholar] [CrossRef] [Green Version]
- Worby, C.J.; Chang, H.-H.; Hanage, W.P.; Lipsitch, M. The distribution of pairwise genetic distances: A tool for investigating disease transmission. Genetics 2014, 198, 1395–1404. [Google Scholar] [CrossRef] [Green Version]
- Famulare, M.; Hu, H. Extracting transmission networks from phylogeographic data for epidemic and endemic diseases: Ebola virus in Sierra Leone, 2009 H1N1 pandemic influenza and polio in Nigeria. Int. Health 2015, 7, 130–138. [Google Scholar] [CrossRef]
- Eldholm, V.; Rieux, A.; Monteserin, J.; Lopez, J.M.; Palmero, D.; Lopez, B.; Ritacco, V.; Didelot, X.; Balloux, F. Impact of HIV co-infection on the evolution and transmission of multidrug-resistant tuberculosis. eLife 2016, 5, e16644. [Google Scholar] [CrossRef] [Green Version]
- Didelot, X.; Fraser, C.; Gardy, J.; Colijn, C. Genomic infectious disease epidemiology in partially sampled and ongoing outbreaks. Mol. Biol. Evol. 2017, 34, 997–1007. [Google Scholar] [CrossRef] [Green Version]
- De Maio, N.; Wu, C.-H.; Wilson, D. SCOTTI: Efficient reconstruction of transmission within outbreaks with the structured coalescent. PLoS Comput. Biol. 2016, 12, e1005130. [Google Scholar] [CrossRef] [PubMed]
- Lau, M.S.Y.; Marion, G.; Streftaris, G.; Gibson, G. A systematic Bayesian integration of epidemiological and genetic data. PLoS Comput. Biol. 2015, 11, e1004633. [Google Scholar] [CrossRef] [PubMed]
- Montazeri, H.; Little, S.; Mozaffarilegha, M.; Beerenwinkel, N.; DeGruttola, V. Bayesian reconstruction of transmission trees from genetic sequences and uncertain infection times. Stat. Appl. Genet. Mol. Biol. 2020, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, J. Optimum branchings. J. Res. Natl. Bur. Stand. Sect. B Math. Math. Phys. 1967, 71B, 233. [Google Scholar] [CrossRef]
- Hughes, J.; Allen, R.C.; Baguelin, M.; Hampson, K.; Baillie, G.J.; Elton, D.; Newton, J.R.; Kellam, P.; Wood, J.L.N.; Holmes, E.C.; et al. Transmission of equine influenza virus during an outbreak is characterized by frequent mixed infections and loose transmission bottlenecks. PLoS Pathog. 2012, 8, e1003081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerra-Assunção, J.A.; Crampin, A.; Houben, R.M.G.J.; Mzembe, T.; Mallard, K.; Coll, F.; Khan, P.; Banda, L.; Chiwaya, A.; Pereira, R.P.A.; et al. Large-scale whole genome sequencing of M. tuberculosis provides insights into transmission in a high prevalence area. eLife 2015, 4, e05166. [Google Scholar] [CrossRef]
- Spencer, M.D.; Winglee, K.; Passaretti, C.; Earl, A.M.; Manson, A.L.; Mulder, H.P.; Sautter, R.L.; Fodor, A.A. Whole genome sequencing detects inter-facility transmission of carbapenem-resistant Klebsiella pneumoniae. J. Infect. 2019, 78, 187–199. [Google Scholar] [CrossRef] [Green Version]
- Séraphin, M.N.; Didelot, X.; Nolan, D.J.; May, J.R.; Khan, S.R.; Murray, E.R.; Salemi, M.; Morris, J.G., Jr.; Lauzardo, M. Genomic investigation of a Mycobacterium tuberculosis outbreak involving prison and community cases in Florida, United States. Am. J. Trop. Med. Hyg. 2018, 99, 867–874. [Google Scholar] [CrossRef]
- Xu, Y.; Cancino-Muñoz, I.; Torres-Puente, M.; Villamayor, L.M.; Borrás, R.; Borrás-Máñez, M.; Bosque, M.; Camarena, J.J.; Colomer-Roig, E.; Colomina, J.; et al. High-resolution mapping of tuberculosis transmission: Whole genome sequencing and phylogenetic modelling of a cohort from Valencia Region, Spain. PLoS Med. 2019, 16, e1002961. [Google Scholar] [CrossRef] [Green Version]
- Sobkowiak, B.; Banda, L.; Mzembe, T.; Crampin, A.C.; Glynn, J.R.; Clark, T. Bayesian reconstruction of Mycobacterium tuberculosis transmission networks in a high incidence area over two decades in Malawi reveals associated risk factors and genomic variants. Microb. Genom. 2020, 6, mgen000361. [Google Scholar] [CrossRef]
- Kwong, J.; Lane, C.R.; Romanes, F.; da Silva, A.G.; Easton, M.; Cronin, K.; Waters, M.J.; Tomita, T.; Stevens, K.; Schultz, M.; et al. Translating genomics into practice for real-time surveillance and response to carbapenemase-producing Enterobacteriaceae: Evidence from a complex multi-institutional KPC outbreak. PeerJ 2018, 6, e4210. [Google Scholar] [CrossRef]
- Van Dorp, L.; Wang, Q.; Shaw, L.P.; Acman, M.; Brynildsrud, O.; Eldholm, V.; Wang, R.; Gao, H.; Yin, Y.; Chen, H.; et al. Rapid phenotypic evolution in multidrug-resistant Klebsiella pneumoniae hospital outbreak strains. Microb. Genom. 2019, 5, e000263. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Didelot, X.; Yang, J.; Wong, G.; Shi, Y.; Liu, W.; Gao, G.F.; Bi, Y. Inference of person-to-person transmission of COVID-19 reveals hidden super-spreading events during the early outbreak phase. Nat. Commun. 2020, 11, 5006. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, P.J.; Eshaghi, A.; Seo, C.Y.; Wilson, S.; Harris, T.; Deeks, S.L.; Bolotin, S.; Goneau, L.W.; Gubbay, J.B.; Patel, S.N. Evaluating the use of whole genome sequencing for the investigation of a large mumps outbreak in Ontario, Canada. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Stockdale, J.E.; Naidu, V.; Hatherell, H.; Stimson, J.; Stagg, H.R.; Abubakar, I.; Colijn, C. Transmission analysis of a large tuberculosis outbreak in London: A mathematical modelling study using genomic data. Microb. Genom. 2020, 6, e000450. [Google Scholar] [CrossRef]
- Hayama, Y.; Firestone, S.M.; Stevenson, M.A.; Yamamoto, T.; Nishi, T.; Shimizu, Y.; Tsutsui, T. Reconstructing a transmission network and identifying risk factors of secondary transmissions in the 2010 foot-and-mouth disease outbreak in Japan. Transbound. Emerg. Dis. 2019, 66, 2074–2086. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.C. Inferring transmission routes of avian influenza during the H5N8 outbreak of South Korea in 2014 using epidemiological and genetic data. Korean J. Microbiol. 2018, 54, 254–265. [Google Scholar] [CrossRef]
- De Maio, N.; Wu, C.-H.; O’Reilly, K.; Wilson, D.J. New routes to phylogeography: A Bayesian structured coalescent approximation. PLoS Genet. 2015, 11, e1005421. [Google Scholar] [CrossRef] [Green Version]
- Alexandersen, S.; Zhang, Z.; Donaldson, A.; Garland, A. The pathogenesis and diagnosis of foot-and-mouth disease. J. Comp. Pathol. 2003, 129, 1–36. [Google Scholar] [CrossRef]
- Azarian, T.; Maraqa, N.F.; Cook, R.L.; Johnson, J.A.; Bailey, C.; Wheeler, S.; Nolan, D.; Rathore, M.H.; Morris, J.G., Jr.; Salemi, M. Genomic epidemiology of methicillin-resistant Staphylococcus aureus in a neonatal intensive care unit. PLoS ONE 2016, 11, e0164397. [Google Scholar] [CrossRef]
- De Maio, N.; Worby, C.; Wilson, D.; Stoesser, N. Bayesian reconstruction of transmission within outbreaks using genomic variants. PLoS Comput. Biol. 2018, 14, e1006117. [Google Scholar] [CrossRef] [Green Version]
- Alamil, M.; Hughes, J.; Berthier, K.; Desbiez, C.; Thébaud, G.; Soubeyrand, S. Inferring epidemiological links from deep sequencing data: A statistical learning approach for human, animal and plant diseases. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180258. [Google Scholar] [CrossRef] [Green Version]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Jukes, T.H.; Cantor, C.R. Evolution of protein molecules. In Mammalian Protein Metabolism; Munro, H.N., Ed.; Academic Press: New York, NY, USA, 1969; Volume 3, pp. 21–132. [Google Scholar]
- Kingman, J. The coalescent. Stoch. Process. Appl. 1982, 13, 235–248. [Google Scholar] [CrossRef] [Green Version]
- Woolhouse, M. Quantifying transmission. Microbiol. Spectr. 2017, 5, 279–289. [Google Scholar] [CrossRef]
- Van Seventer, J.M.; Hochberg, N.S. Principles of infectious diseases: Transmission, diagnosis, prevention, and control. Int. Encycl. Public Health 2017, 6, 22–39. [Google Scholar] [CrossRef]
- Svensson, Å. A note on generation times in epidemic models. Math. Biosci. 2007, 208, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Scaduto, D.I.; Brown, J.; Haaland, W.C.; Zwickl, D.J.; Hillis, D.M.; Metzker, M.L. Source identification in two criminal cases using phylogenetic analysis of HIV-1 DNA sequences. Proc. Natl. Acad. Sci. USA 2010, 107, 21242–21247. [Google Scholar] [CrossRef] [Green Version]
- Schürch, A.; Kremer, K.; Daviena, O.; Kiers, A.; Boeree, M.J.; Siezen, R.J.; van Soolingen, D. High-resolution typing by integration of genome sequencing data in a large tuberculosis cluster. J. Clin. Microbiol. 2010, 48, 3403–3406. [Google Scholar] [CrossRef] [Green Version]
- Shiino, T. Phylodynamic analysis of a viral infection network. Front. Microbiol. 2012, 3, 278. [Google Scholar] [CrossRef] [Green Version]
- Zarrabi, N.; Prosperi, M.; Belleman, R.G.; Colafigli, M.; de Luca, A.; Sloot, P. Combining epidemiological and genetic networks signifies the importance of early treatment in HIV-1 transmission. PLoS ONE 2012, 7, e46156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, S.J.; Zhang, X.; Romero-Severson, E.O.; Henry, C.; Zhong, L.; Volz, E.; Brenner, B.G.; Koopman, J.S. Detectable signals of episodic risk effects on acute HIV transmission: Strategies for analyzing transmission systems using genetic data. Epidemics 2013, 5, 44–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stack, J.C.; Murcia, P.R.; Grenfell, B.T.; Wood, J.L.N.; Holmes, E.C. Inferring the inter-host transmission of influenza A virus using patterns of intra-host genetic variation. Proc. R. Soc. B Boil. Sci. 2013, 280, 20122173. [Google Scholar] [CrossRef] [PubMed]
- Gavryushkina, A.; Welch, D.; Stadler, T.; Drummond, A.J. Bayesian inference of sampled ancestor trees for epidemiology and fossil calibration. PLoS Comput. Biol. 2014, 10, e1003919. [Google Scholar] [CrossRef] [Green Version]
- Mehaffy, C.; Guthrie, J.; Alexander, D.C.; Stuart, R.; Rea, E.; Jamieson, F.B. Marked microevolution of a unique mycobacterium tuberculosis strain in 17 years of ongoing transmission in a high risk population. PLoS ONE 2014, 9, e112928. [Google Scholar] [CrossRef] [Green Version]
- Numminen, E.; Chewapreecha, C.; Sirén, J.; Turner, C.; Turner, P.; Bentley, S.D.; Corander, J. Two-phase importance sampling for inference about transmission trees. Proc. R. Soc. B Boil. Sci. 2014, 281, 20141324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croucher, N.; Didelot, X. The application of genomics to tracing bacterial pathogen transmission. Curr. Opin. Microbiol. 2015, 23, 62–67. [Google Scholar] [CrossRef]
- Janies, D.A.; Pomeroy, L.W.; Krueger, C.; Zhang, Y.; Senturk, I.; Kaya, K.; Çatalyürek, Ü.V. Phylogenetic visualization of the spread of H7 influenza A viruses. Cladistics 2015, 31, 679–691. [Google Scholar] [CrossRef]
- Valdazo-González, B.; Kim, J.T.; Soubeyrand, S.; Wadsworth, J.; Knowles, N.J.; Haydon, D.T.; King, D.P. The impact of within-herd genetic variation upon inferred transmission trees for foot-and-mouth disease virus. Infect. Genet. Evol. 2015, 32, 440–448. [Google Scholar] [CrossRef]
- Folarin, O.A.; Ehichioya, D.; Schaffner, S.F.; Winnicki, S.M.; Wohl, S.; Eromon, P.; West, K.L.; Gladden-Young, A.; Oyejide, N.E.; Matranga, C.; et al. Ebola virus epidemiology and evolution in Nigeria. J. Infect. Dis. 2016, 214, S102–S109. [Google Scholar] [CrossRef] [Green Version]
- Hall, M.; Woolhouse, M.; Rambaut, A. Using genomics data to reconstruct transmission trees during disease outbreaks. Rev. Sci. Tech. Int. Off. Epizoot. 2016, 35, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Luo, Y.; Monday, S.R.; Gonzalez-Escalona, N.; Ottesen, A.R.; Muruvanda, T.; Wang, C.; Kastanis, G.; Keys, C.; Janies, D.; et al. Tracing origins of the Salmonella bareilly strain causing a food-borne outbreak in the United States. J. Infect. Dis. 2015, 213, 502–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenah, E.; Britton, T.; Halloran, M.E.; Longini, I.M. Molecular infectious disease epidemiology: Survival analysis and algorithms linking phylogenies to transmission trees. PLoS Comput. Biol. 2016, 12, e1004869. [Google Scholar] [CrossRef] [Green Version]
- Parratt, S.R.; Numminen, E.; Laine, A.-L. Infectious disease dynamics in heterogeneous landscapes. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 283–306. [Google Scholar] [CrossRef]
- Ray, B.; Ghedin, E.; Chunara, R. Network inference from multimodal data: A review of approaches from infectious disease transmission. J. Biomed. Inform. 2016, 64, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Jin, Y.; Hu, M.; Zhou, J.; Song, T.; Huang, Z.; Li, B.; Li, K.; Zhou, W.; Dai, H.; et al. Ecological dynamics of influenza A viruses: Cross-species transmission and global migration. Sci. Rep. 2016, 6, 36839. [Google Scholar] [CrossRef]
- Wohl, S.; Schaffner, S.F.; Sabeti, P.C. Genomic analysis of viral outbreaks. Annu. Rev. Virol. 2016, 3, 173–195. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Berhane, Y.; Dubé, C.; Liang, B.; Pasick, J.; van Domselaar, G.; Alexandersen, S. Epidemiological and evolutionary inference of the transmission network of the 2014 highly pathogenic avian influenza H5N2 outbreak in British Columbia, Canada. Sci. Rep. 2016, 6, 30858. [Google Scholar] [CrossRef] [Green Version]
- Agoti, C.N.; Munywoki, P.K.; Phan, M.V.T.; Otieno, J.R.; Kamau, E.; Bett, A.; Kombe, I.; Githinji, G.; Medley, G.F.; Cane, P.A.; et al. Transmission patterns and evolution of respiratory syncytial virus in a community outbreak identified by genomic analysis. Virus Evol. 2017, 3, vex006. [Google Scholar] [CrossRef] [Green Version]
- Baele, G.; Suchard, M.A.; Rambaut, A.; Lemey, P. Emerging concepts of data integration in pathogen phylodynamics. Syst. Biol. 2016, 66, e47–e65. [Google Scholar] [CrossRef] [Green Version]
- Glebova, O.; Knyazev, S.; Melnyk, A.; Artyomenko, A.; Khudyakov, Y.; Zelikovsky, A.; Skums, P. Inference of genetic relatedness between viral quasispecies from sequencing data. BMC Genom. 2017, 18, 918. [Google Scholar] [CrossRef] [Green Version]
- Snitkin, E.S.; Won, S.; Pirani, A.; Lapp, Z.; Weinstein, R.A.; Lolans, K.; Hayden, M.K. Integrated genomic and interfacility patient-transfer data reveal the transmission pathways of multidrug-resistant Klebsiella pneumoniae in a regional outbreak. Sci. Transl. Med. 2017, 9, eaan0093. [Google Scholar] [CrossRef] [Green Version]
- Worby, C.J.; Lipsitch, M.; Hanage, W.P. Shared genomic variants: Identification of transmission routes using pathogen deep-sequence data. Am. J. Epidemiol. 2017, 186, 1209–1216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, F.; Didelot, X.; Fitzjohn, R.; Ferguson, N.; Cori, A.; Jombart, T. Outbreaker2: A modular platform for outbreak reconstruction. BMC Bioinform. 2018, 19, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.C. Genomic epidemiology for microbial evolutionary studies and the use of Oxford Nanopore sequencing technology. Korean J. Microbiol. 2018, 54, 188–199. [Google Scholar] [CrossRef]
- Ezeoke, I.; Galac, M.R.; Lin, Y.; Liem, A.T.; Roth, P.A.; Kilianski, A.; Gibbons, H.S.; Bloch, D.; Kornblum, J.; del Rosso, P.; et al. Tracking a serial killer: Integrating phylogenetic relationships, epidemiology, and geography for two invasive meningococcal disease outbreaks. PLoS ONE 2018, 13, e0202615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gotoh, Y.; Taniguchi, T.; Yoshimura, D.; Katsura, K.; Saeki, Y.; Hirabara, Y.; Fukuda, M.; Takajo, I.; Tomida, J.; Kawamura, Y.; et al. Multi-step genomic dissection of a suspected intra-hospital Helicobacter cinaedi outbreak. Microb. Genom. 2019, 5, 1–11. [Google Scholar] [CrossRef]
- Kendall, M.; Ayabina, D.; Xu, Y.; Stimson, J.; Colijn, C. Estimating transmission from genetic and epidemiological data: A metric to compare transmission trees. Stat. Sci. 2018, 33, 70–85. [Google Scholar] [CrossRef] [Green Version]
- Leitner, T.; Romero-Severson, E. Phylogenetic patterns recover known HIV epidemiological relationships and reveal common transmission of multiple variants. Nat. Microbiol. 2018, 3, 983–988. [Google Scholar] [CrossRef]
- Meehan, C.J.; Moris, P.; Kohl, T.A.; Pečerska, J.; Akter, S.; Merker, M.; Utpatel, C.; Beckert, P.; Gehre, F.; Lempens, P.; et al. The relationship between transmission time and clustering methods in Mycobacterium tuberculosis epidemiology. EBioMedicine 2018, 37, 410–416. [Google Scholar] [CrossRef] [Green Version]
- Payne, D.C.; Biggs, H.M.; Al-Abdallat, M.M.; Alqasrawi, S.; Lu, X.; Abedi, G.R.; Haddadin, A.; Iblan, I.; Alsanouri, T.; Al Nsour, M.; et al. Multihospital outbreak of a middle east respiratory syndrome coronavirus deletion variant, Jordan: A molecular, serologic, and epidemiologic investigation. Open Forum Infect. Dis. 2018, 5, ofy095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blackburn, R.M.; Frampton, D.; Smith, C.M.; Fragaszy, E.B.; Watson, S.J.; Ferns, R.B.; Binter, S.; Coen, P.G.; Grant, P.; Shallcross, L.; et al. Nosocomial transmission of influenza: A retrospective cross-sectional study using next generation sequencing at a hospital in England (2012–2014). Influ. Other Respir. Viruses 2019, 13, 556–563. [Google Scholar] [CrossRef]
- DeSilva, M.B.; Styles, T.; Basler, C.; Moses, F.L.; Husain, F.; Reichler, M.; Whitmer, S.; McAuley, J.; Belay, E.; Friedman, M.; et al. Introduction of Ebola virus into a remote border district of Sierra Leone, 2014: Use of field epidemiology and RNA sequencing to describe chains of transmission. Epidemiol. Infect. 2019, 147, e88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Firestone, S.M.; Hayama, Y.; Bradhurst, R.; Yamamoto, T.; Tsutsui, T.; Stevenson, M.A. Reconstructing foot-and-mouth disease outbreaks: A methods comparison of transmission network models. Sci. Rep. 2019, 9, 2074–2086. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, J.L.; Strudwick, L.; Roberts, B.; Allen, M.; McFadzen, J.; Roth, D.; Jorgensen, D.; Rodrigues, M.; Tang, P.; Hanley, B.; et al. Whole genome sequencing for improved understanding of Mycobacterium tuberculosis transmission in a remote circumpolar region. Epidemiol. Infect. 2019, 147, e188. [Google Scholar] [CrossRef] [Green Version]
- Hall, M.D.; Colijn, C. Transmission trees on a known pathogen phylogeny: Enumeration and sampling. Mol. Biol. Evol. 2019, 36, 1333–1343. [Google Scholar] [CrossRef] [Green Version]
- Hall, M.D.; Holden, M.T.; Srisomang, P.; Mahavanakul, W.; Wuthiekanun, V.; Limmathurotsakul, D.; Fountain, K.; Parkhill, J.; Nickerson, E.K.; Peacock, S.J.; et al. Improved characterisation of MRSA transmission using within-host bacterial sequence diversity. eLife 2019, 8, e46402. [Google Scholar] [CrossRef]
- Ratmann, O.; PANGEA Consortium and Rakai Health Sciences Program; Grabowski, M.K.; Hall, M.; Golubchik, T.; Wymant, C.; Abeler-Dörner, L.; Bonsall, D.; Hoppe, A.; Brown, A.L.; et al. Inferring HIV-1 transmission networks and sources of epidemic spread in Africa with deep-sequence phylogenetic analysis. Nat. Commun. 2019, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Sashittal, P.; El-Kebir, M. SharpTNI: Counting and Sampling Parsimonious Transmission Networks under a Weak Bottleneck. Article Number (bioRxiv: 842237). Available online: https://www.biorxiv.org/content/10.1101/842237v1 (accessed on 4 February 2022).
- Van Beek, J.; Räisänen, K.; Broas, M.; Kauranen, J.; Kähkölä, A.; Laine, J.; Mustonen, E.; Nurkkala, T.; Puhto, T.; Sinkkonen, J.; et al. Tracing local and regional clusters of carbapenemase-producing Klebsiella pneumoniae ST512 with whole genome sequencing, Finland, 2013 to 2018. Eurosurveillance 2019, 24, 1800522. [Google Scholar] [CrossRef]
- Vaughan, T.G.; Leventhal, G.E.; Rasmussen, D.A.; Drummond, A.J.; Welch, D.; Stadler, T. Estimating epidemic incidence and prevalence from genomic data. Mol. Biol. Evol. 2019, 36, 1804–1816. [Google Scholar] [CrossRef] [Green Version]
- Bbosa, N.; Ssemwanga, D.; Ssekagiri, A.; Xi, X.; Mayanja, Y.; Bahemuka, U.; Seeley, J.; Pillay, D.; Abeler-Dörner, L.; Golubchik, T.; et al. Phylogenetic and demographic characterization of directed HIV-1 transmission using deep sequences from high-risk and general population cohorts/groups in Uganda. Viruses 2020, 12, 331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, A.D.B.; Ford, C.T.; Hostager, R.; Williams, J.; Cioce, M.; Çatalyürek, Ü.V.; Wertheim, J.O.; Janies, D. StrainHub: A phylogenetic tool to construct pathogen transmission networks. Bioinformatics 2020, 36, 945–947. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Zhang, C.; Mandoiu, I.I.; Bansal, M.S. TNet: Transmission network inference using within-host strain diversity and its application to geographical tracking of COVID-19 spread. IEEE ACM Trans. Comput. Biol. Bioinform. 2022, 19, 230–242. [Google Scholar] [CrossRef]
- Nelson, K.N.; Gandhi, N.R.; Mathema, B.; Lopman, B.A.; Brust, J.C.M.; Auld, S.C.; Ismail, N.; Omar, S.V.; Brown, T.S.; Allana, S.; et al. Modeling missing cases and transmission links in networks of extensively drug-resistant tuberculosis in KwaZulu-Natal, South Africa. Am. J. Epidemiol. 2020, 189, 735–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, K.N.; Jenness, S.M.; Mathema, B.; Lopman, B.A.; Auld, S.C.; Shah, N.S.; Brust, J.C.M.; Ismail, N.; Omar, S.V.; Brown, T.S.; et al. Social mixing and clinical features linked with transmission in a network of extensively drug-resistant tuberculosis cases in KwaZulu-Natal, South Africa. Clin. Infect. Dis. 2019, 70, 2396–2402. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, Q.; He, Y.; Liu, L.; Ma, X.; Wei, X.; Jiang, N.; Liang, L.; Zheng, Y.; Ma, L.; et al. Nosocomial outbreak of COVID-19 pneumonia in Wuhan, China. Eur. Respir. J. 2020, 55, 2000544. [Google Scholar] [CrossRef] [PubMed]
- Worobey, M.; Pekar, J.; Larsen, B.B.; Nelson, M.I.; Hill, V.; Joy, J.B.; Rambaut, A.; Suchard, M.A.; Wertheim, J.O.; Lemey, P. The emergence of SARS-CoV-2 in Europe and North America. Science 2020, 370, 564–570. [Google Scholar] [CrossRef]
- Bataille, A.; van der Meer, F.; Stegeman, A.; Koch, G. Evolutionary analysis of inter-farm transmission dynamics in a highly pathogenic avian influenza epidemic. PLoS Pathog. 2011, 7, e1002094. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.J.D.; Vijaykrishna, D.; Bahl, J.; Lycett, S.J.; Worobey, M.; Pybus, O.G.; Ma, S.K.; Cheung, C.L.; Raghwani, J.; Bhatt, S.; et al. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature 2009, 459, 1122–1125. [Google Scholar] [CrossRef] [Green Version]
- Briand, F.-X.; Niqueux, E.; Schmitz, A.; Martenot, C.; Cherbonnel, M.; Massin, P.; Kerbrat, F.; Chatel, M.; Guillemoto, C.; Guillou-Cloarec, C.; et al. Highly pathogenic avian influenza A(H5N8) virus spread by short- and long-range transmission, France, 2016–17. Emerg. Infect. Dis. 2021, 27, 508–516. [Google Scholar] [CrossRef]
- Murcia, P.R.; Wood, J.L.N.; Holmes, E. Genome-scale evolution and phylodynamics of equine H3N8 influenza A virus. J. Virol. 2011, 85, 5312–5322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biek, R.; Henderson, J.C.; Waller, L.A.; Rupprecht, C.E.; Real, L.A. A high-resolution genetic signature of demographic and spatial expansion in epizootic rabies virus. Proc. Natl. Acad. Sci. USA 2007, 104, 7993–7998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vega, V.B.; Ruan, Y.; Liu, J.; Lee, W.H.; Wei, C.L.; Se-Thoe, S.Y.; Tang, K.F.; Zhang, T.; Kolatkar, P.R.; Ooi, E.E.; et al. Mutational dynamics of the SARS coronavirus in cell culture and human populations isolated in 2003. BMC Infect. Dis. 2004, 4, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, Q.; Li, X.; Chen, W.; Liu, D.; Chen, Y.; Li, H.; Li, D.; Tian, M.; Tan, W.; Zai, J. Phylogenetic and phylodynamic analyses of SARS-CoV-2. Virus Res. 2020, 287, 198098. [Google Scholar] [CrossRef]
- Vrancken, B.; Rambaut, A.; Suchard, M.A.; Drummond, A.; Baele, G.; Derdelinckx, I.; van Wijngaerden, E.; Vandamme, A.-M.; van Laethem, K.; Lemey, P. The genealogical population dynamics of HIV-1 in a large transmission chain: Bridging within and among host evolutionary rates. PLoS Comput. Biol. 2014, 10, e1003505. [Google Scholar] [CrossRef] [Green Version]
- Gire, S.K.; Goba, A.; Andersen, K.G.; Sealfon, R.S.G.; Park, D.J.; Kanneh, L.; Jalloh, S.; Momoh, M.; Fullah, M.; Dudas, G.; et al. Genomic surveillance elucidates Ebola virus origin and transmission during the 2014 outbreak. Science 2014, 345, 1369–1372. [Google Scholar] [CrossRef] [Green Version]
- Burns, C.C.; Shaw, J.; Jorba, J.; Bukbuk, D.N.; Adu, F.; Gumede, N.; Pate, M.A.; Abanida, E.A.; Gasasira, A.; Iber, J.; et al. Multiple independent emergences of type 2 vaccine-derived polioviruses during a large outbreak in Northern Nigeria. J. Virol. 2013, 87, 4907–4922. [Google Scholar] [CrossRef] [Green Version]
- Devold, M.; Karlsen, M.; Nylund, A. Sequence analysis of the fusion protein gene from infectious salmon anemia virus isolates: Evidence of recombination and reassortment. J. Gen. Virol. 2006, 87, 2031–2040. [Google Scholar] [CrossRef]
- Kibenge, F.S.B.; Kibenge, M.J.T.; Wang, Y.; Qian, B.; Hariharan, S.; McGeachy, S. Mapping of putative virulence motifs on infectious salmon anemia virus surface glycoprotein genes. J. Gen. Virol. 2007, 88, 3100–3111. [Google Scholar] [CrossRef]
- Karami-Zarandi, M.; Douraghi, M.; Vaziri, B.; Adibhesami, H.; Rahbar, M.; Yaseri, M. Variable spontaneous mutation rate in clinical strains of multidrug-resistant Acinetobacter baumannii and differentially expressed proteins in a hypermutator strain. Mutat. Res. Mol. Mech. Mutagen. 2017, 800-802, 37–45. [Google Scholar] [CrossRef]
- Harris, S.R.; Feil, E.J.; Holden, M.T.G.; Quail, M.A.; Nickerson, E.K.; Chantratita, N.; Gardete, S.; Tavares, A.; Day, N.; Lindsay, J.A.; et al. Evolution of MRSA during hospital transmission and intercontinental spread. Science 2010, 327, 469–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero-Severson, E.; Nasir, A.; Leitner, T. What should health departments do with HIV sequence data? Viruses 2020, 12, 1018. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duault, H.; Durand, B.; Canini, L. Methods Combining Genomic and Epidemiological Data in the Reconstruction of Transmission Trees: A Systematic Review. Pathogens 2022, 11, 252. https://doi.org/10.3390/pathogens11020252
Duault H, Durand B, Canini L. Methods Combining Genomic and Epidemiological Data in the Reconstruction of Transmission Trees: A Systematic Review. Pathogens. 2022; 11(2):252. https://doi.org/10.3390/pathogens11020252
Chicago/Turabian StyleDuault, Hélène, Benoit Durand, and Laetitia Canini. 2022. "Methods Combining Genomic and Epidemiological Data in the Reconstruction of Transmission Trees: A Systematic Review" Pathogens 11, no. 2: 252. https://doi.org/10.3390/pathogens11020252







