Lineages, Virulence Gene Associated and Integrons among Extended Spectrum β-Lactamase (ESBL) and CMY-2 Producing Enterobacteriaceae from Bovine Mastitis, in Tunisia
Abstract
:1. Introduction
2. Results
2.1. Antibiotic Resistance Rates for ESBL/pAmpC Enterobacteriaceae Isolates
2.2. Genetic Support of ESBL/p(AmpC) Enterobacteriaceae Isolates
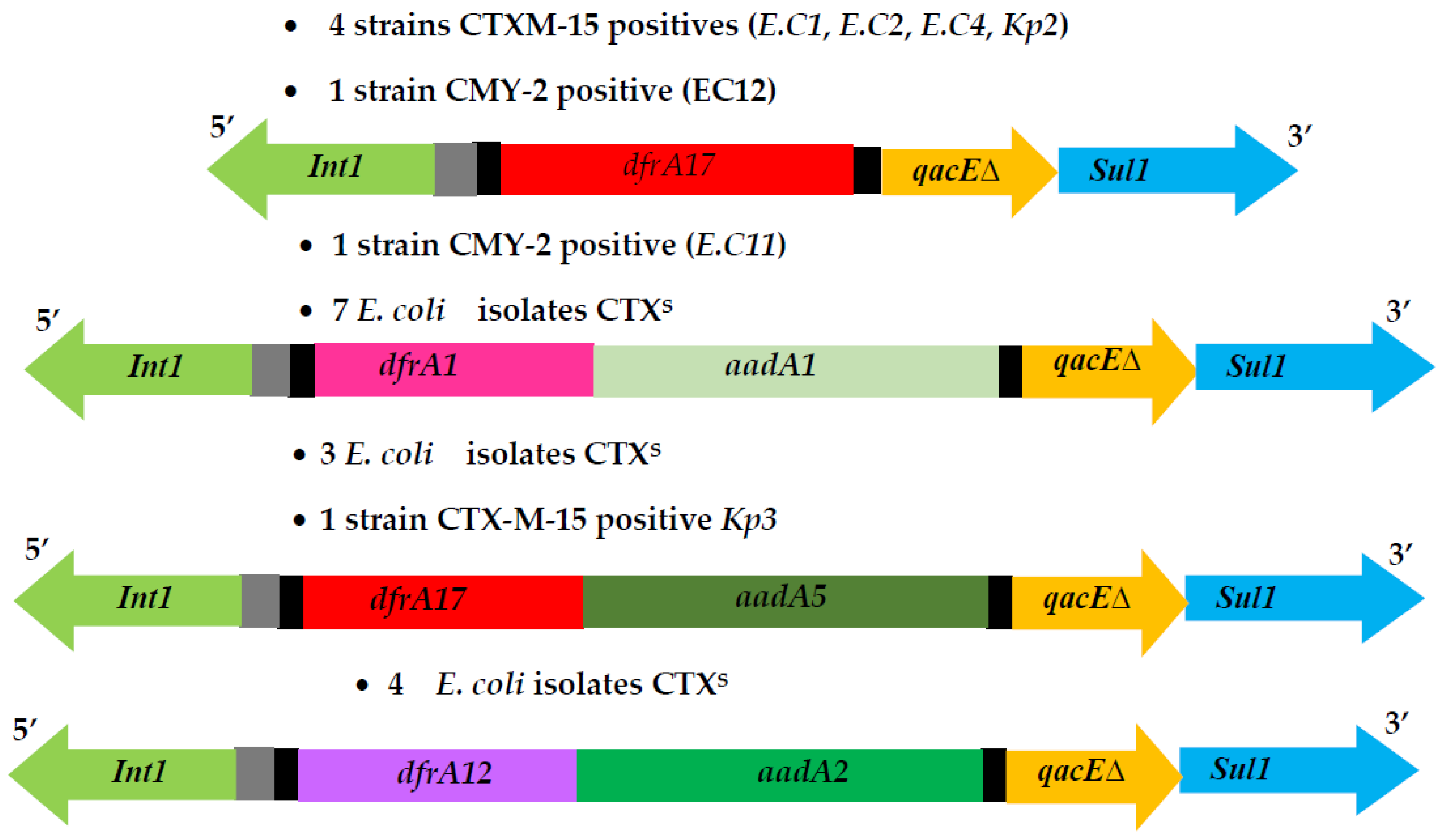
2.3. Integrons and Resistance Mechanisms to Non β-Lactam Antimicrobials Agents of ESBL-EB and p(AmpC)-Producer E. coli Strains
2.4. Molecular Typing, Virulence Factors and Phylotyping of ESBL–EB and p(AmpC)-Producer E. coli Strains
2.5. Antibiotic Resistance Rates for CTXS E. coli Isolates
2.6. Genotypic Features of CTXS E. coli Isolates
3. Discussion
4. Conclusions
5. Methods
5.1. Strain Collection
5.2. Antimicrobial Susceptibility Testing and AmpC Confirmation
5.3. Genomic Extraction, Polymerase Chain Reaction and Sequencing
5.4. Characterization of β-Lactamase Genes and Their Genetic Environment
5.5. Characterization of Integrons and Resistance Mechanisms to Non-b-Lactam Antibiotic
5.6. Virulence Factors and Phylogeny Groups
5.7. Molecular Typing of ESBL-p(AmpC) Enterobacteriaceae Isolates
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seegers, H.; Fourichon, C.; Beaudeau, F. Production effects related to mastitis and mastitis economics in dairy cattle herds. Vet. Res. 2003, 34, 475–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benić, M.; Macesic, N.; Cvetnić, L.; Habrun, B.; Cvetnić, Ž.; Turk, R.; Đuričić, D.; Lojkić, M.; Dobranić, V.; Valpotić, H.; et al. Bovine mastitis: A persistent and evolving problem requiring novel approaches for its control—A review. Vet. Arh. 2018, 88, 535–557. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.P.; Yang, F.; Luo, J.Y.; Wang, X.R.; Liu, L.H.; Li, H.S. Influences of season, parity, lactation, udder area, milk yield, and clinical symptoms on intramammary infection in dairy cows. J. Dairy Sci. 2016, 99, 6484–6493. [Google Scholar] [CrossRef] [PubMed]
- Shpigel, N.Y.; Elazar, S.; Rosenshine, I. mammary pathogenic Escherichia coli. Curr. Opin. Microbiol. 2008, 11, 60–65. [Google Scholar] [CrossRef]
- Wenz, J.R.; Barrington, G.M.; Garry, F.B.; Dinsmore, R.P.; Callan, R.J. Use of systemic disease signs to assess disease severity in dairy cows with acute coliform mastitis. J. Am. Vet. Med. Assoc. 2001, 218, 567–572. [Google Scholar] [CrossRef]
- Wenz, J.R.; Barrington, G.M.; Garry, F.B.; Ellis, R.P.; Magnuson, R.J. Escherichia coli isolates’ serotypes, genotypes, and virulence genes and clinical coliform mastitis severity. J. Dairy Sci. 2006, 89, 3408–3412. [Google Scholar] [CrossRef] [Green Version]
- Timofte, D.; Maciuca, I.E.; Evans, N.J.; Williams, H.; Wattret, A.; Fick, J.C.; Williams, N.J. Detection and molecular characterization of escherichia coli ctx-m-15 and klebsiella pneumoniae shv-12 β-lactamases from bovine mastitis isolates in the United Kingdom. Antimicrob. Agents Chemother. 2014, 58, 789–794. [Google Scholar] [CrossRef] [Green Version]
- Momtaz, H.; Dehkordi, F.S.; Taktaz, T.; Rezvani, A.; Yarali, S. Shiga toxin-producing Escherichia coli isolated from bovine mastitic milk: Serogroups, virulence factors, and antibiotic resistance properties. Sci. World J. 2012, 2012, 618709. [Google Scholar] [CrossRef] [Green Version]
- Kobori, D.; Rigobelo, E.; Macedo, C.; Marin, J.; Avila, F. Virulence properties of Shiga toxin-producing Escherichia coli isolated from cases of bovine mastitis in Brazil. Rev. Elevage Med. Vet. Pays Trop. 2004, 57, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Guerra, S.T.; Dalanezi, F.M.; de Paula, C.L.; Hernandes, R.T.; Pantoja, J.C.F.; Listoni, F.J.P.; Langoni, H.; Ribeiro, M.G. Putative virulence factors of extra-intestinal Escherichia coli isolated from bovine mastitis with different clinical scores. Lett. Appl. Microbiol. 2019, 68, 403–408. [Google Scholar] [CrossRef]
- Alonso, C.A.; Zarazaga, M.; Ben Sallem, R.; Jouini, A.; Ben Slama, K.; Torres, C. Antibiotic resistance in Escherichia coli in husbandry animals: The African perspective. Lett. Appl. Microbiol. 2017, 64, 318–334. [Google Scholar] [CrossRef] [Green Version]
- Destoumieux-Garzón, D.; Mavingui, P.; Boetsch, G.; Boissier, J.; Darriet, F.; Duboz, P.; Fritsch, C.; Giraudoux, P.; Le Roux, F.; Morand, S.; et al. The one health concept: 10 years old and a long road ahead. Front. Vet. Sci. 2018, 5, 14. [Google Scholar] [CrossRef] [Green Version]
- Ali, T.; Rahman, S.U.; Zhang, L.; Shahid, M.; Han, D.; Gao, J.; Zhang, S.; Ruegg, P.L.; Saddique, U.; Han, B. Characteristics and genetic diversity of multi-drug resistant extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli isolated from bovine mastitis. Oncotarget 2017, 8, 90144–90163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafiq, M.; Huang, J.; Shah, J.M.; Wang, X.; Rahman, S.U.; Ali, I.; Chen, L.; Wang, L. Characterization and virulence factors distribution of bla CTX-M and mcr-1 carrying Escherichia coli isolates from bovine mastitis. J. Appl. Microbiol. 2021, 131, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, M.; Okatani, A.T.; Harada, K.; Sawada, T.; Marumo, K.; Murakami, M.; Sato, R.; Esaki, H.; Shimura, K.; Kato, H.; et al. Genetic characteristics of CTX-M-type extended-spectrum-β-lactamase (ESBL)-producing enterobacteriaceae involved in mastitis cases on Japanese dairy farms, 2007 to 2011. J. Clin. Microbiol. 2013, 51, 3117–3122. [Google Scholar] [CrossRef] [Green Version]
- Endimiani, A.; Doi, Y.; Bethel, C.R.; Taracila, M.; Adams-Haduch, J.M.; O’Keefe, A.; Hujer, A.M.; Paterson, D.L.; Skalweit, M.J.; Page, M.G.P.; et al. Enhancing resistance to cephalosporins in class C β-lactamases: Impact of Gly214Glu in CMY-2. Biochemistry 2010, 49, 1014–1023. [Google Scholar] [CrossRef] [Green Version]
- Madec, J.-Y.; Haenni, M.; Nordmann, P.; Poirel, L. Extended-spectrum β-lactamase/AmpC- and carbapenemase-producing enterobacteriaceae in animals: A threat for humans? Clin. Microbiol. Infect. 2017, 23, 826–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maamar, E.; Alonso, C.A.; Hamzaoui, Z.; Dakhli, N.; Abbassi, M.S.; Ferjani, S.; Saidani, M.; Boubaker, I.B.-B.; Torres, C. Emergence of plasmid-mediated colistin-resistance in CMY-2-producing Escherichia coli of lineage ST2197 in a Tunisian poultry farm. Int. J. Food Microbiol. 2018, 269, 60–63. [Google Scholar] [CrossRef]
- Jouini, A.; Klibi, A.; Elarbi, I.; Chaabene, M.B.; Hamrouni, S.; Souiai, O.; Hanachi, M.; Ghram, A.; Maaroufi, A. First detection of human ST131-CTX-M-15-O25-B2 clone and high-risk clonal lineages of ESBL/pAmpC-producing E. coli isolates from diarrheic poultry in Tunisia. Antibiotics 2021, 10, 670. [Google Scholar] [CrossRef]
- Saidani, M.; Messadi, L.; Soudani, A.; Daaloul-Jedidi, M.; Châtre, P.; Ben Chehida, F.; Mamlouk, A.; Mahjoub, W.; Madec, J.-Y.; Haenni, M. Epidemiology, antimicrobial resistance, and extended-spectrum beta-lactamase-producing enterobacteriaceae in clinical bovine mastitis in Tunisia. Microb. Drug Resist. 2018, 24, 1242–1248. [Google Scholar] [CrossRef]
- Tahar, S.; Nabil, M.M.; Safia, T.; Ngaiganam, E.P.; Omar, A.; Hafidha, C.; Hanane, Z.; Rolain, J.-M.; Diene, S.M. Molecular characterization of multidrug-resistant Escherichia coli isolated from milk of dairy cows with clinical mastitis in Algeria. J. Food Prot. 2020, 83, 2173–2178. [Google Scholar] [CrossRef]
- Ahmed, W.; Neubauer, H.; Tomaso, H.; El Hofy, F.I.; Monecke, S.; El-Tawab, A.A.; Hotzel, H. Characterization of Enterococci- and ESBL-Producing Escherichia coli Isolated from Milk of Bovides with Mastitis in Egypt. Pathogens 2021, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Klibi, A.; Jouini, A.; El Andolsi, R.B.; Kmiha, S.; Ben Hamda, C.; Ghedira, K.; Hamrouni, S.; Ghram, A.; Maaroufi, A. Epidemiology of β-lactamase-producing staphylococci and gram negative bacteria as cause of clinical bovine mastitis in Tunisia. BioMed Res. Int. 2019, 2019, 2165316. [Google Scholar] [CrossRef] [Green Version]
- Bevan, E.R.; Jones, A.M.; Hawkey, P.M. Global epidemiology of CTX-M β-lactamases: Temporal and geographical shifts in genotype. J. Antimicrob. Chemother. 2017, 72, 2145–2155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sghaier, S.; Abbassi, M.S.; Pascual, A.; Serrano, L.; De-Alba, P.D.; Ben Said, M.; Hassen, B.; Ibrahim, C.; Hassen, A.; López-Cerero, L. Extended-spectrum β-lactamase-producing enterobacteriaceae from animal origin and wastewater in Tunisia: First detection of O25b-B23-CTX-M-27-ST131 Escherichia coli and CTX-M-15/OXA-204-producing citrobacter freundii from wastewater. J. Glob. Antimicrob. Resist. 2019, 17, 189–194. [Google Scholar] [CrossRef]
- Pehlivanoglu, F.; Turutoglu, H.; Ozturk, D. CTX-M-15-type extended-spectrum beta-lactamase-producing Escherichia coli as causative agent of bovine mastitis. Foodborne Pathog. Dis. 2016, 13, 477–482. [Google Scholar] [CrossRef]
- Freitag, C.; Michael, G.B.; Kadlec, K.; Hassel, M.; Schwarz, S. Detection of plasmid-borne extended-spectrum β-lactamase (ESBL) genes in Escherichia coli isolates from bovine mastitis. Vet. Microbiol. 2017, 200, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Dahmen, S.; Métayer, V.; Gay, E.; Madec, J.-Y.; Haenni, M. Characterization of extended-spectrum beta-lactamase (ESBL)-carrying plasmids and clones of Enterobacteriaceae causing cattle mastitis in France. Vet. Microbiol. 2013, 162, 793–799. [Google Scholar] [CrossRef]
- Tsuka, T.; Ozaki, H.; Saito, D.; Murase, T.; Okamoto, Y.; Azuma, K.; Osaki, T.; Ito, N.; Murahata, Y.; Imagawa, T. Genetic characterization of CTX-M-2-producing Klebsiella pneumoniae and Klebsiella oxytoca associated with bovine mastitis in Japan. Front. Vet. Sci. 2021, 8, 659222. [Google Scholar] [CrossRef]
- De Angelis, G.; Del Giacomo, P.; Posteraro, B.; Sanguinetti, M.; Tumbarello, M. Molecular mechanisms, epidemiology, and clinical importance of β-lactam resistance in Enterobacteriaceae. Int. J. Mol. Sci. 2020, 21, 5090. [Google Scholar] [CrossRef]
- Jouini, A.; Ben Slama, K.; Vinué, L.; Ruiz, E.; Saenz, Y.; Somalo, S.; Klibi, N.; Zarazaga, M.; Ben Moussa, M.; Boudabous, A.; et al. Detection of unrelated Escherichia coli strains harboring genes of CTX-M-15, OXA-1, and AAC(6′)-Ib-cr enzymes in a Tunisian hospital and characterization of their integrons and virulence factors. J. Chemother. 2010, 22, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Ben Slama, K.; Ben Sallem, R.; Jouini, A.; Rachid, S.; Moussa, L.; Sáenz, Y.; Estepa, V.; Somalo, S.; Boudabous, A.; Torres, C. Diversity of genetic lineages among CTX-M-15 and CTX-M-14 producing Escherichia coli strains in a Tunisian hospital. Curr. Microbiol. 2011, 62, 1794–1801. [Google Scholar] [CrossRef] [PubMed]
- Abboud, Z.; Galuppo, L.; Tolone, M.; Vitale, M.; Puleio, R.; Osman, M.; Loria, G.R.; Hamze, M. Molecular characterization of antimicrobial resistance and virulence genes of bacterial pathogens from bovine and caprine mastitis in Northern Lebanon. Microorganisms 2021, 9, 1148. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, S.; Shang, X.; Wang, L.; Li, H.; Wang, X. Characteristics of quinolone-resistant Escherichia coli isolated from bovine mastitis in China. J. Dairy Sci. 2018, 101, 6244–6252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Y.; Yu, C.-Y.; Tsai, Y.; Wang, S.-H.; Lee, C.; Chu, C. Fluoroquinolone-resistant and extended-spectrum β-lactamase-producing Escherichia coli from the milk of cows with clinical mastitis in Southern Taiwan. J. Microbiol. Immunol. Infect. 2016, 49, 892–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endimiani, A.; Bertschy, I.; Perreten, V. Escherichia coli producing CMY-2 β-lactamase in bovine mastitis milk. J. Food Prot. 2012, 75, 137–138. [Google Scholar] [CrossRef]
- Dziri, O.; Dziri, R.; Maraoub, A.; Chouchani, C. Characterization of O25b-ST131 Escherichia coli clone producing CTX-M-15, DHA-4, and CMY-42 in urinary tract infections in a Tunisian Island. Microb. Drug Resist. 2020, 26, 741–746. [Google Scholar] [CrossRef]
- Awandkar, S.P.; Kulkarni, M.B.; Khode, N.V. Bacteria from bovine clinical mastitis showed multiple drug resistance. Vet. Res. Commun. 2022, 46, 147–158. [Google Scholar] [CrossRef]
- Kuipers, A.; Koops, W.J.; Wemmenhove, H. Antibiotic use in dairy herds in the netherlands from 2005 to 2012. J. Dairy Sci. 2016, 99, 1632–1648. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Fan, C.; Zhang, Z.; Li, S.; Xu, C.; Zhao, Y.; Han, L.; Zhang, D.; Liu, M. Enterococcal Isolates from bovine subclinical and clinical mastitis: Antimicrobial resistance and integron-gene cassette distribution. Microb. Pathog. 2019, 129, 82–87. [Google Scholar] [CrossRef]
- Li, L.; Zhao, X. Characterization of the resistance class 1 integrons in Staphylococcus aureus isolates from milk of lactating dairy cattle in Northwestern China. BMC Vet. Res. 2018, 14, 59. [Google Scholar] [CrossRef] [Green Version]
- Ben Sallem, R.; Ben Slama, K.; Sáenz, Y.; Rojo-Bezares, B.; Estepa, V.; Jouini, A.; Gharsa, H.; Klibi, N.; Boudabous, A.; Torres, C. Prevalence and characterization of extended-spectrum beta-lactamase (ESBL)– and CMY-2–producing Escherichia Coli isolates from healthy food-producing animals in Tunisia. Foodborne Pathog. Dis. 2012, 9, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Clermont, O.; Bonacorsi, S.; Bingen, E. Rapid and simple determination of the Escherichia Coli phylogenetic group. Appl. Environ. Microbiol. 2000, 66, 4555–4558. [Google Scholar] [CrossRef] [Green Version]
- Kaipainen, T.; Pohjanvirta, T.; Shpigel, N.Y.; Shwimmer, A.; Pyörälä, S.; Pelkonen, S. Virulence factors of Escherichia Coli isolated from bovine clinical mastitis. Vet. Microbiol. 2002, 85, 37–46. [Google Scholar] [CrossRef]
- Marashifard, M.; Aliabad, Z.K.; Hosseini, S.A.A.M.; Darban-Sarokhalil, D.; Mirzaii, M.; Khoramrooz, S.S. Determination of antibiotic resistance pattern and virulence genes in Escherichia Coli isolated from bovine with subclinical mastitis in Southwest of Iran. Trop. Anim. Health Prod. 2019, 51, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Bag, A.S.; Khan, S.R.; Sami, D.H.; Begum, F.; Islam, S.; Rahman, M.; Rahman, T.; Hassan, J. Virulence determinants and antimicrobial resistance of E. coli isolated from bovine clinical mastitis in some selected dairy farms of Bangladesh. Saudi J. Biol. Sci. 2021, 28, 6317–6323. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Liu, H.; Meng, L.; Xing, M.; Dong, L.; Gu, M.; Wang, J.; Zheng, N. Antimicrobial susceptibility, phylotypes, and virulence genes of Escherichia coli from clinical bovine mastitis in five provinces of China. Food Agric. Immunol. 2020, 31, 406–423. [Google Scholar] [CrossRef] [Green Version]
- Alghoribi, M.F.; Gibreel, T.M.; Farnham, G.; Al Johani, S.M.; Balkhy, H.H.; Upton, M. Antibiotic-resistant ST38, ST131 and ST405 strains are the leading uropathogenic Escherichia coli clones in Riyadh, Saudi Arabia. J. Antimicrob. Chemother. 2015, 70, 2757–2762. [Google Scholar] [CrossRef] [Green Version]
- Paulin-Curlee, G.G.; Singer, R.S.; Sreevatsan, S.; Isaacson, R.; Reneau, J.; Foster, D.; Bey, R. Genetic Diversity of mastitis-associated klebsiella pneumoniae in dairy cows. J. Dairy Sci. 2007, 90, 3681–3689. [Google Scholar]
- CLSI. Susceptibility Testing Twenty-Fourth International Supplement. CLSI Document M100-S24, 34,1; Clinical Laboratory Standard Institute: Wayne, PA, USA, 2013. [Google Scholar]
- Studies Pages, Lahey Health. Available online: http://www.lahey.org/Studies/webt.html (accessed on 17 June 2019).
- Clermont, O.; Lavollay, M.; Vimont, S.; Deschamps, C.; Forestier, C.; Branger, C.; Denamur, E.; Arlet, G. The CTX-M-15-producing Escherichia Coli diffusing clone belongs to a highly virulent B2 phylogenetic subgroup. J. Antimicrob. Chemother. 2008, 61, 1024–1028. [Google Scholar] [CrossRef]
- Blanco, M.; Alonso, M.P.; Nicolas-Chanoine, M.-H.; Dahbi, G.; Mora, A.; Blanco, J.E.; López, C.; Cortés, P.; Llagostera, M.; Leflon-Guibout, V.; et al. Molecular epidemiology of Escherichia Coli producing extended-spectrum {beta}-lactamases in Lugo (Spain): Dissemination of clone O25b:H4-ST131 producing CTX-M-15. J. Antimicrob. Chemother. 2009, 63, 1135–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Strains | Farm | Genes Encoding Beta-Lactamases | blaCTX-M Genetic Environment | Phenotypes of Resistance to Non β-Lactams | Other Resistance Genes Detected Outside Integrons | Gene Cassette Arrays in Class 1 Integron | Phylogroup and Virulence Genes | ST |
|---|---|---|---|---|---|---|---|---|
| E. C 1 | 1 | blaCTX-M-15 + blaOXA-1 | ISEcp1-orf477 | TET NAL SUL SXT | tetB, sul3, sul2, aac(6′)-Ib-cr | Int1, dfrA17 | A | 405 |
| E. C 2 | 5 | blaCTX-M-15 + blaOXA-1 | ISEcp1-orf477 | TET SXT SUL | tetB, sul2 | Int1, dfrA17 | B2, cnf1, stx2, aer | 405 |
| E. C 3 | 8 | blaCTX-M-15 + blaOXA-1 + blaTEM-1b | ISEcp1-orf477 | TET NAL | tetB, aac(6′)-Ib-cr | − | B2, aer, fimA, stx2 | 58 |
| E. C 4 | 2 | blaCTX-M-15 + blaOXA-1 + blaTEM-1b | IS26/ISEcp1-orf477 | TET SUL NAL SXT | tetB, sul3, sul2, aac(6′)-Ib-cr | Int1, dfrA17 | D, aer, stx2 | 58 |
| E. C 5 | 10 | blaCTX-M-15 | ISEcp1-orf477 | TET STR | tetB, strA | − | B1 | 405 |
| E. C 6 | 6 | blaCTX-M-15 | ISEcp1-orf477 | TET | tetB | − | A | 405 |
| E. C 7 | 18 | blaCTX-M-15 | IS26/ISEcp-orf477 | CIP NAL SUL | qnrA, sul2 | − | A | 155 |
| E. C 8 | 20 | blaCTX-M-15 | IS26/ISEcp1-orf477 | CIP NAL SUL | qnrA, sul3 | − | A | 10 |
| E. C 9 | 23 | blaCTX-M-15 | ISEcp1-orf477 | NAL SUL TET CHL STR SXT | tetB, sul3, sul2, strA | ND | A | 155 |
| E. C 10 | 9 | blaCTX-M-15 | ISEcp1-orf477 | SUL TET | tetB, strA | − | A | 58 |
| E. C 11 * | 11 | blaCMY-2 | ISEcp1-orf477 | TET SULNAL SXT STR | tetA, sul2 | Int1, aadA1 + dfrA1 | B1 | 155 |
| E. C 12 * | 14 | blaCMY-2 | ISEcp1-orf477 | TET SUL SXT | tetA | Int1, dfrA17 | B1 | 399 |
| E. C 13 * | 16 | blaCMY-2 | ISEcp1-orf477 | TET SUL NAL CIP | tetB, qnrA, sul2 | − | B1 | 617 |
| Kp1 | 1 | blaCTX-M-15 + blaTEM-1b | IS26/ISEcp1-orf477 | TET NAL SUL | tet A, sul1, sul2, qnrB | − | − | 471 |
| Kp2 | 2 | blaCTX-M-15 + blaTEM-1b | ISEcp1-orf477 | CIP NAL SUL SXT | qnrB, sul2 | Int1, dfrA17 | − | 471 |
| Kp3 | 3 | blaCTX-M-15 + blaTEM-1b | ISEcp1-orf477 | TET SUL SXT STR | tetA, sul1, sul2 | Int1, dfrA17 + aadA5 | − | 101 |
| Kp4 | 4 | blaCTX-M-15 + blaTEM-1b | IS26/ISEcp1-orf477 | TET NAL CIP | tetA, aac(6′)-Ib-cr | − | − | 101 |
| Strains | Antibiotic Resistance Phenotypes | Resistance Genes | Class 1 Integron | Gene Cassette Arrays in Class 1 Integron | Phylogroup and Virulence Genes |
|---|---|---|---|---|---|
| E. C 168 | TET AMP SXT TIC SUL FOX STR CHL | TEM-1b, tetA, catA | + | dfrA12 + aadA2 | B1 |
| E. C 3 | TET AMP SUL NAL CIP TIC SXT | TEM-1b, tetA, qnrA, sul2 | ND | − | B1 |
| E. C 78 | TET AMP SXT TIC SUL STR CHL | TEM-1b, tetA, catA, strA | + | dfrA 1 + aadA1 | B2, sxt2, cnf1 |
| E. C 115 | TET AMP SXT TIC SUL STR | TEM-1b, tetA | + | dfrA1 + aadA1 | B1 |
| E. C 81 | TET AMP SXT TIC SUL STR | TEM-1b, tetA, strA, dfrA1a | ND | − | B2, fimA, stx2 |
| E. C 82 | TET AMP SXT TIC SUL STR | TEM-1b, tetA | + | dfrA12 + aadA2 | B2, stx2, cnf1 |
| E. C 85 | TET AMP SXT TIC SUL STR | TEM-1b, tetB, strA | ND | − | A |
| E. C 91 | TET AMP SXT TIC SUL STR | TEM-1b, tetA | + | dfrA12 + aadA2 | B1 |
| E. C 134 | TET AMP SXT TIC SUL STR | TEM-1b | + | dfrA1 + aadA1 | B2, aer, fimA, cnf1 |
| E. C 162 | TET AMP SXT TIC SUL STR | TEM-1b, tetB | + | dfrA1 + aadA1 | B2, cnf1, stx2 |
| E. C 164 | TET AMP SXT TIC SUL STR | TEM-1b, tetA | + | dfrA1 + aadA1 | B2, cnf1 |
| E. C 167 | TET AMP SXT TIC SUL STR | TEM-1b, tetA | + | dfrA1 + aadA1 | B1 |
| E. C 42 | TET AMP SXT TIC SUL STR | TEM-1b, tetA, strA | + | dfrA 12 + aadA2 | B1 |
| E. C 184 | TET AMP SXT TIC SUL STR | TEM-1b, tetA | + | dfrA17 + aadA5 | B1 |
| E. C 186 | TET AMP SXT TIC SUL STR | TEM-1b, tetA | + | dfrA17 + aadA5 | B1 |
| E. C 196 | TET AMP SXT TIC SUL STR | TEM-1b, tetB | + | dfrA17 + aadA5 | B1 |
| E. C 116 | TET SXT SUL NAL CIP STR | tetA, qnrA | + | dfrA1 + aadA1 | B1 |
| E. C 123 | TET AMP CHL NAL CIP SXT | TEM-1b, tetA, catA, qnrA, dfrA1 a | ND | − | B2, cnf1 |
| E. C 10 | TET AMP SXT TIC SUL | TEM-1b, tetA, sul2, dfrA12 a | ND | − | B2, aer, cnf1 |
| E. C 28 | TET AMP SXT TIC SUL | TEM-1b, tetA, sul2, dfrA1 a | ND | − | A |
| E. C 29 | TET AMP SXT TIC SUL | TEM-1b, tetA, dfrA1 a | ND | − | B1 |
| E. C 40 | TET AMP SXT TIC SUL | TEM-1b, tetA, dfrA1 a | ND | − | B1 |
| E. C 171 | TET AMP SXT TIC SUL | TEM-1b, tetA, dfrA1 a | ND | − | B2, stx2, fimA |
| E. C 175 | TETAMP SXT TIC SUL | TEM-1b, tetA, dfrA1 a | ND | − | B1 |
| E. C 30 | TET AMP SXT TIC SUL | TEM-1b, tetA | ND | − | B2, stx2, aer, fimA |
| E. C 17 | AMP TIC NAL CIP | TEM-1b, qnrA | − | − | A |
| E. C 18 | AMP TIC NAL CIP | TEM-1b, qnrA | − | − | A |
| E. C 195 | AMP TIC TET STR | TEM-1b, tetB, strA | − | − | A |
| E. C 137 | AMP SUL STR | TEM-1b, sul2, strA | − | − | A |
| E. C 14 | TET AMP TIC | TEM-1b, tetA | − | − | A |
| E. C 25 | TET AMP TIC | TEM-1b, tetA | − | − | B1 |
| E. C 39 | TET AMP TIC | TEM-1b, tetB | − | − | B1 |
| E. C 70 | TET AMP TIC | TEM-1b, tetB | − | − | B1 |
| E. C 31 | TET AMP TIC | TEM-1b, tetB | − | − | B2, stx2, aer, cnf1 |
| E. C 32 | TET AMP TIC | TEM-1b, tetB | − | − | A |
| E. C 135 | TET SUL STR | tetB, sul2, strA | B1 | ||
| E. C 183 | FOX AMP | TEM-1b | − | − | A |
| E. C 23 | AMP TIC | TEM-1b | − | − | A |
| E. C 179 | AMP STR | TEM-1b, strA | − | − | A |
| E. C 190 | TET STR | tetB, strA | A | ||
| E. C 119 | AMP | TEM-1b | − | − | A |
| E. C 146 | FOX | − | − | − | A |
| E. C 147 | FOX | − | − | − | B1 |
| E. C 163 | AMP | TEM-1b | − | − | A |
| E. C 194 *(n = 6) | TET | tetB | − | − | A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jouini, A.; Klibi, A.; Kmiha, S.; Hamrouni, S.; Ghram, A.; Maaroufi, A. Lineages, Virulence Gene Associated and Integrons among Extended Spectrum β-Lactamase (ESBL) and CMY-2 Producing Enterobacteriaceae from Bovine Mastitis, in Tunisia. Pathogens 2022, 11, 948. https://doi.org/10.3390/pathogens11080948
Jouini A, Klibi A, Kmiha S, Hamrouni S, Ghram A, Maaroufi A. Lineages, Virulence Gene Associated and Integrons among Extended Spectrum β-Lactamase (ESBL) and CMY-2 Producing Enterobacteriaceae from Bovine Mastitis, in Tunisia. Pathogens. 2022; 11(8):948. https://doi.org/10.3390/pathogens11080948
Chicago/Turabian StyleJouini, Ahlem, Amira Klibi, Souhir Kmiha, Safa Hamrouni, Abdeljelil Ghram, and Abderrazak Maaroufi. 2022. "Lineages, Virulence Gene Associated and Integrons among Extended Spectrum β-Lactamase (ESBL) and CMY-2 Producing Enterobacteriaceae from Bovine Mastitis, in Tunisia" Pathogens 11, no. 8: 948. https://doi.org/10.3390/pathogens11080948






