Porcine Circoviruses and Herpesviruses Are Prevalent in an Austrian Game Population
Abstract
:1. Introduction
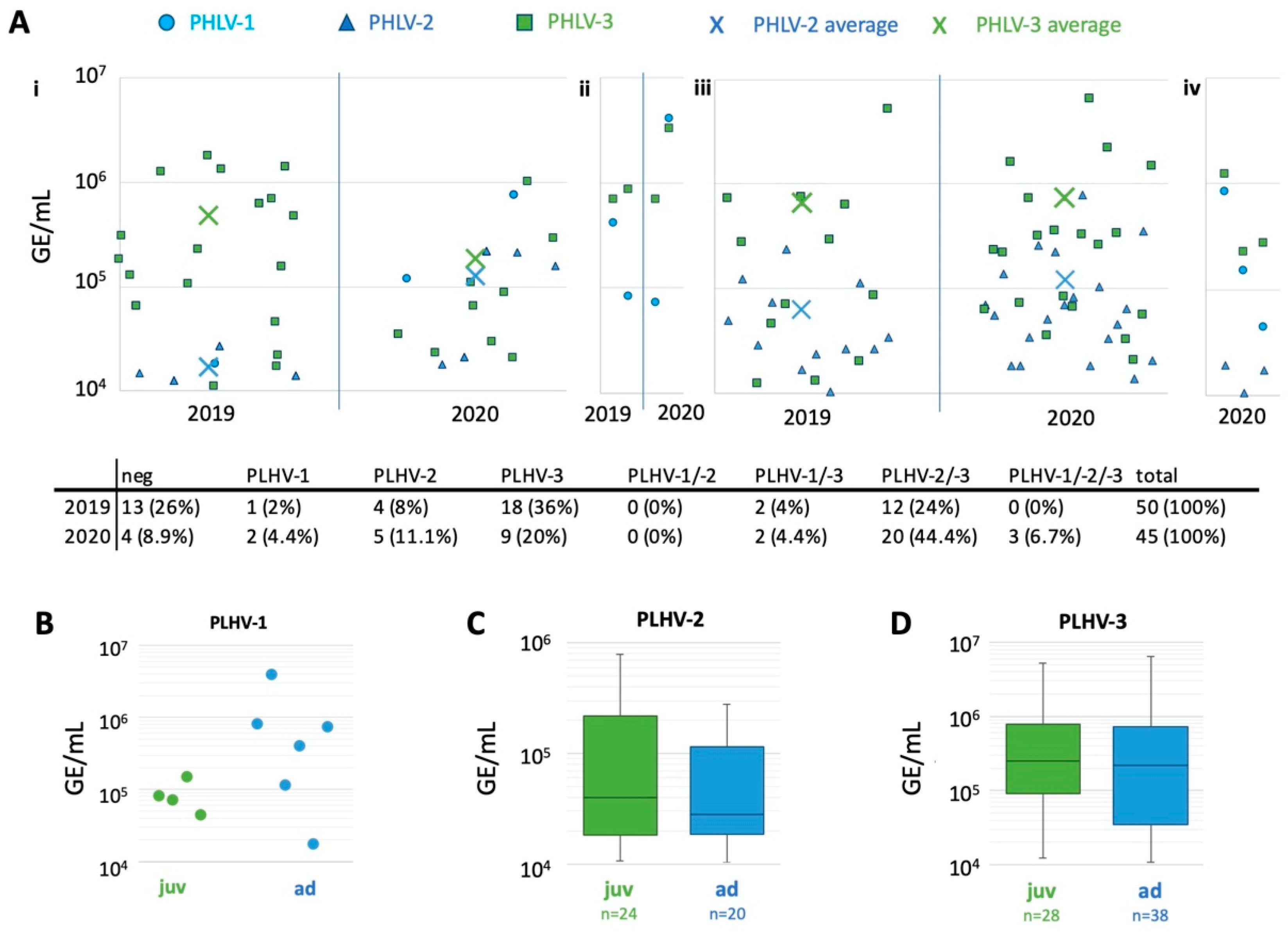
2. Results
3. Discussion
4. Materials and Methods
4.1. Animals and Samples
4.2. Nucleic Acid Extraction and PCRs
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Segalés, J.; Piella, J.; Marco, E.; Mateu-de-Antonio, E.M.; Espuña, E.; Domingo, M. Porcine dermatitis and nephropathy syndrome in Spain. Vet. Rec. 1998, 142, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Segalés, J.; Rosell, C.; Domingo, M. Pathological findings associated with naturally acquired porcine circovirus type 2 associated disease. Vet. Microbiol. 2004, 98, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.; Krakowka, S.; Lairmore, M.; Haines, D.; Bratanich, A.; Clark, E.; Allan, G.; Konoby, C.; Hassard, L.; Meehan, B.; et al. Reproduction of lesions of postweaning multisystemic wasting syndrome in gnotobiotic piglets. J. Vet. Diagn. Investig. 1999, 11, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.C.S. The clinical expression and emergence of porcine circovirus 2. Vet. Microbiol. 2004, 98, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Chae, C. A review of porcine circovirus 2-associated syndromes and diseases. Vet. J. 2005, 169, 326–336. [Google Scholar] [CrossRef]
- Ellis, J.; Spinato, M.; Yong, C.; West, K.; McNeilly, F.; Meehan, B.; Kennedy, S.; Clark, E.; Krakowka, S.; Allan, G. Porcine circovirus 2-associated disease in Eurasian wild boar. J. Vet. Diagn. Investig. 2003, 15, 364–368. [Google Scholar] [CrossRef] [Green Version]
- Toplak, I.; Grom, J.; Hostnik, P.; Barlič-Maganja, D. Phylogenetic analysis of type 2 porcine circoviruses indentified in wild boar in Slovenia. Vet. Rec. 2004, 155, 178–180. [Google Scholar] [CrossRef]
- Vicente, J.; Segalés, J.; Höfle, U.; Balasch, M.; Plana-Durán, J.; Domingo, M.; Gortázar, C. Epidemiological study on porcine circovirus type 2 (PCV 2) infection in the European wild boar (Sus scrofa). Vet. Res. 2004, 35, 243–253. [Google Scholar] [CrossRef] [Green Version]
- Cságola, A.; Kecskeméti, S.; Kardos, G.; Kiss, I.; Tuboly, T. Genetic characterization of type 2 porcine circoviruses detected in Hungarian wild boars. Arch. Virol. 2006, 151, 495–507. [Google Scholar] [CrossRef]
- Cadar, D.; Cságola, A.; Spinu, M.; Dán, D.; Ursu, K.; Lorincz, M.; Tuboly, T. Prevalence of porcine circoviruses in Transylvanian wild boars, detected by real-time PCR—Short communication. Acta Vet. Hung. 2010, 58, 475–481. [Google Scholar] [CrossRef]
- Reiner, G.; Bronnert, B.; Hohloch, C.; Fresen, C.; Haack, I.; Willems, H.; Reinacher, M. Qualitative and quantitative distribution of PCV2 in wild boars and domestic pigs in Germany. Vet. Microbiol. 2010, 145, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Boadella, M.; Ruiz-Fons, J.F.; Vicente, J.; Martín, M.; Segalés, J.; Gortazar, C. Seroprevalence evolution of selected pathogens in iberian wild boar. Transbound. Emerg. Dis. 2012, 59, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Hammer, R.; Ritzmann, M.; Palzer, A.; Lang, C.; Hammer, B.; Pesch, S.; Ladinig, A. Porcine Reproductive and Respiratory Syndrome Virus and Porcine Circovirus Type 2 Infections in Wild Boar (Sus Scrofa) in Southwestern Germany. J. Wildl. Dis. 2012, 48, 87–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabisiak, M.; Podgórska, K.; Skrzypiec, E.; Szczotka, A.; Stadejek, T. Detection of porcine circovirus type 2 (PCV2) and porcine reproductive and respiratory syndrome virus (PRRSV) antibodies in meat juice samples from Polish wild boar (Sus scrofa L.). Acta Vet. Hung. 2013, 61, 529–536. [Google Scholar] [CrossRef]
- Prinz, C.; Stillfried, M.; Neubert, L.K.; Denner, J. Detection of PCV3 in German wild boars. Virol. J. 2019, 16, 25. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, T.; Niu, G.; Liu, X.; Zhang, X.; Zhang, Y.; Ren, L. Recent progress on porcine circovirus type 3. Infect. Genet. Evol. 2019, 73, 227–233. [Google Scholar] [CrossRef]
- Phan, T.G.; Giannitti, F.; Rossow, S.; Marthaler, D.; Knutson, T.; Li, L.; Deng, X.; Resende, T.; Vannucci, F.; Delwart, E. Detection of a novel circovirus PCV3 in pigs with cardiac and multi-systemic inflammation. Virol. J. 2016, 13, 184. [Google Scholar] [CrossRef] [Green Version]
- Faccini, S.; Barbieri, I.; Gilioli, A.; Sala, G.; Gibelli, L.R.; Moreno, A.; Sacchi, C.; Rosignoli, C.; Franzini, G.; Nigrelli, A. Detection and genetic characterization of Porcine circovirus type 3 in Italy. Transbound. Emerg. Dis. 2017, 64, 1661–1664. [Google Scholar] [CrossRef]
- Wen, S.; Sun, W.; Li, Z.; Zhuang, X.; Zhao, G.; Xie, C.; Zheng, M.; Jing, J.; Xiao, P.; Wang, M.; et al. The detection of porcine circovirus 3 in Guangxi, China. Transbound. Emerg. Dis. 2018, 65, 27–31. [Google Scholar] [CrossRef]
- Yuzhakov, A.G.; Raev, S.A.; Alekseev, K.P.; Grebennikova, T.V.; Verkhovsky, O.A.; Zaberezhny, A.D.; Aliper, T.I. First detection and full genome sequence of porcine circovirus type 3 in Russia. Virus Genes 2018, 54, 608–611. [Google Scholar] [CrossRef]
- Ye, X.; Berg, M.; Fossum, C.; Wallgren, P.; Blomström, A.L. Detection and genetic characterisation of porcine circovirus 3 from pigs in Sweden. Virus Genes 2018, 54, 466–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arruda, B.; Piñeyro, P.; Derscheid, R.; Hause, B.; Byers, E.; Dion, K.; Long, D.; Sievers, C.; Tangen, J.; Williams, T.; et al. PCV3-associated disease in the United States swine herd. Emerg. Microbes Infect. 2019, 8, 684–698. [Google Scholar] [CrossRef] [Green Version]
- Deim, Z.; Dencso, L.; Erdélyi, I.; Valappil, S.K.; Varga, C.; Pósa, A.; Makrai, L.; Rákhely, G. Porcine circovirus type 3 detection in a Hungarian pig farm experiencing reproductive failures. Vet. Rec. 2019, 185, 84. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Luo, H.; Liu, Y.; Chen, C.; Xu, W.; Chen, Y.; Li, X.; Fang, W. Prevalence of porcine circovirus type 3 in pigs in the southeastern Chinese province of Zhejiang. BMC Vet. Res. 2019, 15, 244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Wang, D.; Wang, J.; Zhu, S.; She, R.; Ren, X.; Tian, J.; Quan, R.; Hou, L.; Li, Z.; et al. Induction of Porcine Dermatitis and Nephropathy Syndrome in Piglets by Infection with Porcine Circovirus Type 3. J. Virol. 2019, 93, e02045-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sukmak, M.; Thanantong, N.; Poolperm, P.; Boonsoongnern, A.; Ratanavanichrojn, N.; Jirawattanapong, P.; Woonwong, Y.; Soda, N.; Kaminsonsakul, T.; Phuttapatimok, S.; et al. The retrospective identification and molecular epidemiology of porcine circovirus type 3 (PCV3) in swine in Thailand from 2006 to 2017. Transbound. Emerg. Dis. 2019, 66, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Palinski, R.; Piñeyro, P.; Shang, P.; Yuan, F.; Guo, R.; Fang, Y.; Byers, E.; Hause, B.M. A Novel Porcine Circovirus Distantly Related to Known Circoviruses Is Associated with Porcine Dermatitis and Nephropathy Syndrome and Reproductive Failure. J. Virol. 2017, 91, e01879-16. [Google Scholar] [CrossRef] [Green Version]
- Kwon, T.; Yoo, S.J.; Park, C.K.; Lyoo, Y.S. Prevalence of novel porcine circovirus 3 in Korean pig populations. Vet. Microbiol. 2017, 207, 178–180. [Google Scholar] [CrossRef]
- Stadejek, T.; Woźniak, A.; Miłek, D.; Biernacka, K. First detection of porcine circovirus type 3 on commercial pig farms in Poland. Transbound. Emerg. Dis. 2017, 64, 1350–1353. [Google Scholar] [CrossRef]
- Zhai, S.L.; Zhou, X.; Zhang, H.; Hause, B.M.; Lin, T.; Liu, R.; Chen, Q.L.; Wei, W.K.; Lv, D.H.; Wen, X.H.; et al. Comparative epidemiology of porcine circovirus type 3 in pigs with different clinical presentations. Virol. J. 2017, 14, 4–9. [Google Scholar] [CrossRef] [Green Version]
- Franzo, G.; Legnardi, M.; Hjulsager, C.K.; Klaumann, F.; Larsen, L.E.; Segales, J.; Drigo, M. Full-genome sequencing of porcine circovirus 3 field strains from Denmark, Italy and Spain demonstrates a high within-Europe genetic heterogeneity. Transbound. Emerg. Dis. 2018, 65, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Ohshima, Y.; Furuya, Y.; Nagao, A.; Oroku, K.; Tsutsumi, N.; Sasakawa, C.; Sato, T. First detection of porcine circovirus type 3 in Japan. J. Vet. Med. Sci. 2018, 80, 1468–1472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kedkovid, R.; Woonwong, Y.; Arunorat, J.; Sirisereewan, C.; Sangpratum, N.; Lumyai, M.; Kesdangsakonwut, S.; Teankum, K.; Jittimanee, S.; Thanawongnuwech, R. Porcine circovirus type 3 (PCV3) infection in grower pigs from a Thai farm suffering from porcine respiratory disease complex (PRDC). Vet. Microbiol. 2018, 215, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Tochetto, C.; Lima, D.A.; Varela, A.P.M.; Loiko, M.R.; Paim, W.P.; Scheffer, C.M.; Herpich, J.I.; Cerva, C.; Schmitd, C.; Cibulski, S.P.; et al. Full-Genome Sequence of Porcine Circovirus type 3 recovered from serum of sows with stillbirths in Brazil. Transbound. Emerg. Dis. 2018, 65, 5–9. [Google Scholar] [CrossRef]
- Klaumann, F.; Dias-Alves, A.; Cabezón, O.; Mentaberre, G.; Castillo-Contreras, R.; López-Béjar, M.; Casas-Díaz, E.; Sibila, M.; Correa-Fiz, F.; Segalés, J. Porcine circovirus 3 is highly prevalent in serum and tissues and may persistently infect wild boar (Sus scrofa scrofa). Transbound. Emerg. Dis. 2019, 66, 91–101. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Liu, Z.; Zou, Y.; Zhang, N.; Wang, D.; Tu, D.; Yang, L.; Deng, Z.; Yang, Y.; Jiang, P.; et al. First molecular detection of porcine circovirus type 3 in dogs in China. Virus Genes 2018, 54, 140–144. [Google Scholar] [CrossRef]
- Franzo, G.; Grassi, L.; Tucciarone, C.M.; Drigo, M.; Martini, M.; Pasotto, D.; Mondin, A.; Menandro, M.L. A wild circulation: High presence of Porcine circovirus 3 in different mammalian wild hosts and ticks. Transbound. Emerg. Dis. 2019, 66, 1548–1557. [Google Scholar] [CrossRef]
- Jiang, S.; Zhou, N.; Li, Y.; An, J.; Chang, T. Detection and sequencing of porcine circovirus 3 in commercially sourced laboratory mice. Vet. Med. Sci. 2019, 5, 176–181. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, W.; Xu, X.; Wang, W.; He, K.; Fan, H. Phylogenetic analysis of two goat-origin PCV2 isolates in China. Gene 2018, 651, 57–61. [Google Scholar] [CrossRef]
- Wang, W.; Sun, W.; Cao, L.; Zheng, M.; Zhu, Y.; Li, W.; Liu, C.; Zhuang, X.; Xing, J.; Lu, H.; et al. An epidemiological investigation of porcine circovirus 3 infection in cattle in Shandong province, China. BMC Vet. Res. 2019, 15, 60. [Google Scholar] [CrossRef]
- Zhai, S.L.; Lu, S.S.; Wei, W.K.; Lv, D.H.; Wen, X.H.; Zhai, Q.; Chen, Q.L.; Sun, Y.W.; Xi, Y. Reservoirs of Porcine Circoviruses: A Mini Review. Front. Vet. Sci. 2019, 6, 319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinrigl, A.; Revilla-Fernández, S.; Kolodziejek, J.; Wodak, E.; Bagó, Z.; Nowotny, N.; Schmoll, F.; Köfer, J. Detection and molecular characterization of Suid herpesvirus type 1 in Austrian wild boar and hunting dogs. Vet. Microbiol. 2012, 157, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Meier, R.K.; Ruiz-Fons, F.; Ryser-Degiorgis, M.P. A picture of trends in Aujeszky’s disease virus exposure in wild boar in the Swiss and European contexts. BMC Vet. Res. 2015, 11, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caruso, C.; Vitale, N.; Prato, R.; Radaelli, M.C.; Zoppi, S.; Possidente, R.; Dondo, A.; Chiavacci, L.; Moreno Martin, A.M.; Masoero, L. Pseudorabies virus in North-West Italian wild boar (Sus scrofa) populations: Prevalence and risk factors to support a territorial risk-based surveillance. Vet. Ital. 2018, 54, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Denzin, N.; Conraths, F.J.; Mettenleiter, T.C.; Freuling, C.M.; Müller, T. Monitoring of pseudorabies in wild boar of Germany—A spatiotemporal analysis. Pathogens 2020, 9, 276. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Hahn, E.C.; Tottewitz, F.; Kramer, M.; Klupp, B.G.; Mettenleiter, T.C.; Freuling, C. Pseudorabies virus in wild swine: A global perspective. Arch. Virol. 2011, 156, 1691–1705. [Google Scholar] [CrossRef]
- Laval, K.; Enquist, L.W. The neuropathic itch caused by pseudorabies virus. Pathogens 2020, 9, 254. [Google Scholar] [CrossRef] [Green Version]
- Ulrich, S.; Goltz, M.; Ehlers, B. Characterization of the DNA polymerase loci of the novel porcine lymphotropic herpesviruses 1 and 2 in domestic and feral pigs. J. Gen. Virol. 1999, 80, 3199–3205. [Google Scholar] [CrossRef]
- Chmielewicz, B.; Goltz, M.; Franz, T.; Bauer, C.; Brema, S.; Ellerbrok, H.; Beckmann, S.; Rziha, H.J.; Lahrmann, K.H.; Romero, C.; et al. A novel porcine gammaherpesvirus. Virology 2003, 308, 317–329. [Google Scholar] [CrossRef] [Green Version]
- Franzo, G.; Drigo, M.; Legnardi, M.; Grassi, L.; Menandro, M.L.; Pasotto, D.; Cecchinato, M.; Tucciarone, C.M. Porcine gammaherpesviruses in italian commercial swine population: Frequent but harmless. Pathogens 2021, 10, 47. [Google Scholar] [CrossRef]
- Ehlers, B.; Dural, G.; Yasmum, N.; Lembo, T.; de Thoisy, B.; Ryser-Degiorgis, M.-P.; Ulrich, R.G.; McGeoch, D.J. Novel Mammalian Herpesviruses and Lineages within the Gammaherpesvirinae: Cospeciation and Interspecies Transfer. J. Virol. 2008, 82, 3509–3516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plowright, W.; Ferris, R.D.; Scott, G.R. Blue wildebeest and the Ætiological agent of bovine malignant catarrhal fever. Nature 1960, 188, 1167–1169. [Google Scholar] [CrossRef] [PubMed]
- Plowright, W. Malignant Catarrhal Fever Virus. In Virus Infections of Ruminants; Elsevier: Amsterdam, The Netherlands, 1990. [Google Scholar]
- Løken, T.; Aleksandersen, M.; Reid, H.W.; Pow, I. Malignant catarrhal fever caused by ovine herpesvirus-2 in pigs in Norway. Vet. Rec. 1998, 143, 464–467. [Google Scholar] [CrossRef] [PubMed]
- Crawford, T.B.; Li, H.; Rosenburg, S.R.; Norhausen, R.W.; Garner, M.M. Mural folliculitis and alopecia caused by infection with goat-associated malignant catarrhal fever virus in two sika deer. J. Am. Vet. Med. Assoc. 2002, 221, 843–847. [Google Scholar] [CrossRef]
- O’Toole, D.; Li, H. The Pathology of Malignant Catarrhal Fever, with an Emphasis on Ovine Herpesvirus 2. Vet. Pathol. 2014, 51, 437–452. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Huang, Q.; Hu, X.; Chu, W.; Zhang, J.; Jiang, L.; Yu, X.; Zhang, X.; Cheng, S. Caprine herpesvirus 2-associated malignant catarrhal fever of captive sika deer (Cervus nippon) in an intensive management system. BMC Vet. Res. 2018, 14, 38. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Gailbreath, K.; Flach, E.J.; Taus, N.S.; Cooley, J.; Keller, J.; Russell, G.C.; Knowles, D.P.; Haig, D.M.; Oaks, J.L.; et al. A novel subgroup of rhadinoviruses in ruminants. J. Gen. Virol. 2005, 86, 3021–3026. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, H.; Yu, X.; Zhang, J.; Jiang, L.; Chen, G.; Feng, Z.; Li, Y.; Feng, T.; Zhang, X. Evidence of two genetically different lymphotropic herpesviruses present among red deer, sambar, and milu herds in China. J. Vet. Sci. 2018, 19, 716–720. [Google Scholar] [CrossRef]
- Krametter, R.; Nielsen, S.S.; Loitsch, A.; Froetscher, W.; Benetka, V.; Moestl, K.; Baumgartner, W. Pestivirus exposure in free-living and captive deer in Austria. J. Wildl. Dis. 2004, 40, 791–795. [Google Scholar] [CrossRef] [Green Version]
- Casaubon, J.; Vogt, H.R.; Stalder, H.; Hug, C.; Ryser-Degiorgis, M.P. Bovine viral diarrhea virus in free-ranging wild ruminants in Switzerland: Low prevalence of infection despite regular interactions with domestic livestock. BMC Vet. Res. 2012, 8, 204. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Aguilar, X.; López-Olvera, J.R.; Marco, I.; Rosell, R.; Colom-Cadena, A.; Soto-Heras, S.; Lavín, S.; Cabezón, O. Pestivirus in alpine wild ruminants and sympatric livestock from the Cantabrian Mountains, Spain. Vet. Rec. 2016, 178, 586. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Prieto, V.; Kukielka, D.; Rivera-Arroyo, B.; Martínez-López, B.; de las Heras, A.I.; Sánchez-Vizcaíno, J.M.; Vicente, J. Evidence of shared bovine viral diarrhea infections between red deer and extensively raised cattle in south-central Spain. BMC Vet. Res. 2016, 12, 11. [Google Scholar] [CrossRef] [Green Version]
- Unterweger, C.; Brunthaler, R.; Auer, A.; Fux, R.; Weissenbacher-Lang, C.; Ladinig, A. Reconsideration of the diagnostic criteria required for PCV2 reproductive disease. Vet. J. 2021, 272, 105660. [Google Scholar] [CrossRef]
- Franzo, G.; Tucciarone, C.M.; Drigo, M.; Cecchinato, M.; Martini, M.; Mondin, A.; Menandro, M.L. First report of wild boar susceptibility to Porcine circovirus type 3: High prevalence in the Colli Euganei Regional Park (Italy) in the absence of clinical signs. Transbound. Emerg. Dis. 2018, 65, 957–962. [Google Scholar] [CrossRef]
- Tan, C.Y.; Lin, C.; Ooi, P.T. What do we know about porcine circovirus 3 (PCV3) diagnosis so far? A review. Transbound. Emerg. Dis. 2021, 68, 2915–2935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.H.; Hu, W.Q.; Li, J.Y.; Liu, T.N.; Zhou, J.Y.; Opriessnig, T.; Xiao, C.T. Novel circovirus species identified in farmed pigs designated as Porcine circovirus 4, Hunan province, China. Transbound. Emerg. Dis. 2020, 67, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Franzo, G.; Ruiz, A.; Grassi, L.; Sibila, M.; Drigo, M.; Segalés, J. Lack of porcine circovirus 4 genome detection in pig samples from Italy and Spain. Pathogens 2020, 9, 433. [Google Scholar] [CrossRef]
- McMahon, K.J.; Minihan, D.; Campion, E.M.; Loughran, S.T.; Allan, G.; McNeilly, F.; Walls, D. Infection of pigs in Ireland with lymphotropic γ-herpesviruses and relationship to postweaning multisystemic wasting syndrome. Vet. Microbiol. 2006, 116, 60–68. [Google Scholar] [CrossRef]
- Reid, H.W.; Buxton, D.; Corrigall, W.; Hunter, A.R.; McMartin, D.A.; Rushton, R. An outbreak of malignant catarrhal fever in red deer (Cervus elephus). Vet. Rec. 1979, 104, 120–123. [Google Scholar] [CrossRef]
- Albini, S.; Zimmermann, W.; Neff, F.; Ehlers, B.; Häni, H.; Li, H.; Hüssy, D.; Casura, C.; Engels, M.; Ackermann, M. Porcines Bösartiges Katarrhalfieber: Diagnostische Befunde und erstmaliger Nachweis des Erregers bei erkrankten Schweinen in der Schweiz. Schweiz. Arch. Tierheilkd. 2003, 145, 61–68. [Google Scholar] [CrossRef]
- Glawischnig, W.; Schoepf, K.; Matt, M. Monitoring for bovine viral diarrhea virus in austrian red deer (Cervus elaphus elaphus) by using ear-notch samples. J. Wildl. Dis. 2010, 46, 1269–1273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandevanter, D.R.; Warrener, P.; Bennett, L.; Schultz, E.R.; Coulter, S.; Garber, R.L.; Rose, T.M. Detection and analysis of diverse herpesviral species by consensus primer PCR. J. Clin. Microbiol. 1996, 34, 1666–1671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mengeling, W.L.; Lager, K.M.; Volz, D.M.; Brockmeier, S.L. Effect of various vaccination procedures on shedding, latency, and reactivation of attenuated and virulent pseudorabies virus in swine. Am. J. Vet. Res. 1992, 53, 2164–2173. [Google Scholar] [PubMed]
- Kadir, Y.; Christine, F.; Barbara, B.W.; Zeki, Y.; Feray, A.; Aykut, O.; Ibrahim, B.; Sibilina Cedillo, R.; Heinz-Jürgen, T.; Matthias, K. Genetic heterogeneity of bovine viral diarrhoea virus (BVDV) isolates from Turkey: Identification of a new subgroup in BVDV-1. Vet. Microbiol. 2008, 130, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, B. AVID-Methode_VIR02-pCV-2-qPCR-FLI. Available online: http://avid.dvg.net/fileadmin/Bilder/PDF_AVID_Alt/website/Methoden_ab_2016/AVID-Methode_VIR02_porcines_Circovirus_2_final.pdf (accessed on 9 December 2021).

| 2019 | 2020 | |
|---|---|---|
| fallow deer herpesvirus | 3 (7.7%) | 7 (19.4%) |
| elk gamma herpesvirus | 1 (2.6%) | 1 (2.8%) |
| no herpesvirus | 35 (89.7%) | 28 (77.8%) |
| sum | 39 | 36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Auer, A.; Schweitzer, L.; Kübber-Heiss, A.; Posautz, A.; Dimmel, K.; Seitz, K.; Beiglböck, C.; Riedel, C.; Rümenapf, T. Porcine Circoviruses and Herpesviruses Are Prevalent in an Austrian Game Population. Pathogens 2022, 11, 305. https://doi.org/10.3390/pathogens11030305
Auer A, Schweitzer L, Kübber-Heiss A, Posautz A, Dimmel K, Seitz K, Beiglböck C, Riedel C, Rümenapf T. Porcine Circoviruses and Herpesviruses Are Prevalent in an Austrian Game Population. Pathogens. 2022; 11(3):305. https://doi.org/10.3390/pathogens11030305
Chicago/Turabian StyleAuer, Angelika, Lea Schweitzer, Anna Kübber-Heiss, Annika Posautz, Katharina Dimmel, Kerstin Seitz, Christoph Beiglböck, Christiane Riedel, and Till Rümenapf. 2022. "Porcine Circoviruses and Herpesviruses Are Prevalent in an Austrian Game Population" Pathogens 11, no. 3: 305. https://doi.org/10.3390/pathogens11030305






