Preparation and Storage of Cryoprecipitate Derived from Amotosalen and UVA-Treated Apheresis Plasma and Assessment of In Vitro Quality Parameters
Abstract
:1. Introduction
2. Results
2.1. Coagulation Factor levels
2.1.1. Apheresis Plasma Pre- and Post-Pathogen-Reduction Treatment
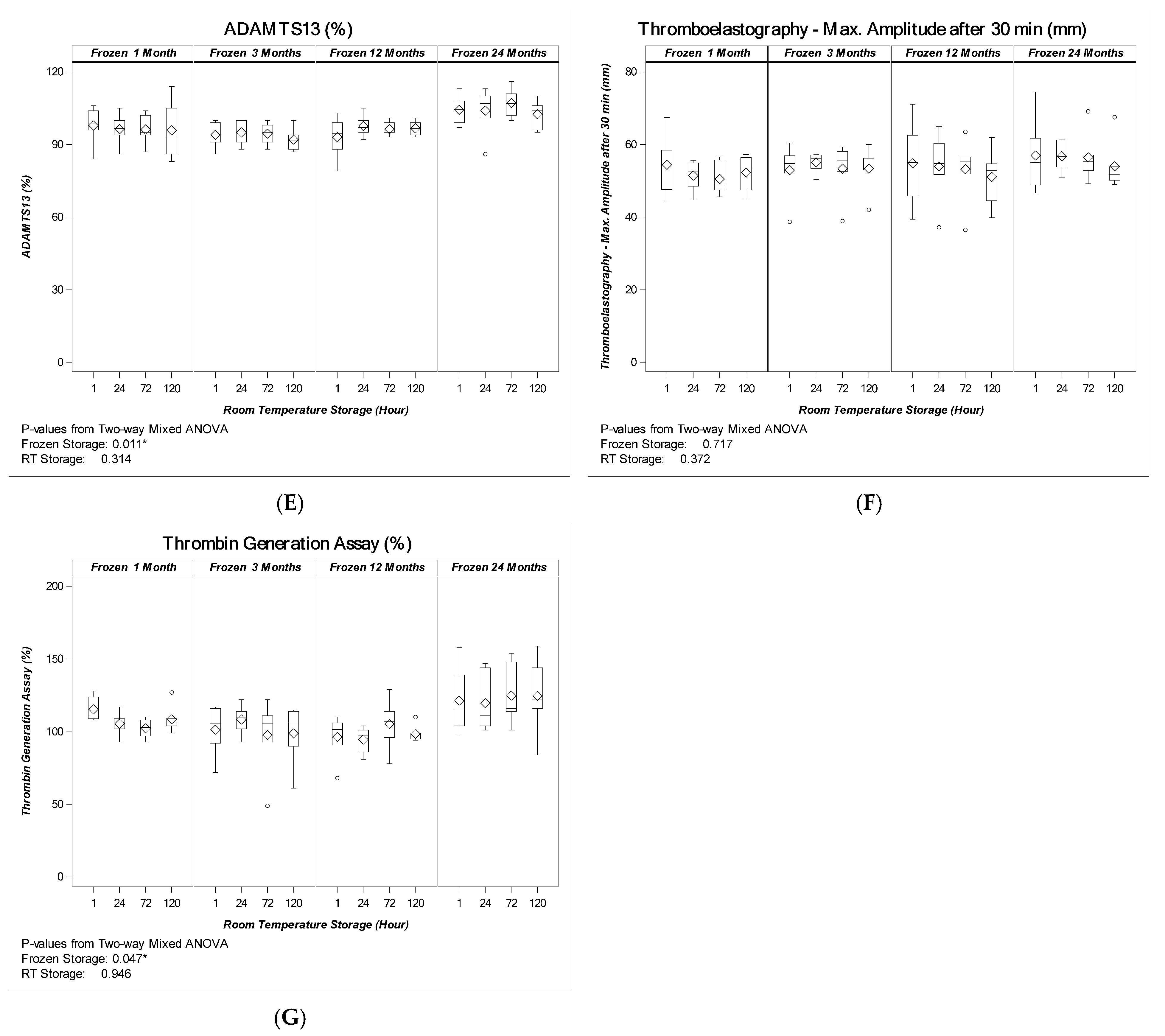
2.1.2. Cryoprecipitate
3. Discussion
4. Materials and Methods
4.1. Plasma Preparation and Pathogen Reduction with Amotosalen/UVA
4.2. Frozen Storage, Thawing and Sampling of PR-Cryo
4.3. Residual Cells
4.4. Coagulation Testing
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kasper, G.K. Judith Graham Pool and the discovery of cryoprecipitate. Haemophilia 2012, 18, 833–835. [Google Scholar] [CrossRef] [PubMed]
- Callum, J.L.; Karkouti, K.; Lin, Y. Cryoprecipitate: The current state of knowledge. Transfus. Med. Rev. 2009, 23, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Idris, S.F.; Hadjinicolaou, A.V.; Sweeney, M.; Winthrop, C.; Balendran, G.; Besser, M. The efficacy and safety of cryoprecipitate in the treatment of acquired hypofibrinogenaemia. Br. J. Haematol. 2014, 166, 458–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curry, N.; Rourke, C.; Davenport, R.; Beer, S.; Pankhurst, L.; Deary, A.; Thomas, H.; Llewelyn, C.; Green, L.; Doughty, H.; et al. Early cryoprecipitate for major haemorrhage in trauma: A randomised controlled feasibility trial. Br. J. Anaesth. 2015, 115, 76–83. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, B.; Goodnough, L.; Levy, J. Cryoprecipitate therapy. Br. J. Anaesth. 2014, 113, 922–934. [Google Scholar] [CrossRef] [Green Version]
- Green, L.; Bolton-Maggs, P.; Beattie, C.; Cardigan, R.; Kallis, Y.; Stanworth, S.J.; Thachil, J.; Zahra, S. British Society of Haematology Guidelines on the spectrum of fresh frozen plasma and cryoprecipitate products: Their handling and use in various patient groups in the absence of major bleeding. Br. J. Haematol. 2018, 181, 54–67. [Google Scholar] [CrossRef] [Green Version]
- Sørensen, B.; Bevan, D. A critical evaluation of cryoprecipitate for replacement of fibrinogen. Br. J. Haematol. 2010, 149, 834–843. [Google Scholar] [CrossRef] [Green Version]
- Backholer, L.; Wiltshire, M.; Proffitt, S.; Cookson, P.; Cardigan, R. Paired comparison of methylene blue- and amotosale treated plasma and cryoprecipitate. Vox Sang. 2016, 110, 352–361. [Google Scholar] [CrossRef]
- Burnouf, T.; Goubran, H.A.; Radosevich, M.; Sayed, M.A.; Gorgy, G.; El-Ekiaby, M. A minipool process for solvent-detergent treatment of cryoprecipitate at blood centres using a disposable bag system. Vox Sang. 2006, 91, 56–62. [Google Scholar] [CrossRef]
- Cid, J.; Caballo, C.; Pino, M.; Galan, A.M.; Martínez, N.; Escolar, G.; Diaz-Ricart, M. Quantitative and qualitative analysis of coagulation factors in cryoprecipitate prepared from fresh-frozen plasma inactivated with amotosalen and ultraviolet A light. Transfusion 2013, 53, 600–605. [Google Scholar] [CrossRef]
- European Committee on Blood Transfusion. Guide to Preparation, Use Ans Quality Assurance of Blood Components, 20th ed.; European Committee on Blood Transfusion: Strasbourg, France, 2020. [Google Scholar]
- Lokhandwala, P.M.; O’Neal, A.; Patel, E.U.; Brunker, P.A.R.; Gehrie, E.A.; Zheng, G.; Kickler, T.S.; Ness, P.M.; Tobian, A.A.R. Hemostatic profile and safety of pooled cryoprecipitate up to 120 hours after thawing. Transfusion 2018, 58, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Sheffield, W.P.; Bhakta, V. Stability of coagulation protein activities in single units or pools of cryoprecipitate during storage at 20–24 °C for up to 24 h. Vox Sang. 2016, 110, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Green, L.; Backholer, L. The hemostatic properties of thawed pooled cryoprecipitate up to 72 hours. Transfusion 2016, 56, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Cushing, M.M.; Asmis, L.M. Efficacy of a new pathogen-reduced cryoprecipitate stored 5 days after thawing to correct dilutional coagulopathy in vitro. Transfusion 2019, 59, 1818–1826. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.A.; Shea, S.M.; Spinella, P.C. Effects of pathogen reduction technology and storage duration on the ability of cryoprecipitate to rescue induced coagulopathies in vitro. Transfusion 2021, 61, 1943–1954. [Google Scholar] [CrossRef] [PubMed]
- Thomson, C.; Sobieraj-Teague, M. Extending the post-thaw viability of cryoprecipitate. Transfusion 2021, 61, 1578–1585. [Google Scholar] [CrossRef]
- Singh, Y.; Sawyer, L.S. Photochemical treatment of plasma with amotosalen and long-wavelength ultraviolet light inactivates pathogens while retaining coagulation function. Transfusion 2006, 46, 1168–1177. [Google Scholar] [CrossRef]
- Schlenke, P.; Hervig, T. Photochemical treatment of plasma with amotosalen and UVA light: Process validation in three European blood centers. Transfusion 2008, 48, 697–705. [Google Scholar] [CrossRef]
- Larrea, L.; Ortiz-de-Salazar, M. Quantitative analysis of plasma proteins in whole blood-derived fresh frozen plasma prepared with three pathogen reduction technologies. Transfus. Apher. Sci. 2015, 52, 305–310. [Google Scholar] [CrossRef]
- Larrea, L.; Calabuig, M. The influence of riboflavin photochemistry on plasma coagulation factors. Transfus. Apher. Sci. 2009, 41, 199–204. [Google Scholar] [CrossRef] [Green Version]
- Hornsey, V.S.; Drummond, O. Pathogen reduction of fresh plasma using riboflavin and ultraviolet light: Effects on plasma coagulation proteins. Transfusion 2009, 49, 2167–2172. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Rock, G. Protein quality in Mirasol pathogen reduction technology-treated, apheresis-derived fresh-frozen plasma. Transfusion 2010, 50, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Kamyszek, R.W.; Foster, M.W.; Evans, B.A.; Stoner, K.; Poisson, J.; Srinivasan, A.J.; Thompson, J.W.; Moseley, M.A.; Mooberry, M.J.; Welsby, I.J. The effect of pathogen inactivation on cryoprecipitate: A functional and quantitative evaluation. Blood Transfus. 2020, 18, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Arcos, S.; Jenkins, C. Bacteria can proliferate in thawed cryoprecipitate stored at room temperature for longer than 4 h. Vox Sang. 2017, 112, 477–479. [Google Scholar] [CrossRef] [PubMed]
- Curry, N.; Foley, C. Early fibrinogen concentrate therapy for major haemorrhage in trauma (E-FIT 1): Results from a UK multi-centre, randomised, double blind, placebo-controlled pilot trial. Crit. Care 2018, 22, 164. [Google Scholar] [CrossRef] [Green Version]
- Okerberg, C.K.; Williams, L.A., III. Cryoprecipitate AHF vs. fibrinogen concentrates for fibrinogen replacement in acquired bleeding patients—An economic evaluation. Vox Sang. 2016, 111, 292–298. [Google Scholar] [CrossRef]
- Novak, A.; Stanworth, S.J. Do we still need cryoprecipitate? Cryoprecipitate and fibrinogen concentrate as treatments for major hemorrhage—How do they compare? Expert Rev. Hematol. 2018, 11, 351–360. [Google Scholar] [CrossRef]


| Parameter | Pre- Treatment | Post- Treatment | Loss (%) | Significance (p) |
|---|---|---|---|---|
| RBC × 106/L | 10 ± 7 | |||
| WBC × 106/U | 0.1 ± 0.1 | |||
| PLT × 109/L | 6 ± 3 | |||
| Fibrinogen mg/dL | 270 ± 40 | 234 ± 31 | 13 ± 5 | <0.001 * |
| FVIII IU/dL | 128 ± 30 | 101 ± 21 | 21 ± 7 | <0.001 * |
| vWF Ac IU/dL | 114 ± 30 | 107 ± 29 | 5 ± 9 | 0.008 * |
| FXIII Ac IU/dL | 109 ± 15 | 104 ± 15 | 4 ± 3 | <0.001 * |
| ADAMTS13% | 93 ± 6 | 92 ± 7 | 1 ± 6 | 0.359 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovacic Krizanic, K.; Prüller, F.; Rosskopf, K.; Payrat, J.-M.; Andresen, S.; Schlenke, P. Preparation and Storage of Cryoprecipitate Derived from Amotosalen and UVA-Treated Apheresis Plasma and Assessment of In Vitro Quality Parameters. Pathogens 2022, 11, 805. https://doi.org/10.3390/pathogens11070805
Kovacic Krizanic K, Prüller F, Rosskopf K, Payrat J-M, Andresen S, Schlenke P. Preparation and Storage of Cryoprecipitate Derived from Amotosalen and UVA-Treated Apheresis Plasma and Assessment of In Vitro Quality Parameters. Pathogens. 2022; 11(7):805. https://doi.org/10.3390/pathogens11070805
Chicago/Turabian StyleKovacic Krizanic, Katarina, Florian Prüller, Konrad Rosskopf, Jean-Marc Payrat, Silke Andresen, and Peter Schlenke. 2022. "Preparation and Storage of Cryoprecipitate Derived from Amotosalen and UVA-Treated Apheresis Plasma and Assessment of In Vitro Quality Parameters" Pathogens 11, no. 7: 805. https://doi.org/10.3390/pathogens11070805






