Both Neisseria gonorrhoeae and Neisseria sicca Induce Cytokine Secretion by Infected Human Cells, but Only Neisseria gonorrhoeae Upregulates the Expression of Long Non-Coding RNAs
Abstract
:1. Introduction
2. Results and Discussion
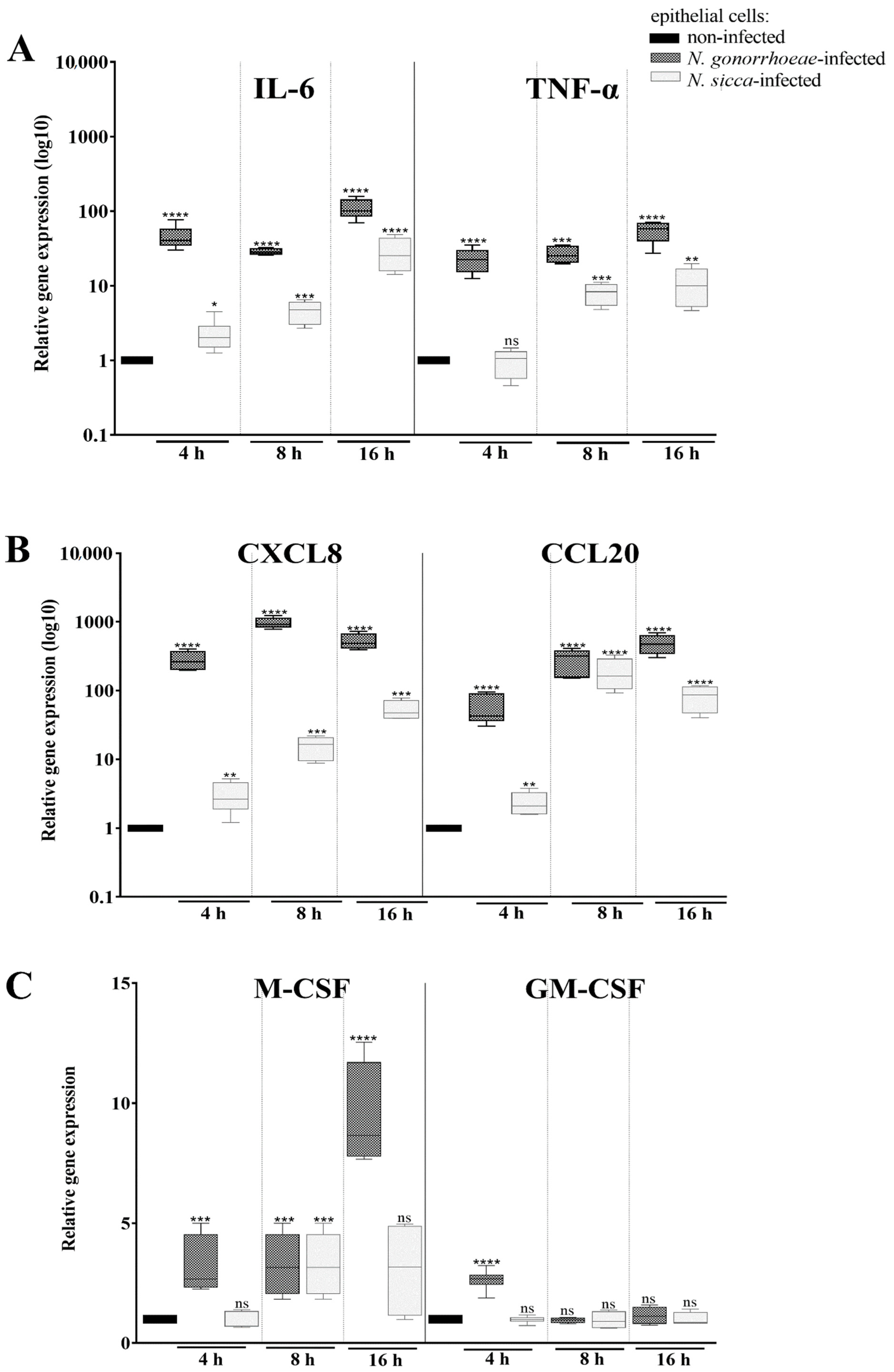
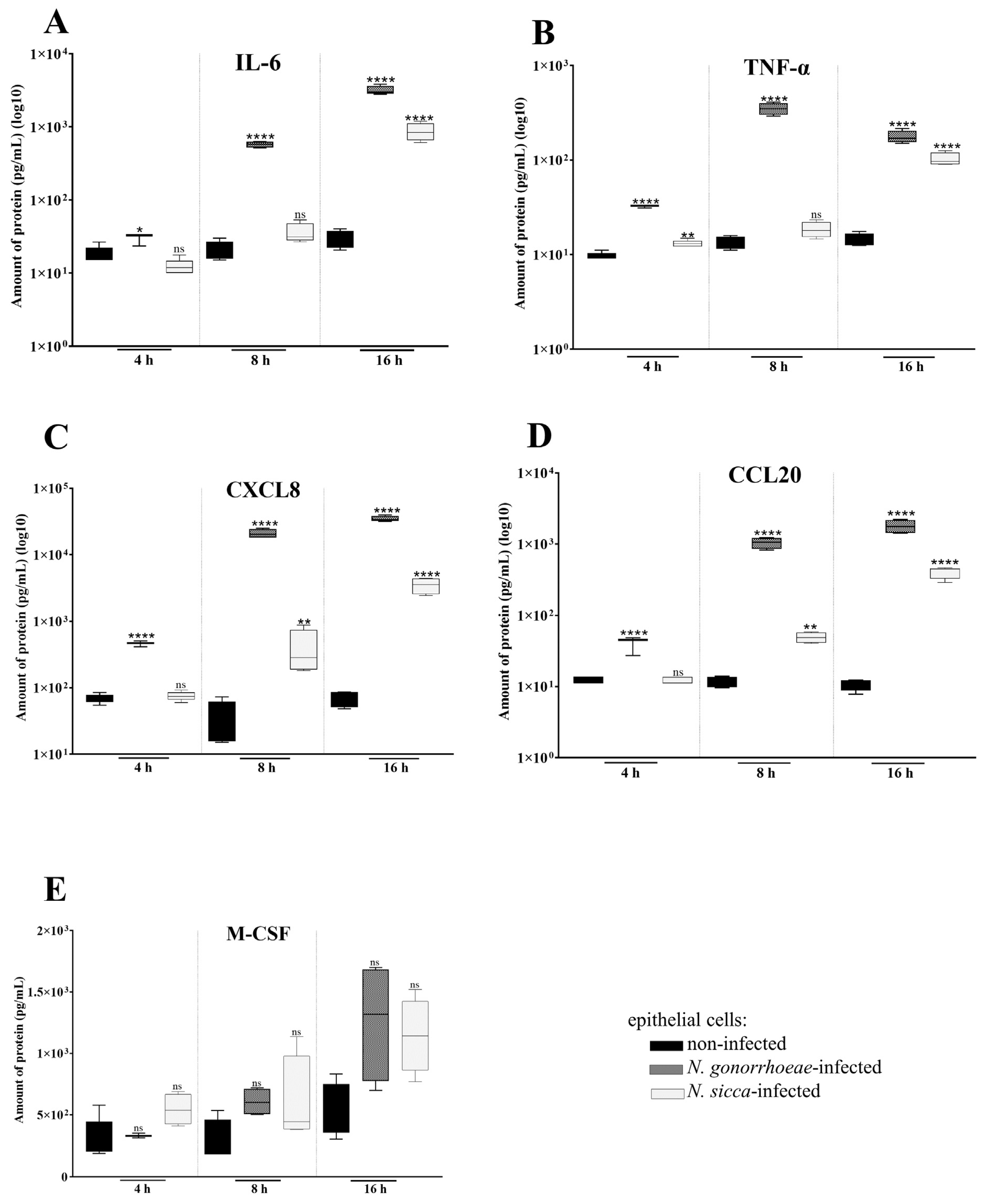
2.1. N. gonorrhoeae and N. sicca Induce an Immune Response in Human Epithelial Cells
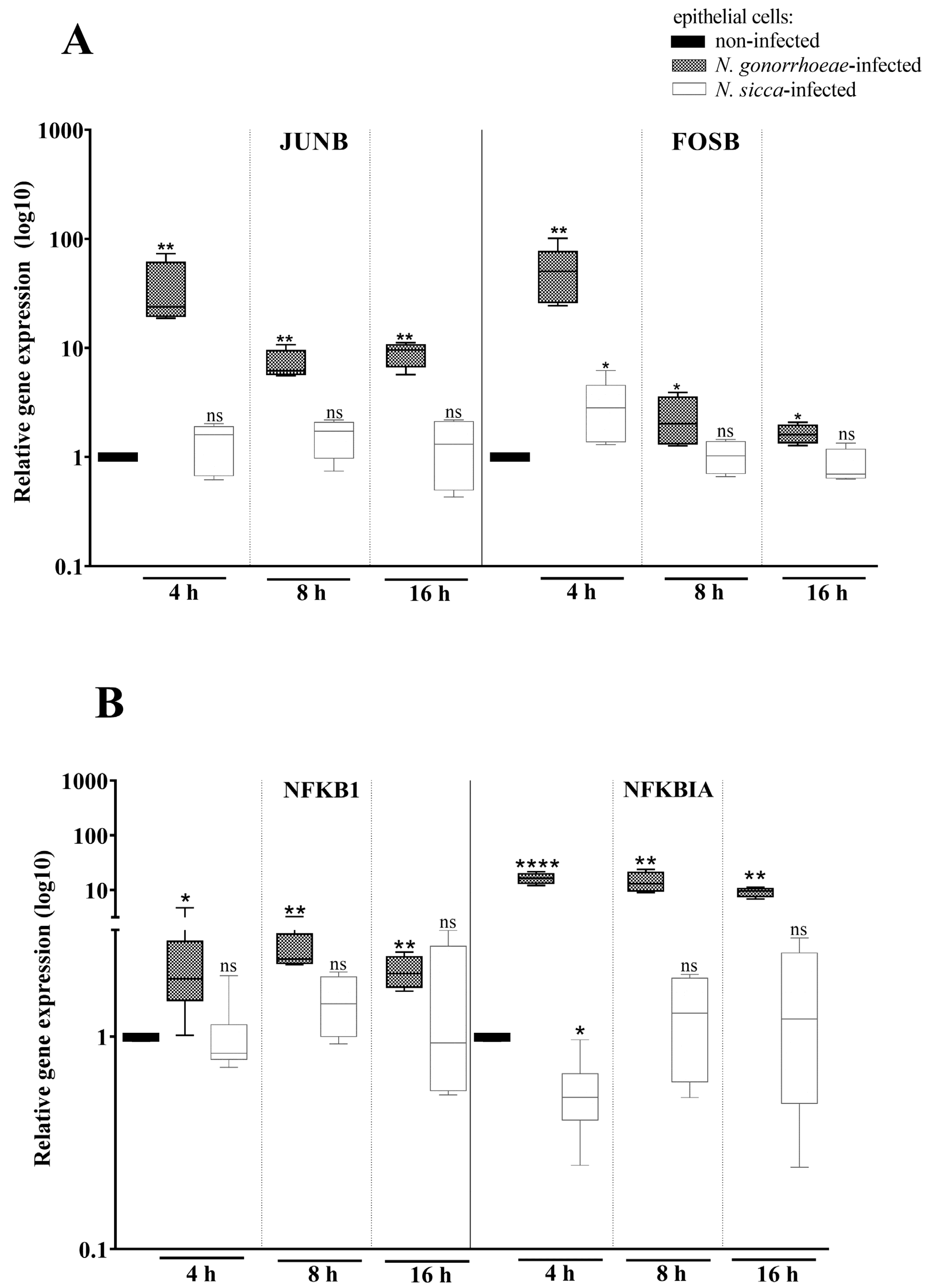
2.2. Expression of AP-1 and NF-κB Transcription Factors Is Affected by the Presence of N. gonorrhoeae and N. sicca
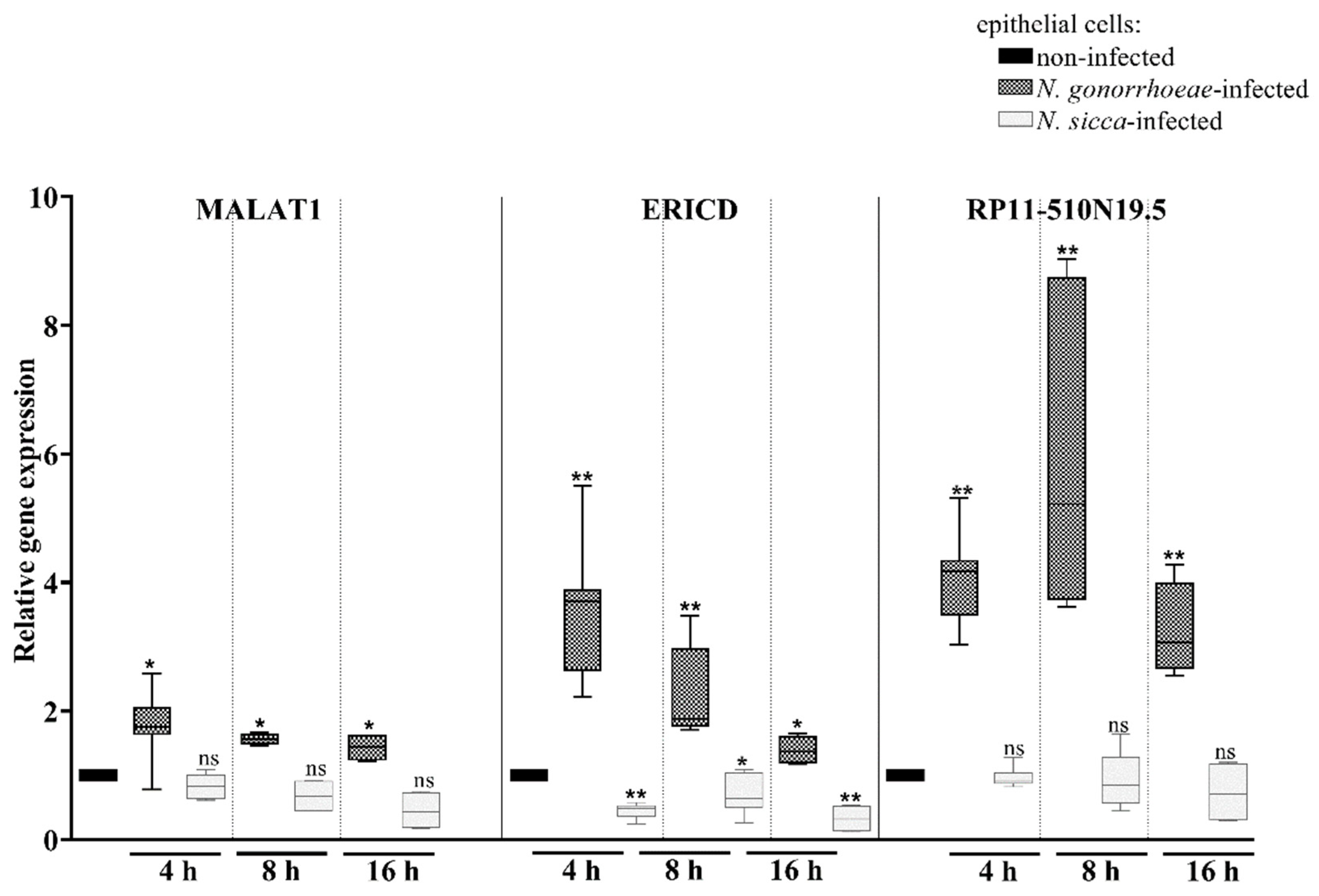
2.3. Long Non-Coding RNAs Are Differentially Expressed Mostly in Human Cells Treated by N. gonorrhoeae Compared to Those Treated by N. sicca
3. Materials and Methods
3.1. Bacterial Culture
3.2. Human Cell Culture and Infection
3.3. Adhesion Assays of Neisseria spp. to Human Epithelial Cells
3.4. RNA Extraction
3.5. Real-Time RT-qPCR
3.6. Cytokine Assay
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hill, S.A.; Masters, T.L.; Wachter, J. Gonorrhea—An evolving disease of the new millennium. Microb. Cell 2016, 3, 371–389. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; World Health Organization: Geneva, Switzerland, 2017; pp. 1–7. [Google Scholar]
- Ding, J.; Rapista, A.; Teleshova, N.; Mosoyan, G.; Jarvis, G.A.; Klotman, M.E.; Chang, T.L. Neisseria gonorrhoeae enhances HIV-1 infection of primary resting CD4+ T cells through TLR2 activation. J. Immunol. 2010, 184, 2814–2824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Abreu, A.L.; Malaguti, N.; Souza, R.P.; Uchimura, N.S.; Ferreira, É.C.; Pereira, M.W.; Carvalho, M.D.; Pelloso, S.M.; Bonini, M.G.; Gimenes, F.; et al. Association of human papillomavirus, Neisseria gonorrhoeae and Chlamydia trachomatis co-infections on the risk of high-grade squamous intraepithelial cervical lesion. Am. J. Cancer Res. 2016, 6, 1371–1383. [Google Scholar]
- Liu, G.; Tang, C.M.; Exley, R.M. Non-pathogenic Neisseria: Members of an abundant, multi-habitat, diverse genus. Microbiology 2015, 161, 1297–1312. [Google Scholar] [CrossRef]
- Kim, W.J.; Higashi, D.; Goytia, M.; Rendón, M.A.; Pilligua-Lucas, M.; Bronnimann, M.; McLean, J.A.; Duncan, J.; Trees, D.; Jerse, A.E.; et al. Commensal Neisseria kill Neisseria gonorrhoeae through a DNA-dependent mechanism. Cell Host Microbe 2019, 26, 228–239. [Google Scholar] [CrossRef]
- Everts, R.J.; Speers, D.; George, S.T.; Ansell, B.J.; Karunajeewa, H.; Ramos, R.D. Neisseria lactamica arthritis and septicemia complicating myeloma. J. Clin. Microbiol. 2010, 48, 2318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Kietzell, M.; Richter, H.; Bruderer, T.; Goldenberger, D.; Emonet, S.; Strahm, C. Meningitis and bacteremia due to Neisseria cinerea following a percutaneous rhizotomy of the trigeminal ganglion. J. Clin. Microbiol. 2016, 54, 233–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abandeh, F.I.; Balada-Llasat, J.M.; Pancholi, P.; Risaliti, C.M.; Maher, W.E.; Bazan, J.A. A rare case of Neisseria bacilliformis native valve endocarditis. Diagn. Microbiol. Infect. Dis. 2012, 73, 378–379. [Google Scholar] [CrossRef]
- Kozlova, A.; Palazzolo, L.; Michael, A. Neisseria sicca: A rare cause of bacterial conjunctivitis. Am. J. Case Rep. 2020, 21, e923135. [Google Scholar] [CrossRef]
- Eser, I.; Akcali, A.; Tatman-Otkun, M.; Taskiran-Comez, A. Conjunctivitis due to Neisseria sicca: A case report. Indian J. Ophthalmol. 2014, 62, 350–352. [Google Scholar] [CrossRef]
- Hadjicharalambous, M.R.; Lindsay, M.A. Long Non-Coding RNAs and the innate immune response. Non-Coding RNA 2019, 5, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duval, M.; Cossart, P.; Lebreton, A. Mammalian microRNAs and long noncoding RNAs in the host-bacterial pathogen crosstalk. Semin. Cell. Dev. Biol. 2017, 65, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Panni, S.; Lovering, R.C.; Porras, P.; Orchard, S. Non-coding RNA regulatory networks. Biochim. Biophys. Acta Gene Regul. Mech. 2020, 1863, 194417. [Google Scholar] [CrossRef] [PubMed]
- Hombach, S.; Kretz, M. Non-coding RNAs: Classification, Biology and Functioning. Adv. Exp. Med. Biol. 2016, 937, 3–17. [Google Scholar]
- Ding, Y.Z.; Zhang, Z.W.; Liu, Y.L.; Shi, C.X.; Zhang, J.; Zhang, Y.G. Relationship of long noncoding RNA and viruses. Genomics 2016, 107, 150–154. [Google Scholar] [CrossRef] [Green Version]
- Naumann, M.; Wessler, S.; Bartsch, C.; Wieland, B.; Meyer, T.F. Neisseria gonorrhoeae epithelial cell interaction leads to the activation of the transcription factors nuclear factor kappaB and activator protein 1 and the induction of inflammatory cytokines. J. Exp. Med. 1997, 186, 247–258. [Google Scholar] [CrossRef]
- Edwards, J.L.; Apicella, M.A. The molecular mechanisms used by Neisseria gonorrhoeae to initiate infection differ between men and women. Clin. Microbiol. Rev. 2004, 17, 965–981. [Google Scholar] [CrossRef] [Green Version]
- Shaw, J.H.; Falkow, S. Model for invasion of human tissue culture cells by Neisseria gonorrhoeae. Infect. Immun. 1988, 56, 1625–1632. [Google Scholar] [CrossRef] [Green Version]
- Hook, E.W., 3rd; Handsfield, H.H. Gonococcal infections in the adult. In Sexually Transmitted Diseases, 3rd ed.; Holmes, K.K., Mårdh, P.A., Sparling, P.F., Lemon, S.M., Stamm, W.E., Piot, P., Wasserheit, J.N., Eds.; McGraw-Hill: New York, NY, USA, 1999; pp. 451–466. [Google Scholar]
- Maisey, K.; Nardocci, G.; Imarai, M.; Cardenas, H.; Rios, M.; Croxatto, H.B.; Heckels, J.E.; Christodoulides, M.; Velasquez, L.A. Expression of proinflammatory cytokines and receptors by human fallopian tubes in organ culture following challenge with Neisseria gonorrhoeae. Infect. Immun. 2003, 71, 527–532. [Google Scholar] [CrossRef] [Green Version]
- Velasquez, L.; García, K.; Morales, F.; Heckels, J.E.; Orihuela, P.; Rodas, P.I.; Christodoulides, M.; Cardenas, H. Neisseria gonorrhoeae pilus attenuates cytokine response of human fallopian tube explants. J. Biomed. Biotechnol. 2012, 2012, 491298. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.C.; Eckmann, L.; Yang, S.K.; Panja, A.; Fierer, J.; Morzycka-Wroblewska, E.; Kagnoff, M.F. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J. Clin. Investig. 1995, 95, 55–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Wetzler, L.M.; Nascimento, L.O.; Massari, P. Human airway epithelial cell responses to Neisseria lactamica and purified porin via Toll-like receptor 2-dependent signaling. Infect. Immun. 2010, 78, 5314–5323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Wetzler, L.M.; Massari, P. The PorB porin from commensal Neisseria lactamica induces Th1 and Th2 immune responses to ovalbumin in mice and is a potential immune adjuvant. Vaccine 2008, 26, 786–796. [Google Scholar] [CrossRef] [Green Version]
- Powell, D.A.; Ma, M.; So, M.; Frelinger, J.A. The commensal Neisseria musculi modulates host innate immunity to promote oral colonization. ImmunoHorizons 2018, 2, 305–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haller, D.; Bode, C.; Hammes, W.P.; Pfeifer, A.M.; Schiffrin, E.J.; Blum, S. Non-pathogenic bacteria elicit a differential cytokine response by intestinal epithelial cell/leucocyte co-cultures. Gut 2000, 47, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Hughes, C.E.; Nibbs, R.J.B. A guide to chemokines and their receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef]
- Sanyal, A.; Shen, C.; Ding, M.; Reinhart, T.A.; Chen, Y.; Sankapal, S.; Gupta, P. Neisseria gonorrhoeae uses cellular proteins CXCL10 and IL8 to enhance HIV-1 transmission across cervical mucosa. Am. J. Reprod. Immunol. 2019, 81, e13111. [Google Scholar] [CrossRef] [Green Version]
- Hess, J.; Angel, P.; Schorpp-Kistner, M. AP-1 subunits: Quarrel and harmony among siblings. J. Cell Sci. 2004, 117, 5965–5973. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, J.C.R.; Acuña, S.M.; Aoki, J.I.; Floeter-Winter, L.M.; Muxel, S.M. Long Non-Coding RNAs in the regulation of gene expression: Physiology and disease. Non-Coding RNA 2019, 5, 17. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Huang, C.; Meng, X.; Li, J. Long Noncoding RNA MALAT1: Insights into its biogenesis and implications in human disease. Curr. Pharm. Des. 2015, 21, 5017–5028. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Su, Z.; Mookhtiar, A.K. Long non-coding RNA: A versatile regulator of the nuclear factor-κB signalling circuit. Immunology 2017, 150, 379–388. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.; Su, Z.; Song, D.; Mao, Y.; Mao, X. The long noncoding RNA MALAT1 regulates the lipopolysaccharide-induced inflammatory response through its interaction with NF-κB. FEBS Lett. 2016, 590, 2884–2895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arman, K.; Saadat, K.; Igci, Y.Z.; Bozgeyik, E.; Ikeda, M.A.; Cakmak, E.A.; Arslan, A. Long noncoding RNA ERICD interacts with ARID3A via E2F1 and regulates migration and proliferation of osteosarcoma cells. Cell Biol. Int. 2020, 44, 2263–2274. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhong, H.; Xie, X.; Chen, C.Y.; Huang, D.; Shen, L.; Zhang, H.; Chen, Z.W.; Zeng, G. Long noncoding RNA derived from CD244 signaling epigenetically controls CD8+ T-cell immune responses in tuberculosis infection. Proc. Natl. Acad. Sci. USA 2015, 112, E3883. [Google Scholar] [CrossRef] [Green Version]
- Kawaguchi, T.; Hirose, T. Chromatin remodeling complexes in the assembly of long noncoding RNA-dependent nuclear bodies. Nucleus 2015, 6, 462–467. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Xu, X.; Xue, J.; Duan, W.; Yi, Z. Deregulated lncRNAs in B cells from patients with active tuberculosis. PLoS ONE 2017, 12, e0170712. [Google Scholar] [CrossRef]
- Sharbati, S.; Ravon, F.; Einspanier, R.; zur Bruegge, J. Mycobacterium smegmatis but not Mycobacterium avium subsp. hominissuis causes increased expression of the Long Non-Coding RNA MEG3 in THP-1-derived human macrophages and associated decrease of TGF-β. Microorganisms 2019, 7, 63. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Jiang, Y.; Xu, X.; Su, X.; Liu, Y.; Ma, Y.; Zhao, Y.; Shen, Z.; Huang, B.; Cao, X. Inducible degradation of lncRNA Sros1 promotes IFN-γ-mediated activation of innate immune responses by stabilizing Stat1 mRNA. Nat. Immunol. 2019, 20, 1621–1630. [Google Scholar] [CrossRef]
- Chan, J.; Atianand, M.; Jiang, Z.; Carpenter, S.; Aiello, D.; Elling, R.; Fitzgerald, K.A.; Caffrey, D.R. Cutting Edge: A natural antisense transcript, AS-IL1α, controls inducible transcription of the proinflammatory cytokine IL-1α. J. Immunol. 2015, 195, 1359–1363. [Google Scholar] [CrossRef] [Green Version]
- Hu, G.; Gong, A.-Y.; Wang, Y.; Ma, S.; Chen, X.; Chen, J.; Su, C.-J.; Shibata, A.; Strauss-Soukup, J.K.; Drescher, K.M.; et al. LincRNA-Cox2 promotes late inflammatory gene transcription in macrophages through modulating SWI/SNF-mediated chromatin remodeling. J. Immunol. 2016, 196, 2799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Shuai, T.; Li, B.; Zhu, L.; Li, X. Long non-coding RNA lnc-GNAT1-1 inhibits gastric cancer cell proliferation and invasion through the Wnt/β-catenin pathway in Helicobacter pylori infection. Mol. Med. Rep. 2018, 18, 4009–4015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawar, K.; Hanisch, C.; Palma Vera, S.E.; Einspanier, R.; Sharbati, S. Down regulated lncRNA MEG3 eliminates mycobacteria in macrophages via autophagy. Sci. Rep. 2016, 6, 19416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, P.; Peter, S.; Jung, M.; Lewin, A.; Hemmrich-Stanisak, G.; Franke, A.; von Kleist, M.; Schütte, C.; Einspanier, R.; Sharbati, S.; et al. Analysis of long non-coding RNA and mRNA expression in bovine macrophages brings up novel aspects of Mycobacterium avium subspecies paratuberculosis infections. Sci. Rep. 2019, 9, 1571. [Google Scholar] [CrossRef] [Green Version]
- Falvo, J.V.; Tsytsykova, A.V.; Goldfeld, A.E. Transcriptional control of the TNF gene. Curr. Dir. Autoimmun. 2010, 11, 27–60. [Google Scholar]
- Dillard, J.P. Genetic Manipulation of Neisseria gonorrhoeae. Curr. Protoc. Microbiol. 2011, 23, 4A-2. [Google Scholar]
- Płaczkiewicz, J.; Adamczyk-Popławska, M.; Lasek, R.; Bącal, P.; Kwiatek, A. Inactivation of genes encoding MutL and MutS proteins influences adhesion and biofilm formation by Neisseria gonorrhoeae. Microorganisms 2019, 7, 647. [Google Scholar] [CrossRef] [Green Version]
- Płaczkiewicz, J.; Chmiel, P.; Malinowska, E.; Bącal, P.; Kwiatek, A. Lactobacillus crispatus and its enolase and glutamine synthetase influence interactions between Neisseria gonorrhoeae and human epithelial cells. J. Microbiol. 2020, 58, 405–414. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Płaczkiewicz, J.; Adamczyk-Popławska, M.; Kozłowska, E.; Kwiatek, A. Both Neisseria gonorrhoeae and Neisseria sicca Induce Cytokine Secretion by Infected Human Cells, but Only Neisseria gonorrhoeae Upregulates the Expression of Long Non-Coding RNAs. Pathogens 2022, 11, 394. https://doi.org/10.3390/pathogens11040394
Płaczkiewicz J, Adamczyk-Popławska M, Kozłowska E, Kwiatek A. Both Neisseria gonorrhoeae and Neisseria sicca Induce Cytokine Secretion by Infected Human Cells, but Only Neisseria gonorrhoeae Upregulates the Expression of Long Non-Coding RNAs. Pathogens. 2022; 11(4):394. https://doi.org/10.3390/pathogens11040394
Chicago/Turabian StylePłaczkiewicz, Jagoda, Monika Adamczyk-Popławska, Ewa Kozłowska, and Agnieszka Kwiatek. 2022. "Both Neisseria gonorrhoeae and Neisseria sicca Induce Cytokine Secretion by Infected Human Cells, but Only Neisseria gonorrhoeae Upregulates the Expression of Long Non-Coding RNAs" Pathogens 11, no. 4: 394. https://doi.org/10.3390/pathogens11040394





