Pathophysiology of Acute Kidney Injury in Malaria and Non-Malarial Febrile Illness: A Prospective Cohort Study
Abstract
:1. Introduction
2. Results
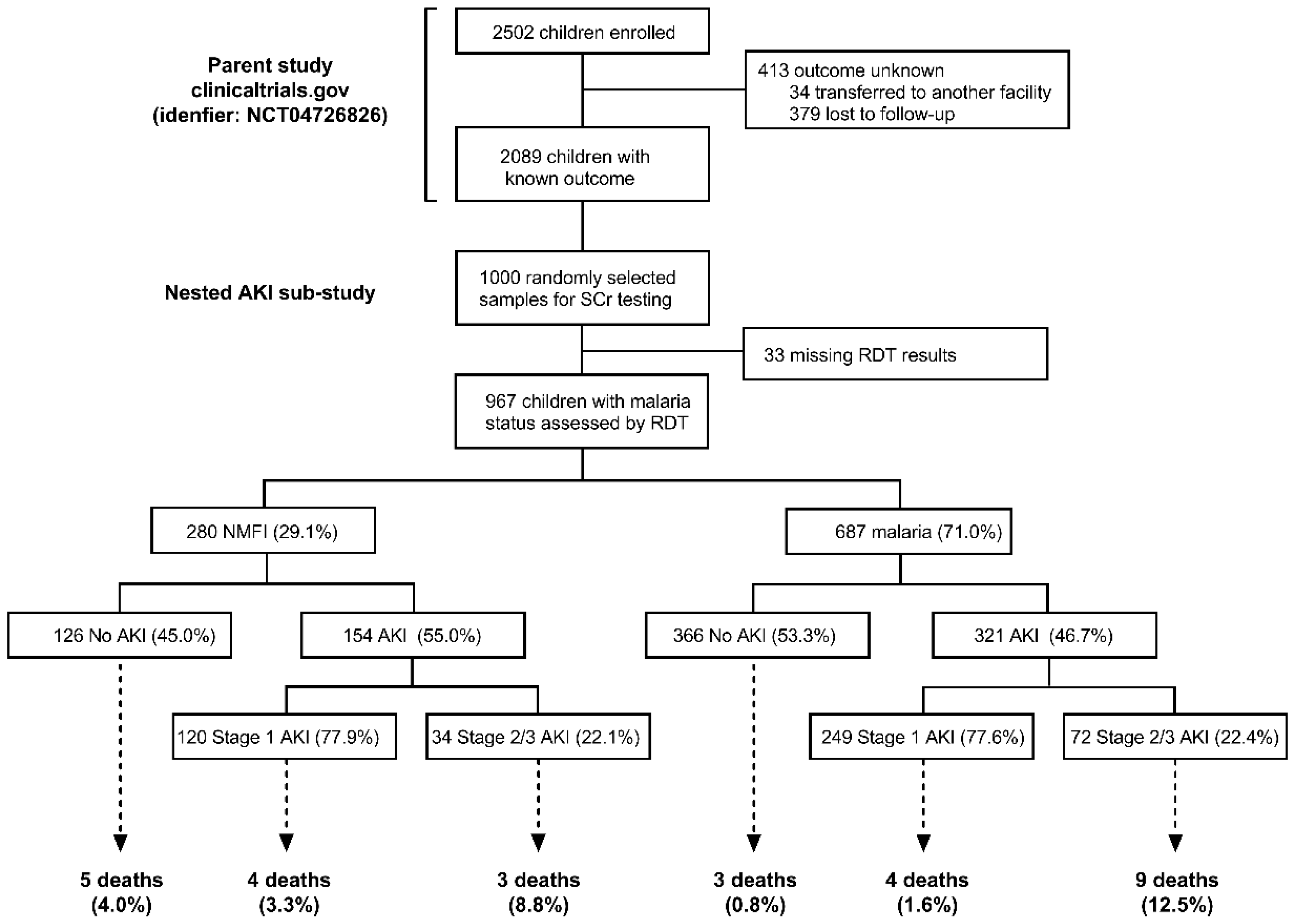
2.1. Prevalence of AKI in Malaria vs. NMFI
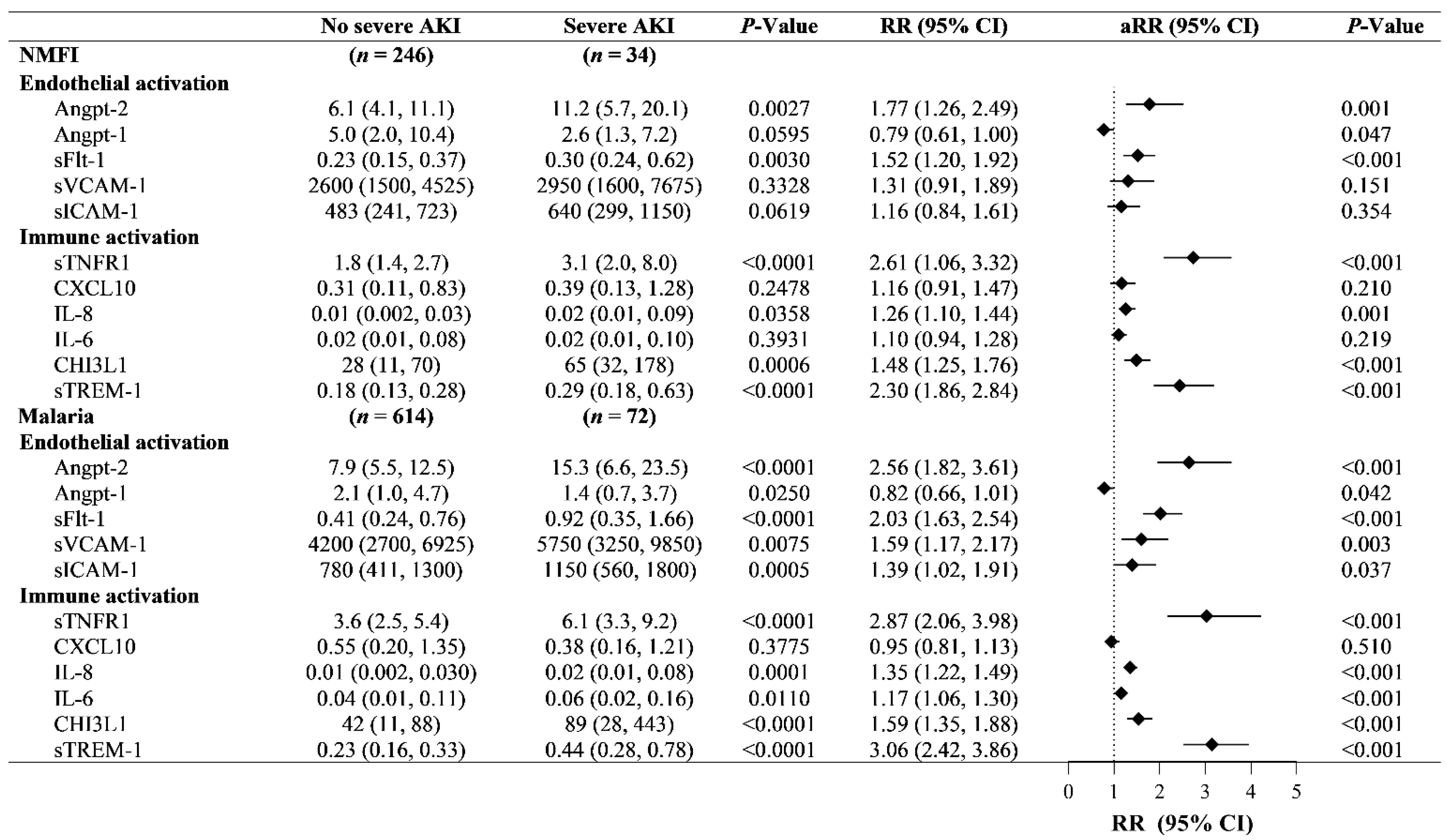
2.2. Immune and Endothelial Activation in AKI in Children with Malaria vs. NMFI
2.3. Relationship between AKI and Mortality
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Study Design
4.3. Defining Acute Kidney Injury
4.4. Measurement of Biomarkers of Host Response to Infection
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehta, R.L.; Cerda, J.; Burdmann, E.A.; Tonelli, M.; Garcia–Garcia, G.; Jha, V.; Susantitaphong, P.; Rocco, M.; Vanholder, R.; Sever, M.S.; et al. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): A human rights case for nephrology. Lancet 2015, 385, 2616–2643. [Google Scholar] [CrossRef]
- Olowu, W.A.; Niang, A.; Osafo, C.; Ashuntantang, G.; Arogundade, F.A.; Porter, J.; Naicker, S.; Luyckx, V.A. Outcomes of acute kidney injury in children and adults in sub–Saharan Africa: A systematic review. Lancet Glob. Health 2016, 4, e242–e250. [Google Scholar] [CrossRef] [Green Version]
- Jha, V.; Parameswaran, S. Community–acquired acute kidney injury in tropical countries. Nat. Rev. Nephrol. 2013, 9, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Conroy, A.L.; Hawkes, M.; Elphinstone, R.E.; Morgan, C.; Hermann, L.; Barker, K.R.; Namasopo, S.; Opoka, R.O.; John, C.C.; Liles, W.C.; et al. Acute Kidney Injury Is Common in Pediatric Severe Malaria and Is Associated With Increased Mortality. Open Forum Infect. Dis. 2016, 3, ofw046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conroy, A.L.; Opoka, R.O.; Bangirana, P.; Idro, R.; Ssenkusu, J.M.; Datta, D.; Hodges, J.S.; Morgan, C.; John, C.C. Acute kidney injury is associated with impaired cognition and chronic kidney disease in a prospective cohort of children with severe malaria. BMC Med. 2019, 17, 98. [Google Scholar] [CrossRef] [PubMed]
- Oshomah–Bello, E.O.; Esezobor, C.I.; Solarin, A.U.; Njokanma, F.O. Acute Kidney Injury in Children with Severe Malaria Is Common and Associated with Adverse Hospital Outcomes. J. Trop. Pediatr. 2020, 66, 218–225. [Google Scholar] [CrossRef]
- Afolayan, F.M.; Adedoyin, O.T.; Abdulkadir, M.B.; Ibrahim, O.R.; Biliaminu, S.A.; Mokuolu, O.A.; Ojuawo, A. Acute Kidney Injuries in Children with Severe Malaria: A comparative study of diagnostic criteria based on serum cystatin C and creatinine levels. Sultan Qaboos Univ. Med. J. 2020, 20, e312–e317. [Google Scholar] [CrossRef] [PubMed]
- Kunuanunua, T.S.; Nsibu, C.N.; Gini–Ehungu, J.L.; Bodi, J.M.; Ekulu, P.M.; Situakibanza, H.; Nseka, N.M.; Magoga, K.; Aloni, M.N. Acute renal failure and severe malaria in Congolese children living in Kinshasa, Democratic Republic of Congo. Nephrol. Ther. 2013, 9, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Batte, A.; Berrens, Z.; Murphy, K.; Mufumba, I.; Sarangam, M.L.; Hawkes, M.T.; Conroy, A.L. Malaria–Associated Acute Kidney Injury in African Children: Prevalence, Pathophysiology, Impact, and Management Challenges. Int. J. Nephrol. Renov. Dis. 2021, 14, 235–253. [Google Scholar] [CrossRef]
- KDIGO. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar] [CrossRef] [Green Version]
- Robinson, C.H.; Jeyakumar, N.; Luo, B.; Wald, R.; Garg, A.X.; Nash, D.M.; McArthur, E.; Greenberg, J.H.; Askenazi, D.; Mammen, C.; et al. Long–Term Kidney Outcomes Following Dialysis–Treated Childhood Acute Kidney Injury: A Population–Based Cohort Study. J. Am. Soc. Nephrol. 2021, 32, 2005–2019. [Google Scholar] [CrossRef] [PubMed]
- Namazzi, R.; Batte, A.; Opoka, R.O.; Bangirana, P.; Schwaderer, A.L.; Berrens, Z.; Datta, D.; Goings, M.; Ssenkusu, J.M.; Goldstein, S.L.; et al. Acute kidney injury, persistent kidney disease, and post–discharge morbidity and mortality in severe malaria in children: A prospective cohort study. eClinicalMedicine 2022, 44, 101292. [Google Scholar] [CrossRef]
- Mammen, C.; Al Abbas, A.; Skippen, P.; Nadel, H.; Levine, D.; Collet, J.P.; Matsell, D.G. Long–term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: A prospective cohort study. Am. J. Kidney Dis. 2012, 59, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Coca, S.G.; Singanamala, S.; Parikh, C.R. Chronic kidney disease after acute kidney injury: A systematic review and meta–analysis. Kidney Int. 2012, 81, 442–448. [Google Scholar] [CrossRef] [Green Version]
- Heung, M.; Chawla, L.S. Predicting progression to chronic kidney disease after recovery from acute kidney injury. Curr. Opin. Nephrol. Hypertens. 2012, 21, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Romagnani, P.; Ashuntantang, G.; Ronco, C.; Zarbock, A.; Anders, H.-J. Acute kidney injury. Nat. Rev. Dis. Primers 2021, 7, 52. [Google Scholar] [CrossRef]
- Fitzgerald, J.C.; Basu, R.K.; Akcan–Arikan, A.; Izquierdo, L.M.; Piñeres Olave, B.E.; Hassinger, A.B.; Szczepanska, M.; Deep, A.; Williams, D.; Sapru, A.; et al. Acute Kidney Injury in Pediatric Severe Sepsis: An Independent Risk Factor for Death and New Disability. Crit. Care Med. 2016, 44, 2241–2250. [Google Scholar] [CrossRef] [PubMed]
- Starr, M.C.; Banks, R.; Reeder, R.W.; Fitzgerald, J.C.; Pollack, M.M.; Meert, K.L.; McQuillen, P.S.; Mourani, P.M.; Chima, R.S.; Sorenson, S.; et al. Severe Acute Kidney Injury Is Associated With Increased Risk of Death and New Morbidity After Pediatric Septic Shock. Pediatr. Crit. Care Med. 2020, 21, e686–e695. [Google Scholar] [CrossRef] [PubMed]
- Hickson, M.R.; Conroy, A.L.; Bangirana, P.; Opoka, R.O.; Idro, R.; Ssenkusu, J.M.; John, C.C. Acute kidney injury in Ugandan children with severe malaria is associated with long–term behavioral problems. PLoS ONE 2019, 14, e0226405. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Monneret, G.; Payen, D. Immunosuppression in sepsis: A novel understanding of the disorder and a new therapeutic approach. Lancet Infect. Dis. 2013, 13, 260–268. [Google Scholar] [CrossRef] [Green Version]
- Leligdowicz, A.; Richard–Greenblatt, M.; Wright, J.; Crowley, V.M.; Kain, K.C. Endothelial Activation: The Ang/Tie Axis in Sepsis. Front. Immunol. 2018, 9, 838. [Google Scholar] [CrossRef] [Green Version]
- Peerapornratana, S.; Manrique–Caballero, C.L.; Gómez, H.; Kellum, J.A. Acute kidney injury from sepsis: Current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019, 96, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Molema, G.; Zijlstra, J.G.; van Meurs, M.; Kamps, J.A.A.M. Renal microvascular endothelial cell responses in sepsis–induced acute kidney injury. Nat. Rev. Nephrol. 2021, 18, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Odum, J.D.; Wong, H.R.; Stanski, N.L. A Precision Medicine Approach to Biomarker Utilization in Pediatric Sepsis–Associated Acute Kidney Injury. Front. Pediatr. 2021, 9, 313. [Google Scholar] [CrossRef]
- Conroy, A.L.; Hawkes, M.; McDonald, C.R.; Kim, H.; Higgins, S.J.; Barker, K.R.; Namasopo, S.; Opoka, R.O.; John, C.C.; Liles, W.C.; et al. Host Biomarkers Are Associated With Response to Therapy and Long–Term Mortality in Pediatric Severe Malaria. Open Forum. Infect. Dis. 2016, 3, ofw134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erdman, L.K.; Dhabangi, A.; Musoke, C.; Conroy, A.L.; Hawkes, M.; Higgins, S.; Rajwans, N.; Wolofsky, K.T.; Streiner, D.L.; Liles, W.C.; et al. Combinations of host biomarkers predict mortality among Ugandan children with severe malaria: A retrospective case–control study. PLoS ONE 2011, 6, e17440. [Google Scholar] [CrossRef] [PubMed]
- Ricciuto, D.R.; dos Santos, C.C.; Hawkes, M.; Toltl, L.J.; Conroy, A.L.; Rajwans, N.; Lafferty, E.I.; Cook, D.J.; Fox-Robichaud, A.; Kahnamoui, K.; et al. Angiopoietin–1 and angiopoietin–2 as clinically informative prognostic biomarkers of morbidity and mortality in severe sepsis. Crit. Care Med. 2011, 39, 702–710. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Severe Malaria. Trop Med. Int. Health 2014, 19, 7–131. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Ouma, B.J.; Ssenkusu, J.M.; Shabani, E.; Datta, D.; Opoka, R.O.; Idro, R.; Bangirana, P.; Park, G.; Joloba, M.L.; Kain, K.C.; et al. Endothelial Activation, Acute Kidney Injury, and Cognitive Impairment in Pediatric Severe Malaria. Crit. Care Med. 2020, 48, e734–e743. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.K.; Wong, H.R.; Krawczeski, C.D.; Wheeler, D.S.; Manning, P.B.; Chawla, L.S.; Devarajan, P.; Goldstein, S.L. Combining functional and tubular damage biomarkers improves diagnostic precision for acute kidney injury after cardiac surgery. J. Am. Coll. Cardiol. 2014, 64, 2753–2762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elphinstone, R.E.; Conroy, A.L.; Hawkes, M.; Hermann, L.; Namasopo, S.; Warren, H.S.; John, C.C.; Liles, W.C.; Kain, K.C. Alterations in Systemic Extracellular Heme and Hemopexin Are Associated With Adverse Clinical Outcomes in Ugandan Children With Severe Malaria. J. Infect. Dis. 2016, 214, 1268–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, S.K.; Das, B.S. Malaria and acute kidney injury. Semin. Nephrol. 2008, 28, 395–408. [Google Scholar] [CrossRef]
- Yeo, T.W.; Lampah, D.A.; Gitawati, R.; Tjitra, E.; Kenangalem, E.; Piera, K.; Price, R.N.; Duffull, S.B.; Celermajer, D.S.; Anstey, N.M. Angiopoietin–2 is associated with decreased endothelial nitric oxide and poor clinical outcome in severe falciparum malaria. Proc. Natl. Acad. Sci. USA 2008, 105, 17097–17102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conroy, A.L.; Glover, S.J.; Hawkes, M.; Erdman, L.K.; Seydel, K.B.; Taylor, T.E.; Molyneux, M.E.; Kain, K.C. Angiopoietin–2 levels are associated with retinopathy and predict mortality in Malawian children with cerebral malaria: A retrospective case–control study*. Crit. Care Med. 2012, 40, 952–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouma, B.J.; Bangirana, P.; Ssenkusu, J.M.; Datta, D.; Opoka, R.O.; Idro, R.; Kain, K.C.; John, C.C.; Conroy, A.L. Plasma angiopoietin–2 is associated with age–related deficits in cognitive sub–scales in Ugandan children following severe malaria. Malar. J. 2021, 20, 17. [Google Scholar] [CrossRef] [PubMed]
- Robinson–Cohen, C.; Katz, R.; Price, B.L.; Harju–Baker, S.; Mikacenic, C.; Himmelfarb, J.; Liles, W.C.; Wurfel, M.M. Association of markers of endothelial dysregulation Ang1 and Ang2 with acute kidney injury in critically ill patients. Crit. Care 2016, 20, 207. [Google Scholar] [CrossRef] [Green Version]
- Bhatraju, P.K.; Cohen, M.; Nagao, R.J.; Morrell, E.D.; Kosamo, S.; Chai, X.Y.; Nance, R.; Dmyterko, V.; Delaney, J.; Christie, J.D.; et al. Genetic variation implicates plasma angiopoietin–2 in the development of acute kidney injury sub–phenotypes. BMC Nephrol. 2020, 21, 284. [Google Scholar] [CrossRef]
- Ostermann, M.; Zarbock, A.; Goldstein, S.; Kashani, K.; Macedo, E.; Murugan, R.; Bell, M.; Forni, L.; Guzzi, L.; Joannidis, M.; et al. Recommendations on Acute Kidney Injury Biomarkers From the Acute Disease Quality Initiative Consensus Conference: A Consensus Statement. JAMA Netw. Open 2020, 3, e2019209. [Google Scholar] [CrossRef]
- Richard–Greenblatt, M.; Boillat–Blanco, N.; Zhong, K.; Mbarack, Z.; Samaka, J.; Mlaganile, T.; Kazimoto, T.; D’Acremont, V.; Kain, K.C. Prognostic Accuracy of Soluble Triggering Receptor Expressed on Myeloid Cells (sTREM–1)–based Algorithms in Febrile Adults Presenting to Tanzanian Outpatient Clinics. Clin. Infect. Dis. 2020, 70, 1304–1312. [Google Scholar] [CrossRef]
- Anderson, B.J.; Calfee, C.S.; Liu, K.D.; Reilly, J.P.; Kangelaris, K.N.; Shashaty, M.G.S.; Lazaar, A.L.; Bayliffe, A.I.; Gallop, R.J.; Miano, T.A.; et al. Plasma sTNFR1 and IL8 for prognostic enrichment in sepsis trials: A prospective cohort study. Crit. Care 2019, 23, 400. [Google Scholar] [CrossRef] [Green Version]
- Conroy, A.L.; Hawkes, M.T.; Elphinstone, R.; Opoka, R.O.; Namasopo, S.; Miller, C.; John, C.C.; Kain, K.C. Chitinase–3–like 1 is a biomarker of acute kidney injury and mortality in paediatric severe malaria. Malar. J. 2018, 17, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erdman, L.K.; Petes, C.; Lu, Z.; Dhabangi, A.; Musoke, C.; Cserti–Gazdewich, C.M.; Lee, C.G.; Liles, W.C.; Elias, J.A.; Kain, K.C. Chitinase 3–like 1 is induced by Plasmodium falciparum malaria and predicts outcome of cerebral malaria and severe malarial anaemia in a case–control study of African children. Malar. J. 2014, 13, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leligdowicz, A.; Conroy, A.L.; Hawkes, M.; Richard–Greenblatt, M.; Zhong, K.; Opoka, R.O.; Namasopo, S.; Bell, D.; Liles, W.C.; da Costa, B.R.; et al. Risk–stratification of febrile African children at risk of sepsis using sTREM–1 as basis for a rapid triage test. Nat. Commun. 2021, 12, 6832. [Google Scholar] [CrossRef] [PubMed]
- Soranno, D.E.; Kirkbride–Romeo, L.; Wennersten, S.A.; Ding, K.; Cavasin, M.A.; Baker, P.; Altmann, C.; Bagchi, R.A.; Haefner, K.R.; Steinkühler, C.; et al. Acute Kidney Injury Results in Long–Term Diastolic Dysfunction That Is Prevented by Histone Deacetylase Inhibition. JACC Basic Transl. Sci. 2021, 6, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Raimann, J.G.; Riella, M.C.; Levin, N.W. International Society of Nephrology’s 0by25 initiative (zero preventable deaths from acute kidney injury by 2025): Focus on diagnosis of acute kidney injury in low–income countries. Clin. Kidney J. 2018, 11, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Conroy, A.L.; Hawkes, M.; Hayford, K.; Namasopo, S.; Opoka, R.O.; John, C.C.; Liles, W.C.; Kain, K.C. Prospective validation of pediatric disease severity scores to predict mortality in Ugandan children presenting with malaria and non–malaria febrile illness. Crit. Care 2015, 19, 47. [Google Scholar] [CrossRef] [Green Version]
- Hawkes, M.; Conroy, A.L.; Opoka, R.O.; Namasopo, S.; Liles, W.C.; John, C.C.; Kain, K.C. Use of a three–band HRP2/pLDH combination rapid diagnostic test increases diagnostic specificity for falciparum malaria in Ugandan children. Malar. J. 2014, 13, 43. [Google Scholar] [CrossRef] [Green Version]
- Batte, A.; Starr, M.C.; Schwaderer, A.L.; Opoka, R.O.; Namazzi, R.; Phelps Nishiguchi, E.S.; Ssenkusu, J.M.; John, C.C.; Conroy, A.L. Methods to estimate baseline creatinine and define acute kidney injury in lean Ugandan children with severe malaria: A prospective cohort study. BMC Nephrol. 2020, 21, 417. [Google Scholar] [CrossRef]
- Kaddourah, A.; Basu, R.K.; Bagshaw, S.M.; Goldstein, S.L.; Investigators, A. Epidemiology of Acute Kidney Injury in Critically Ill Children and Young Adults. N. Engl. J. Med. 2017, 376, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Leligdowicz, A.; Conroy, A.L.; Hawkes, M.; Zhong, K.; Lebovic, G.; Matthay, M.A.; Kain, K.C. Validation of two multiplex platforms to quantify circulating markers of inflammation and endothelial injury in severe infection. PLoS ONE 2017, 12, e0175130. [Google Scholar] [CrossRef] [Green Version]

| N | Cohort (n = 967) | NMFI (n = 280) | Malaria (n = 687) | p-Value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, years | 964 | 1.7 (1.1) | 1.6 (1.1) | 1.7 (1.1) | 0.065 |
| Female sex, n (%) | 957 | 428 (44.7) | 129 (46.2) | 299 (44.1) | 0.55 |
| Weight, kg | 963 | 9.8 (3.1) | 9.5 (2.8) | 9.9 (3.2) | 0.055 |
| Height, cm | 948 | 74.0 (11.6) | 72.8 (11.8) | 74.5 (11.4) | 0.034 |
| Medication history | |||||
| Antimalarial n (%) | 956 | 444 (46.4) | 145 (52.2) | 299 (44.1) | 0.023 |
| Antibiotic, n (%) | 954 | 325 (34.1) | 114 (41.0) | 211 (31.2) | 0.004 |
| Infection status | |||||
| HIV, n (%) | 966 | 20 (2.1) | 12 (4.3) | 8 (1.2) | 0.002 |
| Clinical signs and symptoms | |||||
| Axillary Temperature in °C | 954 | 37.9 (1.2) | 38.0 (1.1) | 37.8 (1.1) | 0.05 |
| Systolic Blood Pressure, mmHg | 931 | 105 (16) | 104 (16) | 105 (15) | 0.14 |
| Diastolic Blood Pressure, mmHg | 929 | 58 (13) | 58 (13) | 57 (13) | 0.35 |
| Heart Rate | 960 | 160 (25) | 156 (25) | 162 (24) | 0.0009 |
| Respiratory Rate | 928 | 46 (15) | 46 (15) | 45 (14) | 0.52 |
| SpO2 % | 960 | 96.8 (5.2) | 95.9 (5.8) | 97.2 (4.9) | 0.0003 |
| Capillary refill time > 2 s, n (%) | 940 | 130 (13.8) | 33 (11.9) | 97 (14.6) | 0.27 |
| Unable to drink or breastfeed, n (%) | 961 | 184 (19.2) | 49 (17.6) | 135 (19.8) | 0.45 |
| Vomiting, n (%) | 963 | 293 (30.4) | 87 (31.2) | 206 (30.1) | 0.74 |
| Diarrhea, n (%) | 964 | 289 (30.0) | 108 (38.9) | 181 (26.4) | <0.0001 |
| Respiratory distress, n (%) | 967 | 309 (32.0) | 88 (31.4) | 221 (32.2) | 0.82 |
| Prostration, n (%) | 964 | 219 (22.7) | 52 (18.6) | 167 (24.4) | 0.049 |
| Coma (BCS < 3) n (%) | 949 | 49 (5.2) | 9 (3.3) | 40 (5.9) | 0.09 |
| Altered consciousness, n (%) | 961 | 134 (13.9) | 30 (10.7) | 104 (15.3) | 0.064 |
| Convulsions, n (%) | 966 | 170 (17.6) | 41 (14.6) | 129 (18.8) | 0.12 |
| Jaundice, n (%) | 966 | 104 (10.8) | 16 (5.7) | 88 (12.8) | 0.001 |
| Severe anemia (Hb < 5.0 g/dL), n (%) | |||||
| No Yes Missing | 967 | 182 (18.8) 203 (21.0) 582 (60.2) | 43 (15.4) 40 (14.3) 197 (70.4) | 139 (20.2) 163 (23.7) 385 (56.0) | <0.0001 |
| AKI, n (%) | 967 | 475 (49.1) | 154 (55.0) | 321 (46.7) | 0.02 |
| Severe AKI (Stage 2 or 3), n (%) | 967 | 106 (11.0) | 34 (12.1) | 72 (10.5) | 0.45 |
| Positive Cystatin C (≥0.8 mg/L), n (%) | 967 | 188 (19.4) | 73 (26.1) | 115 (16.7) | <0.001 |
| LOD Score, n (%) | |||||
| 0 1 2 3 | 965 | 614 (63.6) 180 (18.7) 109 (11.3) 62 (6.4) | 184 (65.7) 58 (20.7) 28 (10.0) 10 (3.6) | 430 (62.8) 122 (17.8) 81 (11.8) 52 (7.6) | 0.08 |
| Outcome | |||||
| Death, n (%) | 966 | 28 (2.9) | 12 (4.3) | 16 (2.3) | 0.10 |
| No severe AKI (n = 861) | Severe AKI (Stage 2 or 3) (n = 106) | |||||
|---|---|---|---|---|---|---|
| NMFI (n = 246) | Malaria (n = 615) | p-Value | NMFI (n = 34) | Malaria (n = 72) | p-Value | |
| Demographics | ||||||
| Age, years | 1.6 (1.1) | 1.7 (1.1) | 0.12 | 1.7 (0.8) | 2.0 (1.0) | 0.16 |
| Female sex, n (%) | 115 (46.9) | 278 (45.7) | 0.75 | 14 (41.2) | 21 (30.1) | 0.26 |
| Weight, kg | 9.4 (2.8) | 9.8 (3.2) | 0.13 | 9.7 (2.7) | 10.8 (3.1) | 0.09 |
| Length, cm | 72.3 (11.8) | 74 (11.3) | 0.04 | 76.6 (11.2) | 78.6 (12.1) | 0.43 |
| Medication history | ||||||
| Antimalarial n (%) | 126 (51.6) | 267 (43.9) | 0.04 | 19 (55.9) | 32 (45.7) | 0.33 |
| Antibiotic, n (%) | 95 (38.9) | 190 (31.4) | 0.03 | 19 (55.9) | 21 (30.0) | 0.01 |
| Infection status | ||||||
| HIV, n (%) | 10 (4.1) | 6 (0.9) | 0.002 | 2 (5.9) | 2 (2.8) | 0.43 |
| Clinical signs and symptoms | ||||||
| Axillary Temperature in °C | 37.9 (1.1) | 37.9 (1.2) | 0.14 | 37.9 (1.2) | 37.5 (1.1) | 0.07 |
| Systolic Blood Pressure, mmHg | 104 (16.8) | 105 (15.1) | 0.15 | 104 (13.5) | 105 (16.3) | 0.83 |
| Diastolic Blood Pressure, mmHg | 58 (13.3) | 58 (13.2) | 0.37 | 56 (10.9) | 55 (12.0) | 0.61 |
| Heart Rate | 156 (25.2) | 162 (24.2) | 0.0003 | 158 (22.9) | 155 (25.4) | 0.66 |
| Respiratory Rate | 46 (15.1) | 45 (14.2) | 0.42 | 45 (12.7) | 45 (16.2) | 0.81 |
| SpO2 % | 96 (6.1) | 97 (5.0) | 0.0002 | 97 (2.5) | 97 (3.5) | 0.91 |
| Capillary refill time > 2 s, n (%) | 27 (11.1) | 81 (13.6) | 0.31 | 6 (18.2) | 16 (23.2) | 0.57 |
| Unable to drink/breastfeed, n (%) | 39 (15.9) | 114 (18.6) | 0.36 | 10 (29.4) | 21 (29.6) | 0.97 |
| Vomiting, n (%) | 72 (29.4) | 173 (28.2) | 0.73 | 15 (44.1) | 33 (46.5) | 0.82 |
| Diarrhea, n (%) | 96 (39.2) | 163 (26.6) | <0.0001 | 12 (36.4) | 18 (25.0) | 0.23 |
| Respiratory distress, n (%) | 75 (30.5) | 188 (30.6) | 0.98 | 13 (38.2) | 33 (45.8) | 0.46 |
| Prostration, n (%) | 41 (16.7) | 133 (21.7) | 0.10 | 11 (32.4) | 34 (48.6) | 0.12 |
| Coma (BCS < 3) n (%) | 7 (2.9) | 27 (4.5) | 0.28 | 2 (6.3) | 13 (18.8) | 0.10 |
| Altered consciousness, n (%) | 21 (8.5) | 82 (133.4) | 0.05 | 9 (26.5) | 22 (30.9) | 0.64 |
| Convulsions, n (%) | 33 (13.4) | 121 (19.7) | 0.03 | 8 (23.5) | 8 (11.1) | 0.10 |
| Jaundice, n (%) | 10 (4.1) | 66 (10.8) | 0.002 | 6 (17.7) | 22 (30.6) | 0.16 |
| Severe anemia 1, n (%) | ||||||
| No Yes Missing | 40 (16.3) 32 (13.0) 174 (70.7) | 121 (19.7) 136 (22.1) 358 (58.2) | 0.001 | 3 (8.8) 8 (23.5) 23 (67.7) | 18 (25.0) 27 (37.5) 27 (37.5) | 0.01 |
| LOD Score, n (%) | ||||||
| 0 1 2 3 | 164 (66.7) 54 (21.9) 21 (8.5) 7 (2.9) | 400 (65.6) 109 (17.5) 71 (11.6) 34 (5.5) | 0.12 | 20 (58.8) 4 (11.8) 7 (20.6) 3 (8.8) | 30 (42.3) 13 (18.3) 10 (14.1) 18 (25.4) | 0.13 |
| Outcome | ||||||
| Death, n (%) | 9 (3.7) | 7 (1.1) | 0.01 | 3 (8.8) | 9 (12.5) | 0.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hawkes, M.T.; Leligdowicz, A.; Batte, A.; Situma, G.; Zhong, K.; Namasopo, S.; Opoka, R.O.; Kain, K.C.; Conroy, A.L. Pathophysiology of Acute Kidney Injury in Malaria and Non-Malarial Febrile Illness: A Prospective Cohort Study. Pathogens 2022, 11, 436. https://doi.org/10.3390/pathogens11040436
Hawkes MT, Leligdowicz A, Batte A, Situma G, Zhong K, Namasopo S, Opoka RO, Kain KC, Conroy AL. Pathophysiology of Acute Kidney Injury in Malaria and Non-Malarial Febrile Illness: A Prospective Cohort Study. Pathogens. 2022; 11(4):436. https://doi.org/10.3390/pathogens11040436
Chicago/Turabian StyleHawkes, Michael T., Aleksandra Leligdowicz, Anthony Batte, Geoffrey Situma, Kathleen Zhong, Sophie Namasopo, Robert O. Opoka, Kevin C. Kain, and Andrea L. Conroy. 2022. "Pathophysiology of Acute Kidney Injury in Malaria and Non-Malarial Febrile Illness: A Prospective Cohort Study" Pathogens 11, no. 4: 436. https://doi.org/10.3390/pathogens11040436






