Direct and Indirect Role of Migratory Birds in Spreading CCHFV and WNV: A Multidisciplinary Study on Three Stop-Over Islands in Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples’ Collection
2.2. Statistical Analyses
2.3. Tick Identification, Nucleic Acids Extraction, and Pathogens’ Detection
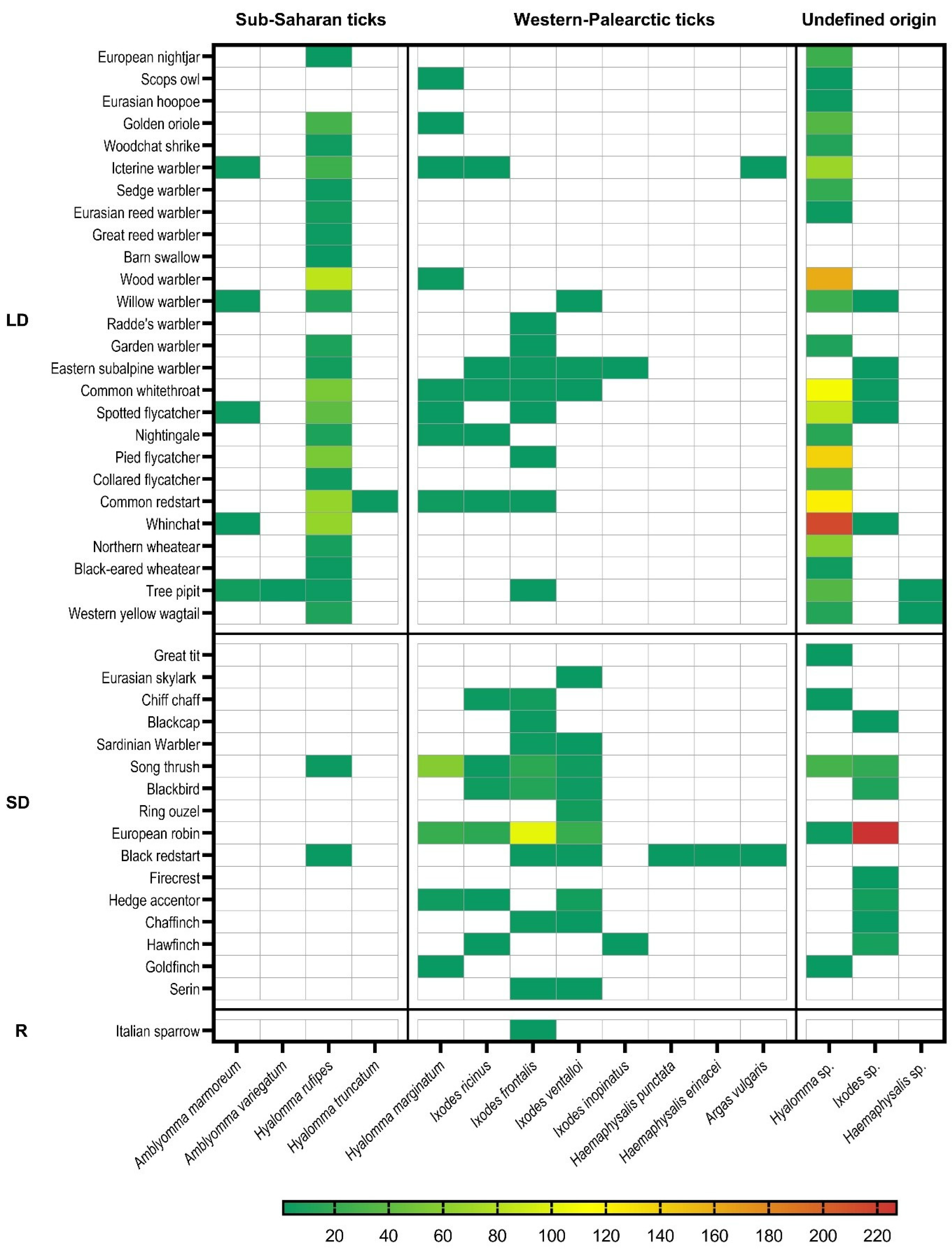
3. Results
3.1. Samples’ Collection
3.2. Infested Bird Species
3.3. Statistical Analysis: Migratory Strategies and Spread of Ticks
3.4. Tick Identification: Species Introduced to Europe with Migratory Birds
3.5. Pathogens’ Detection in Ticks
3.6. Pathogens’ Detection in Birds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Estrada-Peña, A.; Ostfeld, R.S.; Peterson, A.T.; Poulin, R.; de la Fuente, J. Effects of environmental change on zoonotic disease risk: An ecological primer. Trends Parasitol. 2014, 30, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Hasle, G. Transport of ixodid ticks and tick-borne pathogens by migratory birds. Front. Cell. Infect. Microbiol. 2013, 3, 48. [Google Scholar] [CrossRef] [PubMed]
- Nikolay, B. A review of West Nile and Usutu virus co-circulation in Europe: How much do transmission cycles overlap? Trans. R Soc. Trop Med. Hyg. 2015, 109, 609–618. [Google Scholar] [CrossRef]
- Rizzoli, A.; Jiménez-Clavero, A.M.; Barzon, L.; Cordioli, P.; Figuerola, J.; Koraka, P.; Martina, B.; Moreno, A.; Nowotny, N.; Pardigon, N.; et al. The challenge of West Nile virus in Europe: Knowledge gaps and research priorities. Euro Surveill. 2015, 20, 21135. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Annual Review of Diseases Prioritized under the Research and Development Blueprint Informal Consultation 6–7 February 2018, Geneva, Switzerland. Available online: https://www.who.int/news-room/events/detail/2018/02/06/default-calendar/2018-annual-review-of-diseases-prioritized-under-the-research-anddevelopment-blueprint (accessed on 18 July 2022).
- Mehand, M.S.; Al-Shorbaji, F.; Millett, P.; Murgue, B. The WHO R&D Blueprint: 2018 review of emerging infectious diseases requiring urgent research and development efforts. Antivir. Res. 2018, 159, 63–67. [Google Scholar] [PubMed]
- Zeller, H.G.; Cornet, J.-P.; Camicas, J.-L. Experimental Transmission of Crimean-Congo Hemorrhagic Fever Virus by West African Wild Ground-Feeding Birds to Hyalomma marginatum rufipes Ticks. Am. J. Trop. Med. Hyg. 1994, 50, 676–681. [Google Scholar] [CrossRef]
- Shepherd, A.J.; Swanepoel, R.; Shepherd, S.P.; Leman, P.A.; Mathee, O. Viraemic transmission of Crimean-Congo haemorrhagic fever virus to ticks. Epidemiol. Infect. 1991, 106, 373–382. [Google Scholar] [CrossRef]
- Palomar, A.M.; Portillo, A.; Santibáñez, P.; Mazuelas, D.; Arizaga, J.; Crespo, A.; Gutiérrez, O.; Cuadrado, J.F.; Oteo, J.A. Crimean-Congo Hemorrhagic Fever Virus in Ticks from Migratory Birds, Morocco1. Emerg. Infect. Dis. 2013, 19, 260–263. [Google Scholar] [CrossRef]
- Leblebicioglu, H.; Eroglu, C.; Erciyas-Yavuz, K.; Hokelek, M.; Acici, M.; Yilmaz, H. Role of migratory birds in spreading Crimean-Congo hemorrhagic fever, Turkey. Emerg Infect. Dis. 2014, 20, 1331–1334. [Google Scholar] [CrossRef]
- Lindeborg, M.; Barboutis, C.; Ehrenborg, C.; Fransson, T.; Jaenson, T.G.; Lindgren, P.-E.; Lundkvist, P.E.; Nyström, F.; Salaneck, E.; Waldenström, J.; et al. Migratory Birds, Ticks, and Crimean-Congo Hemorrhagic Fever Virus. Emerg. Infect. Dis. 2012, 18, 2095–2097. [Google Scholar]
- Mancuso, E.; Toma, L.; Polci, A.; d’Alessio, S.G.; Di Luca, M.; Orsini, M.; Di Domenico, M.; Marcacci, M.; Mancini, G.; Spina, F.; et al. Crimean-Congo Hemorrhagic Fever Virus Genome in Tick from Migratory Bird, Italy. Emerg. Infect. Dis. 2019, 25, 1418–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estrada-Peña, A.; Palomar, A.M.; Santibáñez, P.; Sánchez, N.; Habela, M.A.; Portillo, A.; Romero, L.; Oteo, J.A. Crimean-Congo Hemorrhagic Fever Virus in Ticks, Southwestern Europe, 2010. Emerg. Infect. Dis. 2012, 18, 179–180. [Google Scholar] [CrossRef] [PubMed]
- Negredo, A.; Habela, M.Á; de Arellano, E.R.; Diez, F.; Lasala, F.; López, P.; Sarriá, A.; Labiod, N.; Calero-Bernal, R.; Arenas, M.; et al. Survey of Crimean‐Congo Hemorrhagic Fever Enzootic Focus, Spain, 2011–2015. Emerg. Infect. Dis. 2019, 25, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Fons, F.; Fernández-De-Mera, I.G.; Acevedo, P.; Höfle, U.; Vicente, J.; de la Fuente, J.; Gortazár, C. Ixodid ticks parasitizing Iberian red deer (Cervus elaphus hispanicus) and European wild boar (Sus scrofa) from Spain: Geographical and temporal distribution. Veter.- Parasitol. 2006, 140, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Nijhof, A.M.; Bodaan, C.; Postigo, M.; Nieuwenhuijs, H.; Opsteegh, M.; Franssen, L.; Jebbink, F.; Jongejan, F. Ticks and Associated Pathogens Collected from Domestic Animals in the Netherlands. Vector-Borne Zoonotic Dis. 2007, 7, 585–596. [Google Scholar] [CrossRef]
- Hornok, S.; Horváth, G. First report of adult Hyalomma marginatum rufipes (vector of Crimean-Congo haemorrhagic fever virus) on cattle under a continental climate in Hungary. Parasites Vectors 2012, 5, 170. [Google Scholar] [CrossRef]
- Chitimia-Dobler, L.; Nava, S.; Bestehorn, M.; Dobler, G.; Wölfel, S. First detection of Hyalomma rufipes in Germany. Ticks Tick-Borne Dis. 2016, 7, 1135–1138. [Google Scholar] [CrossRef]
- Chitimia-Dobler, L.; Schaper, S.; Rieß, R.; Bitterwolf, K.; Frangoulidis, D.; Bestehorn, M.; Springer, A.; Oehme, R.; Drehmann, M.; Lindau, A.; et al. Imported Hyalomma ticks in Germany in 2018. Parasites Vectors 2019, 12, 1–9. [Google Scholar] [CrossRef]
- Hansford, K.M.; Carter, D.; Gillingham, E.L.; Hernandez-Triana, L.M.; Chamberlain, J.; Cull, B.; McGinley, L.; Phipps, L.P.; Medlock, J.M. Hyalomma rufipes on an untraveled horse: Is this the first evidence of Hyalomma nymphs successfully moulting in the United Kingdom? Ticks Tick-borne Dis. 2019, 10, 704–708. [Google Scholar] [CrossRef]
- Grandi, G.; Chitimia-Dobler, L.; Choklikitumnuey, P.; Strube, C.; Springer, A.; Albihn, A.; Jaenson, T.G.; Omazic, A.W. First records of adult Hyalomma marginatum and H. rufipes ticks (Acari: Ixodidae) in Sweden. Ticks Tick-borne Dis. 2020, 11, 101403. [Google Scholar] [CrossRef]
- Hubálek, Z.; Sedláček, P.; Estrada-Peña, A.; Vojtíšek, J.; Rudolf, I. First record of Hyalomma rufipes in the Czech Republic, with a review of relevant cases in other parts of Europe. Ticks Tick-borne Dis. 2020, 11, 101421. [Google Scholar] [CrossRef]
- Lesiczka, P.M.; Daněk, O.; Modrý, D.; Hrazdilová, K.; Votýpka, J.; Zurek, L. A new report of adult Hyalomma marginatum and Hyalomma rufipes in the Czech Republic. Ticks Tick-borne Dis. 2022, 13, 101894. [Google Scholar] [CrossRef]
- Cicculli, V.; de Lamballerie, X.; Charrel, R.; Falchi, A. First molecular detection of Rickettsia africae in a tropical bont tick, Amblyomma variegatum, collected in Corsica, France. Exp. Appl. Acarol. 2019, 77, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Pintore, E.; Olivieri, E.; Floriano, A.M.; Sassera, D.; Sanna, N.; Garippa, G. First detection of Amblyomma variegatum and molecular finding of Rickettsia africae in Sardinia, Italy. Ticks Tick-Borne Dis. 2020, 12, 101561. [Google Scholar] [CrossRef]
- Hoffman, T.; Carra, L.G.; Öhagen, P.; Fransson, T.; Barboutis, C.; Piacentini, D.; Figuerola, J.; Kiat, Y.; Onrubia, A.; Jaenson, T.G.; et al. Association between guilds of birds in the African-Western Palaearctic region and the tick species Hyalomma rufipes, one of the main vectors of Crimean-Congo hemorrhagic fever virus. One Health 2021, 13, 100349. [Google Scholar] [CrossRef] [PubMed]
- Rollins, R.E.; Schaper, S.; Kahlhofer, C.; Frangoulidis, D.; Strauß, A.F.; Cardinale, M.; Springer, A.; Strube, C.; Bakkes, D.K.; Becker, N.S.; et al. Ticks (Acari: Ixodidae) on birds migrating to the island of Ponza, Italy, and the tick-borne pathogens they carry. Ticks Tick-Borne Dis. 2021, 12, 101590. [Google Scholar] [CrossRef] [PubMed]
- Toma, L.; Mancini, F.; Di Luca, M.; Cecere, J.G.; Bianchi, R.; Khoury, C.; Quarchioni, E.; Manzia, F.; Rezza, G.; Ciervo, A. Detection of Microbial Agents in Ticks Collected from Migratory Birds in Central Italy. Vector-Borne Zoonotic Dis. 2014, 14, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Toma, L.; Mancuso, E.; d’Alessio, S.G.; Menegon, M.; Spina, F.; Pascucci, I.; Monaco, F.; Goffredo, M.; Di Luca, M. Tick species from Africa by migratory birds: A 3-year study in Italy. Exp. Appl. Acarol. 2021, 83, 147–164. [Google Scholar] [CrossRef]
- Pascucci, I.; Di Domenico, M.; Capobianco Dondona, G.; Di Gennaro, A.; Polci, A.; Capobianco Dondona, A.; Mancuso, E.; Cammà, C.; Savini, G.; Cecere, J.G.; et al. Assessing the role of migratory birds in the introduction of ticks and tick-borne pathogens from African countries: An Italian experience. Ticks Tick-Borne Dis. 2019, 10, 101272. [Google Scholar] [CrossRef]
- Okely, M.; Anan, R.; Gad-Allah, S.; Samy, A. Mapping the environmental suitability of etiological agent and tick vectors of Crimean-Congo hemorrhagic fever. Acta Trop. 2020, 203, 105319. [Google Scholar] [CrossRef]
- Hoffman, T.; Lindeborg, M.; Barboutis, C.; Erciyas-Yavuz, K.; Evander, M.; Fransson, T.; Figuerola, J.; Jaenson, T.G.; Kiat, Y.; Lindgren, P.-E.; et al. Alkhurma Hemorrhagic Fever Virus RNA in Hyalomma rufipes Ticks Infesting Migratory Birds, Europe and Asia Minor. Emerg. Infect. Dis. 2018, 24, 879–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spina, F.; Massi, A.; Montemaggiori, A.; Baccetti, N. Spring migration across central Mediterranean: General results from the “Progetto Piccole Isole”. Die Vogelwarte 1993, 37, 1–94. [Google Scholar]
- Tenan, S.; Spina, F. Timing and condition-related effects on recapture probability, mass change and stopover length of spring migrating songbirds on A small Mediterranean island. Ardeola 2010, 57, 121–132. [Google Scholar]
- Newton, I. The Migration Ecology of Birds; Elsevier: Cambridge, MA, USA, 2010. [Google Scholar]
- Manilla, G. Fauna d’Italia—Ixodida; Calderini: Edagricole Bologna, Italy, 1998. [Google Scholar]
- Iori, A.; Di Giulio, A.; De Felici, S. Zecche d’Italia parte III. In Mappe Parassitologiche; Rolando Editore: Napoli, Italy, 2005; pp. 52–163. [Google Scholar]
- Wölfel, R.; Paweska, J.T.; Petersen, N.; Grobbelaar, A.A.; Leman, P.A.; Hewson, R.; Georges-Courbot, M.-C.; Papa, A.; Günther, S.; Drosten, C. Virus Detection and Monitoring of Viral Load in Crimean-Congo Hemorrhagic Fever Virus Patients. Emerg. Infect. Dis. 2007, 13, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Del Amo, J.; Sotelo, E.; Fernández-Pinero, J.; Gallardo, C.; Llorente, F.; Agüero, M.; Jiménez-Clavero, M.A. A novel quantitative multiplex real-time RT-PCR for the simultaneous detection and differentiation of West Nile virus lineages 1 and 2, and of Usutu virus. J. Virol. Methods 2013, 189, 321–327. [Google Scholar] [CrossRef]
- Vázquez, A.; Herrero, L.; Negredo, A.; Hernández, L.; Sánchez-Seco, M.P.; Tenorio, A. Real time PCR assay for detection of all known lineages of West Nile virus. J. Virol. Methods 2016, 236, 266–270. [Google Scholar] [CrossRef]
- Cavrini, F.; DELLA Pepa, M.E.; Gaibani, P.; Pierro, A.M.; Rossini, G.; Landini, M.P.; Sambri, V. A rapid and specific real-time RT-PCR assay to identify Usutu virus in human plasma, serum, and cerebrospinal fluid. J. Clin. Virol. 2011, 50, 221–223. [Google Scholar] [CrossRef]
- Dusbábek, F. Argas (Argas) vulgaris Filippova, 1961, a new member of Czechoslovak tick fauna. Folia Parasitol. 1976, 23, 281–283. [Google Scholar]
- Bakirci, S.; Sarali, H.; Aydin, L.; Eren, H.; Karagenc, T.; Bakırcı, S. Distribution and seasonal activity of tick species on cattle in the West Aegean region of Turkey. Exp. Appl. Acarol. 2012, 56, 165–178. [Google Scholar] [CrossRef]
- EFSA Panel On Animal Health and Welfare (AHAW). Scientific Opinion on Geographic Distribution of Tick-borne Infections and their Vectors in Europe and the other Regions of the Mediterranean Basin. EFSA J. 2010, 8, 1723. [Google Scholar] [CrossRef]
- Capek, M.; Literak, I.; Kocianova, E.; Sychra, O.; Najer, T.; Trnka, A.; Kverek, P. Ticks of the Hyalomma marginatum complex transported by migratory birds into Central Europe. Ticks Tick-Borne Dis. 2014, 5, 489–493. [Google Scholar] [CrossRef] [PubMed]
- De Liberato, C.; Frontoso, R.; Magliano, A.; Montemaggiori, A.; Autorino, G.L.; Sala, M.; Bosworth, A.; Scicluna, M.T. Monitoring for the possible introduction of Crimean Congo haemorrhagic fever virus in Italy based on tick sampling on migratory birds and serological survey of sheep flocks. Prevent. Vet. Med. 2018, 149, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.R.; Bouattour, A.; Camicas, J.-L.; Estrada-Peña, A.; Horak, I.G.; Latif, A.A.; Pegram, R.G.; Preston, P.M. Ticks of Domestic Animals in Africa: A Guide to Identification of Species; Bioscience Reports: Edinburgh, UK, 2003. [Google Scholar]
- Farkas, R.; Estrada-Peña, A.; Jaenson, T.G.T.; Pascucci, I.; Madder, M.; Salman, M.; Tarrés-Call, J. Ticks and Tick-Borne Diseases: Geographical Distribution and Control Strategies in the Euro-Asia Region; CAB International: Wallingford, UK, 2012; pp. 6–26. [Google Scholar]
- Hoogstraal, H. Review Article 1: The Epidemiology of Tick-Borne Crimean-Congo Hemorrhagic Fever in Asia, Europe, and Africa. J. Med. Entomol. 1979, 15, 307–417. [Google Scholar] [CrossRef]
- Newton, I. The Migration Ecology of Birds; Academic Press: Cambridge, MA, USA, 2007. [Google Scholar]
- European Centre for Disease Prevention and Control and European Food Safety Authority. Tick Maps [Internet]. Stockholm: ECDC. 2022. Available online: https://ecdc.europa.eu/en/disease-vectors/surveillance-and-disease-data/tick-maps (accessed on 18 July 2022).
- Gargili, A.; Estrada-Peña, A.; Spengler, J.R.; Lukashev, A.; Nuttall, P.A.; Bente, D.A. The role of ticks in the maintenance and transmission of Crimean-Congo hemorrhagic fever virus: A review of published field and laboratory studies. Antivir. Res. 2017, 144, 93–119. [Google Scholar] [CrossRef] [PubMed]
- Spengler, J.R.; Estrada-Peña, A. Host preferences support the prominent role of Hyalomma ticks in the ecology of Crimean-Congo hemorrhagic fever. PLOS Neglected Trop. Dis. 2018, 12, e0006248. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Farkas, R.; Jaenson, T.G.; Madder, M.; Pascucci, I.; Tarrés-Call, J. Scientific opinion on the role of tick vectors in the epidemiology of Crimean-Congo hemorrhagic fever and African swine fever in Eurasia: EFSA Panel on Animal Health and Welfare. EFSA J. 2010, 8, 1–156. [Google Scholar]
- Spengler, J.R.; Estrada-Peña, A.; Garrison, A.R.; Schmaljohn, C.; Spiropoulou, C.F.; Bergeron, É.; Bente, D.A. A chronological review of experimental infection studies of the role of wild animals and livestock in the maintenance and transmission of Crimean-Congo hemorrhagic fever virus. Antivir. Res. 2016, 135, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Grech-Angelini, S.; Lancelot, R.; Ferraris, O.; Peyrefitte, C.N.; Vachiery, N.; Pédarrieu, A.; Peyraud, A.; Rodrigues, V.; Bastron, D.; Libeau, G.; et al. Crimean-Congo Hemorrhagic Fever Virus Antibodies among Livestock on Corsica, France, 2014–2016. Emerg. Infect. Dis. 2020, 26, 1041–1044. [Google Scholar] [CrossRef]
- Formosinho, P.; Santos-Silva, M.M. Experimental infection of Hyalomma marginatum ticks with West Nile virus. Acta Virol. 2006, 50, 175–180. [Google Scholar]
- Lawrie, C.H.; Uzcátegui, N.Y.; Gould, E.A.; Nuttall, P. Ixodid and Argasid Tick Species and West Nile Virus. Emerg. Infect. Dis. 2004, 10, 653–657. [Google Scholar] [CrossRef]
- Kolodziejek, J.; Marinov, M.; Kiss, B.J.; Alexe, V.; Nowotny, N. The Complete Sequence of a West Nile Virus Lineage 2 Strain Detected in a Hyalomma marginatum marginatum Tick Collected from a Song Thrush (Turdus philomelos) in Eastern Romania in 2013 Revealed Closest Genetic Relationship to Strain Volgograd 2007. PLoS ONE 2014, 9, e109905. [Google Scholar] [CrossRef] [PubMed]
- Lwande, O.W.; Venter, M.; Lutomiah, J.; Michuki, G.; Rumberia, C.; Gakuya, F.; Obanda, V.; Tigoi, C.; Odhiambo, C.; Nindo, F.; et al. Whole genome phylogenetic investigation of a West Nile virus strain isolated from a tick sampled from livestock in north eastern Kenya. Parasites Vectors 2014, 7, 542. [Google Scholar] [CrossRef] [PubMed]
- Savini, G.; Capelli, G.; Monaco, F.; Polci, A.; Russo, F.; Di Gennaro, A.; Marini, V.; Teodori, L.; Montarsi, F.; Pinoni, C.; et al. Evidence of West Nile virus lineage 2 circulation in Northern Italy. Veter. Microbiol. 2012, 158, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Zehender, G.; Veo, C.; Ebranati, E.; Carta, V.; Rovida, F.; Percivalle, E.; Moreno, A.; Lelli, D.; Calzolari, M.; Lavazza, A.; et al. Reconstructing the recent West Nile virus lineage 2 epidemic in Europe and Italy using discrete and continuous phylogeography. PLoS ONE 2017, 12, e0179679. [Google Scholar] [CrossRef] [PubMed]
- Mencattelli, G.; Iapaolo, F.; Monaco, F.; Fusco, G.; de Martinis, C.; Portanti, O.; Di Gennaro, A.; Curini, V.; Polci, A.; Berjaoui, S.; et al. West Nile Virus Lineage 1 in Italy: Newly Introduced or a Re-Occurrence of a Previously Circulating Strain? Viruses 2021, 14, 64. [Google Scholar] [CrossRef]

| Common Name | Scientific Name | Migration Strategy | N. Infested Birds | N. Screened Birds | N. Ticks | Prevalence Infested Birds (%) | Abundance of Ticks (%) |
|---|---|---|---|---|---|---|---|
| European nightjar | Caprimulgus europaeus | LD | 3 | 107 | 25 | 2.80 | 23.36 |
| Scops owl | Otus scops | LD | 2 | 78 | 2 | 2.56 | 2.56 |
| Eurasian hoopoe | Upupa epops | LD | 2 | 91 | 2 | 2.20 | 2.20 |
| Golden oriole | Oriolus oriolus | LD | 26 | 418 | 63 | 6.22 | 15.07 |
| Woodchat shrike | Lanius senator | LD | 6 | 190 | 16 | 3.16 | 8.42 |
| Icterine warbler | Hippolais icterina | LD | 60 | 3937 | 98 | 1.52 | 2.49 |
| Sedge warbler | Acrocephalus schoenobaenus | LD | 7 | 343 | 22 | 2.04 | 6.41 |
| Eurasian reed warbler | Acrocephalus scirpaceus | LD | 7 | 351 | 7 | 1.99 | 1.99 |
| Great reed warbler | Acrocephalus arundinaceus | LD | 1 | 81 | 3 | 1.23 | 3.70 |
| Barn swallow | Hirundo rustica | LD | 1 | 818 | 1 | 0.12 | 0.12 |
| Wood warbler | Phylloscopus sibilatrix | LD | 108 | 2781 | 244 | 3.88 | 8.77 |
| Willow warbler | Phylloscopus trochilus | LD | 33 | 4697 | 39 | 0.70 | 0.83 |
| Radde’s warbler | Phylloscopus schwarzi | LD | 1 | 1 | 1 | 100.00 | 100.00 |
| Garden warbler | Sylvia borin | LD | 16 | 5181 | 22 | 0.31 | 0.42 |
| Eastern subalpine warbler | Sylvia cantillans | LD | 12 | 3818 | 12 | 0.31 | 0.31 |
| Common whitethroat | Sylvia communis | LD | 88 | 3779 | 170 | 2.33 | 4.50 |
| Spotted flycatcher | Muscicapa striata | LD | 86 | 2180 | 126 | 3.94 | 5.78 |
| Nightingale | Luscinia megarhynchos | LD | 14 | 744 | 28 | 1.88 | 3.76 |
| Pied flycatcher | Ficedula hypoleuca | LD | 83 | 3563 | 191 | 2.33 | 5.36 |
| Collared flycatcher | Ficedula albicollis | LD | 8 | 571 | 31 | 1.40 | 5.43 |
| Common redstart | Phoenicurus phoenicurus | LD | 121 | 1745 | 202 | 6.93 | 11.58 |
| Whinchat | Saxicola rubetra | LD | 79 | 1210 | 281 | 6.53 | 23.22 |
| Northern wheatear | Oenanthe oenanthe | LD | 10 | 227 | 66 | 4.41 | 29.07 |
| Black-eared wheatear | Oenanthe hispanica | LD | 2 | 25 | 6 | 8.00 | 24.00 |
| Tree pipit | Anthus trivialis | LD | 16 | 618 | 45 | 2.59 | 7.28 |
| Western yellow wagtail | Motacilla flava | LD | 13 | 220 | 25 | 5.91 | 11.36 |
| TOTAL LD | 805 | 37,774 | 1728 | 2.13 | 4.57 | ||
| Great tit | Parus major | SD | 1 | 65 | 1 | 1.54 | 1.54 |
| Eurasian skylark | Alauda arvensis | SD | 1 | 11 | 1 | 9.09 | 9.09 |
| Chiff chaff | Phylloscopus collybita | SD | 8 | 1840 | 8 | 0.43 | 0.43 |
| Blackcap | Sylvia atricapilla | SD | 3 | 1292 | 3 | 0.23 | 0.23 |
| Sardinian Warbler | Sylvia melanocephala | SD | 4 | 319 | 4 | 1.25 | 1.25 |
| Song thrush | Turdus philomelos | SD | 42 | 521 | 128 | 8.06 | 24.57 |
| Blackbird | Turdus merula | SD | 16 | 59 | 31 | 27.12 | 52.54 |
| Ring ouzel | Turdus torquatus | SD | 1 | 6 | 5 | 16.67 | 83.33 |
| European robin | Erithacus rubecula | SD | 172 | 2173 | 391 | 7.92 | 17.99 |
| Black redstart | Phoenicurus ochruros | SD | 7 | 322 | 8 | 2.17 | 2.48 |
| Firecrest | Regulus ignicapilla | SD | 1 | 48 | 1 | 2.08 | 2.08 |
| Hedge accentor | Prunella modularis | SD | 8 | 21 | 17 | 38.10 | 80.95 |
| Chaffinch | Fringilla coelebs | SD | 4 | 85 | 4 | 4.71 | 4.71 |
| Hawfinch | Coccothraustes coccothraustes | SD | 2 | 30 | 9 | 6.67 | 30.00 |
| Goldfinch | Carduelis carduelis | SD | 1 | 88 | 2 | 1.14 | 2.27 |
| Serin | Serinus serinus | SD | 2 | 241 | 2 | 0.83 | 0.83 |
| TOTAL SD | 273 | 7121 | 615 | 3.83 | 8.64 | ||
| Italian sparrow | Passer italiae | R | 1 | 152 | 1 | 0.66 | 0.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancuso, E.; Toma, L.; Pascucci, I.; d’Alessio, S.G.; Marini, V.; Quaglia, M.; Riello, S.; Ferri, A.; Spina, F.; Serra, L.; et al. Direct and Indirect Role of Migratory Birds in Spreading CCHFV and WNV: A Multidisciplinary Study on Three Stop-Over Islands in Italy. Pathogens 2022, 11, 1056. https://doi.org/10.3390/pathogens11091056
Mancuso E, Toma L, Pascucci I, d’Alessio SG, Marini V, Quaglia M, Riello S, Ferri A, Spina F, Serra L, et al. Direct and Indirect Role of Migratory Birds in Spreading CCHFV and WNV: A Multidisciplinary Study on Three Stop-Over Islands in Italy. Pathogens. 2022; 11(9):1056. https://doi.org/10.3390/pathogens11091056
Chicago/Turabian StyleMancuso, Elisa, Luciano Toma, Ilaria Pascucci, Silvio Gerardo d’Alessio, Valeria Marini, Michela Quaglia, Sara Riello, Andrea Ferri, Fernando Spina, Lorenzo Serra, and et al. 2022. "Direct and Indirect Role of Migratory Birds in Spreading CCHFV and WNV: A Multidisciplinary Study on Three Stop-Over Islands in Italy" Pathogens 11, no. 9: 1056. https://doi.org/10.3390/pathogens11091056





