Diversity and Genetic Structure of Theileria annulata in Pakistan and Other Endemic Sites
Abstract
:1. Introduction
2. Results
2.1. Demographic Data
2.2. Diversity of Microsatellites and Minisatellites
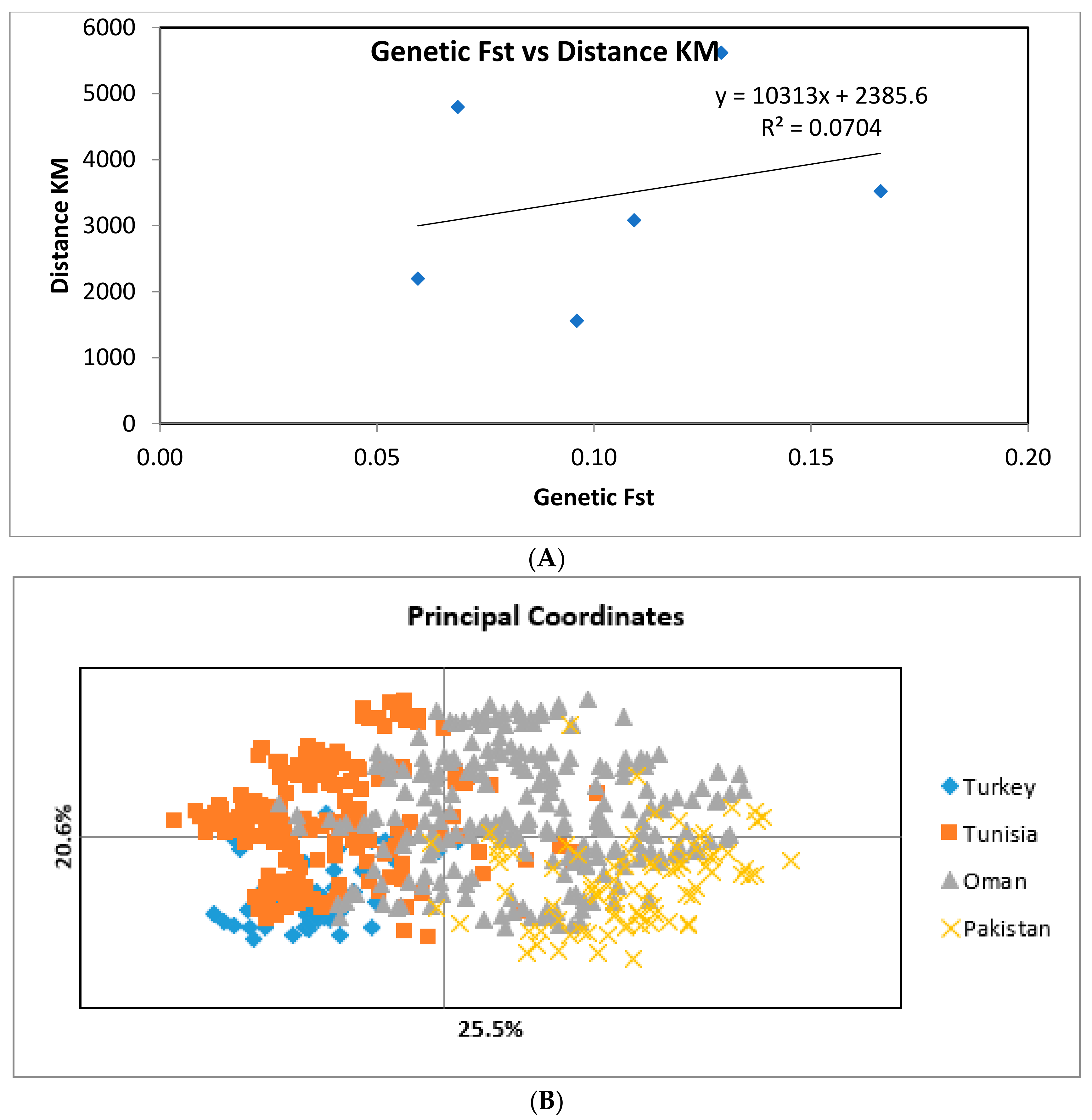
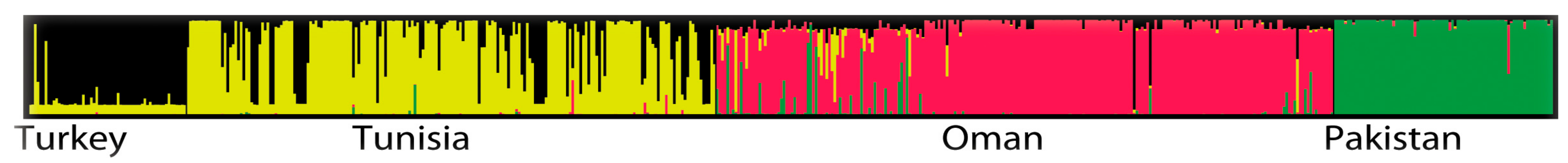
2.3. Population Differentiation
3. Discussion
4. Materials and Methods
4.1. Study Site and Sample Collection
4.2. Identification of Theileria Species and Microsatellite and Minisatellite Genotyping
4.3. Genetic Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gharbi, M.; Darghouth, M.A.; Elati, K.; Al-Hosary, A.A.T.; Ayadi, O.; Salih, D.A.; El Hussein, A.M.; Mhadhbi, M.; Khamassi Khbou, M.; Hassan, S.M.; et al. Current status of tropical theileriosis in Northern Africa: A review of recent epidemiological investigations and implications for control. Transbound. Emerg. Dis. 2020, 67, 8–25. [Google Scholar] [CrossRef] [PubMed]
- Parveen, A.; Ashraf, S.; Aktas, M.; Ozubek, S.; Iqbal, F. Molecular epidemiology of Theileria annulata infection of cattle in Layyah District, Pakistan. Exp. Appl. Acarol. 2021, 83, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Bishop, R.; Musoke, A.; Morzaria, S.; Gardner, M.; Nene, V. Theileria: Intracellular protozoan parasites of wild and domestic ruminants transmitted by ixodid ticks. Parasitol. Res. 2004, 129, S271–S283. [Google Scholar] [CrossRef] [PubMed]
- Gharbi, M.; Sassi, L.; Dorchies, P.; Darghouth, M.A. Infection of calves with theileria annulata in tunisia: Economic analysis and evaluation of the potential benefit of vaccination. Vet. Parasitol. 2006, 137, 231–241. [Google Scholar] [CrossRef]
- Al-Hamidhi, S.; Tageldin, M.H.; Weir, W.; Al-Fahdi, A.; Johnson, E.H.; Bobade, P.; Alqamashoui, B.; Beja-Pereira, A.; Thompson, J.; Kinnaird, J. Genetic diversity and population structure of Theileria annulata in Oman. PLoS ONE 2015, 10, e0139581. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Bhandari, V.; Barman, M.; Kumar, P.; Bhanot, V.; Arora, J.S.; Singh, S.; Sharma, P. Population genetic analysis of the Theileria annulata parasites identified limited diversity and multiplicity of infection in the vaccine from India. Front. Microbiol. 2021, 11, 3477. [Google Scholar] [CrossRef]
- Weir, W.; Ben-Miled, L.; Karagenç, T.; Katzer, F.; Darghouth, M.; Shiels, B.; Tait, A. Genetic exchange and sub-structuring in Theileria annulata populations. Mol. Biochem. Parasitol. 2007, 154, 170–180. [Google Scholar] [CrossRef]
- Babiker, H.; Ranford-Cartwright, L.C.; Currie, D.; Charlwood, J.; Billingsley, P.; Teuscher, T.; Walliker, D. Random mating in a natural population of the malaria parasite Plasmodium falciparum. Parasitology 1994, 109, 413–421. [Google Scholar] [CrossRef]
- Oura, C.; Asiimwe, B.; Weir, W.; Lubega, G.; Tait, A. Population genetic analysis and sub-structuring of Theileria parva in Uganda. Mol. Biochem. Parasitol. 2005, 140, 229–239. [Google Scholar] [CrossRef]
- Awad, H.; Al-Hamidhi, S.; El Hussein, A.-R.M.; Zein Yousif, Y.M.; Taha, K.M.; Salih, D.A.; Weir, W.; Babiker, H.A. Theileria lestoquardi in Sudan is highly diverse and genetically distinct from that in Oman. Infect. Genet. Evol. 2018, 62, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Criscione, C. Parasite co-structure: Broad and local scale approaches. Parasite 2008, 15, 439–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holsinger, K.E.; Weir, B.S. Genetics in geographically structured populations: Defining, estimating and interpreting Fst. Nat. Rev. Genet. 2009, 10, 639–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieberding, C.M.; Durette-Desset, M.-C.; Vanderpoorten, A.; Casanova, J.C.; Ribas, A.; Deffontaine, V.; Feliu, C.; Morand, S.; Libois, R.; Michaux, J.R. Geography and host biogeography matter for understanding the phylogeography of a parasite. Mol. Phylogenetics Evol. 2008, 47, 538–554. [Google Scholar] [CrossRef] [PubMed]
- Malak, A.K.; Mpoke, L.; Banak, J.; Muriuki, S.; Skilton, R.A.; Odongo, D.; Sunter, J.; Kiara, H. Prevalence of livestock diseases and their impact on livelihoods in Central Equatoria State, Southern Sudan. Prev. Vet. Med. 2012, 104, 216–223. [Google Scholar] [CrossRef]
- Marcellino, W.L.; Salih, D.A.; Njahira, M.N.; Ndiwa, N.; Araba, A.; El Hussein, A.; Seitzer, U.; Ahmed, J.; Bishop, R.P.; Skilton, R.A. The emergence of Theileria parva in Jonglei State, South Sudan: Confirmation using molecular and serological diagnostic tools. Transbound. Emerg. Dis. 2017, 64, 1229–1235. [Google Scholar] [CrossRef]
- Pérez-Pardal, L.; Sánchez-Gracia, A.; Álvarez, I.; Traoré, A.; Ferraz, J.B.S.; Fernández, I.; Costa, V.; Chen, S.; Tapio, M.; Cantet, R.J.C.; et al. Legacies of domestication, trade and herder mobility shape extant male zebu cattle diversity in South Asia and Africa. Sci. Rep. 2018, 8, 18027. [Google Scholar] [CrossRef]
- Afridi, M.J.K.; Mian, A.H.; Saqib, M.; Abbas, G.; Ali, J.; Mansoor, M.K.; Sial, A.U.R.; Rasheed, I.; Hussain, M.H. Seroprevalence and risk factors for theileria equi infection in equines from Khyber Pakhtunkhwa province, Pakistan. Iran. J. Parasitol. 2017, 12, 597–605. [Google Scholar]
- Al-Fahdi, A.; Alqamashoui, B.; Al-Hamidhi, S.; Kose, O.; Tageldin, M.H.; Bobade, P.; Johnson, E.H.; Hussain, A.-R.; Karagenc, T.; Tait, A.; et al. Molecular surveillance of Theileria parasites of livestock in Oman. Ticks Tick-Borne Dis. 2017, 8, 741–748. [Google Scholar] [CrossRef] [Green Version]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software structure: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [Green Version]
- Ghafar, A.; Abbas, T.; Rehman, A.; Sandhu, Z.-U.; Cabezas-Cruz, A.; Jabbar, A. Systematic Review of Ticks and Tick-Borne Pathogens of Small Ruminants in Pakistan. Pathogens 2020, 9, 937. [Google Scholar] [CrossRef]
- Al-Hamidhi, S.; Weir, W.; Kinnaird, J.; Tageledin, M.; Beja-Pereira, A.; Morrison, I.; Thompson, J.; Tait, A.; Shiels, B.; Babiker, H.A. Theileria lestoquardi displays reduced genetic diversity relative to sympatric Theileria annulata in Oman. Infect. Genet. Evol. 2016, 43, 297–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weir, W.; Karagenç, T.; Gharbi, M.; Simuunza, M.; Aypak, S.; Aysul, N.; Darghouth, M.A.; Shiels, B.; Tait, A. Population diversity and multiplicity of infection in theileria annulata. Int. J. Parasitol. 2011, 41, 193–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyangiwe, N.; Yawa, M.; Muchenje, V. Driving forces for changes in geographic range of cattle ticks (acari: Ixodidae) in Africa: A review. S. Afr. J. Anim. Sci. 2018, 48, 829. [Google Scholar] [CrossRef] [Green Version]
- Sutton, A.J.; Karagenc, T.; Bakirci, S.; Sarali, H.; Pekel, G.; Medley, G.F. Modelling the transmission dynamics of Theileria annulata: Model structure and validation for the Turkish context. Parasitology 2012, 139, 441–453. [Google Scholar] [CrossRef] [Green Version]
- Rollins, R.E.; Schaper, S.; Kahlhofer, C.; Frangoulidis, D.; Strauß, A.F.; Cardinale, M.; Springer, A.; Strube, C.; Bakkes, D.K.; Becker, N.S.; et al. Ticks (Acari: Ixodidae) on birds migrating to the island of Ponza, Italy, and the tick-borne pathogens they carry. Ticks Tick-Borne Dis. 2020, 12, 101590. [Google Scholar] [CrossRef]
- Abbas, T.; Wilking, H.; Staubach, C.; Ziller, M.; Conraths, F.J. Priority areas for surveillance and prevention of avian influenza during the water-bird migration season in Pakistan. Geospat. Health 2011, 6, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Hoogstraal, H.; Kaiser, M.N.; Traylor, M.A.; Gaber, S.; Guindy, E. Ticks (ixodoidea) on birds migrating from africa to europe and asia. Bull. World Health Organ. 1961, 24, 197. [Google Scholar]
- Kjelland, V.; Stuen, S.; Skarpaas, T.; Slettan, A. Borrelia burgdorferi sensu lato in Ixodes ricinus ticks collected from migratory birds in southern norway. Acta Vet. Scand. 2010, 52, 59. [Google Scholar] [CrossRef] [Green Version]
- Chitimia-Dobler, L.; Rieß, R.; Kahl, O.; Wölfel, S.; Dobler, G.; Nava, S.; Estrada-Peña, A. Ixodes inopinatus—Occurring also outside the Mediterranean region. Ticks Tick-Borne Dis. 2018, 9, 196–200. [Google Scholar] [CrossRef]
- Wilhelmsson, P.; Jaenson, T.G.; Olsen, B.; Waldenström, J.; Lindgren, P.-E. Migratory birds as disseminators of ticks and the tick-borne pathogens borrelia bacteria and tick-borne encephalitis (tbe) virus: A seasonal study at ottenby bird observatory in South-Eastern Sweden. Parasites Vectors 2020, 13, 607. [Google Scholar] [CrossRef]
- Anderson, T.J.C.; Haubold, B.; Williams, J.T.; Estrada-Franco, J.G.; Richardson, L.; Mollinedo, R.; Bockarie, M.; Mokili, J.; Mharakurwa, S.; French, N.; et al. Microsatellite markers reveal a spectrum of population structures in the malaria parasite Plasmodium falciparum. Mol. Biol. Evol. 2000, 17, 1467–1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mobegi, V.A.; Loua, K.M.; Ahouidi, A.D.; Satoguina, J.; Nwakanma, D.C.; Amambua-Ngwa, A.; Conway, D.J. Population genetic structure of Plasmodium falciparum across a region of diverse endemicity in West Africa. Malar. J. 2012, 11, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Lin, B.Z.; Baig, M.; Mitra, B.; Lopes, R.J.; Santos, A.M.; Magee, D.A.; Azevedo, M.; Tarroso, P.; Sasazaki, S.; et al. Zebu cattle are an exclusive legacy of the South Asia neolithic. Mol. Biol. Evol. 2010, 27, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Awad, H.; Gadalla, A.A.; Postigo, M.; Al-Hamidhi, S.; Tageldin, M.H.; Skariah, S.; Sultan, A.A.; Johnson, E.H.; Shiels, B.; Pain, A. Dynamics and within-host interaction of Theileria lestoquardi and T. ovis among naive sheep in Oman. Sci. Rep. 2020, 10, 19802. [Google Scholar] [CrossRef] [PubMed]
- Peakall, R.; Smouse, P.E. Genalex 6.5: Genetic analysis in excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [Green Version]
- Anon, A. The evaluation of forensic DNA evidence. Proc. Natl. Acad. Sci. USA 1996, 94, 5498–5500. [Google Scholar]
- Haubold, B.; Hudson, R.R. Lian 3.0: Detecting linkage disequilibrium in multilocus data. Bioinformatics 2000, 16, 847–849. [Google Scholar] [CrossRef] [PubMed]
- Cockerham, C.C.; Weir, B.S. Covariances of relatives stemming from a population undergoing mixed self and random mating. Biometrics 1984, 40, 157–164. [Google Scholar] [CrossRef]
- Nei, M. Molecular Evolutionary Genetics; Columbia University Press: New York, NY, USA, 1987. [Google Scholar]
- Huelsenbeck, J.P.; Andolfatto, P.; Huelsenbeck, E.T. Structurama: Bayesian inference of population structure. Evol. Bioinform. Online 2011, 7, 55–59. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.; Bryc, K.; Bustamante, C.D. On identifying the optimal number of population clusters via the deviance information criterion. PLoS ONE 2011, 6, e21014. [Google Scholar] [CrossRef] [Green Version]
| n | TS5 | TS6 | TS8 | TS9 | TS12 | TS15 | TS16 | TS20 | TS25 | TS31 | Average He | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pakistan | 82 | 0.627 | 0.941 | 0.960 | 0.922 | 0.935 | 0.638 | 0.613 | 0.743 | 0.348 | 0.704 | 0.743 |
| Oman | 231 | 0.781 | 0.887 | 0.937 | 0.916 | 0.945 | 0.862 | 0.735 | 0.667 | 0.769 | 0.920 | 0.842 |
| Turkey | 59 | 0.827 | 0.941 | 0.953 | 0.931 | 0.936 | 0.829 | 0.828 | 0.897 | 0.669 | 0.836 | 0.865 |
| Tunisia | 198 | 0.826 | 0.943 | 0.957 | 0.900 | 0.957 | 0.815 | 0.803 | 0.851 | 0.658 | 0.950 | 0.866 |
| Turkey | Tunisia | Oman | Pakistan | |
|---|---|---|---|---|
| Turkey | 0 | 2200.9 | 3083.35 | 3526.09 |
| Tunisia | 0.0594 | 0 | 4800.6 | 5621.92 |
| Oman | 0.1092 | 0.06855 | 0 | 1562.12 |
| Pakistan | 0.166 | 0.1293 | 0.096 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Hamidhi, S.; Parveen, A.; Iqbal, F.; Asif, M.; Akhtar, N.; Elshafie, E.I.; Beja-Pereira, A.; Babiker, H.A. Diversity and Genetic Structure of Theileria annulata in Pakistan and Other Endemic Sites. Pathogens 2022, 11, 334. https://doi.org/10.3390/pathogens11030334
Al-Hamidhi S, Parveen A, Iqbal F, Asif M, Akhtar N, Elshafie EI, Beja-Pereira A, Babiker HA. Diversity and Genetic Structure of Theileria annulata in Pakistan and Other Endemic Sites. Pathogens. 2022; 11(3):334. https://doi.org/10.3390/pathogens11030334
Chicago/Turabian StyleAl-Hamidhi, Salama, Asia Parveen, Furhan Iqbal, Muhammad Asif, Naheed Akhtar, Elshafie I. Elshafie, Albano Beja-Pereira, and Hamza A. Babiker. 2022. "Diversity and Genetic Structure of Theileria annulata in Pakistan and Other Endemic Sites" Pathogens 11, no. 3: 334. https://doi.org/10.3390/pathogens11030334







