Morphogenesis Dynamics in Leishmania Differentiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Leishmania Cell Culture and Differentiation
2.3. Cell Viability Assay
2.4. Image Stream Flow Cytometry Analysis
2.5. Isolation of Knockout Mutants ∆ldpkar3 and ∆ldpkac3
3. Results
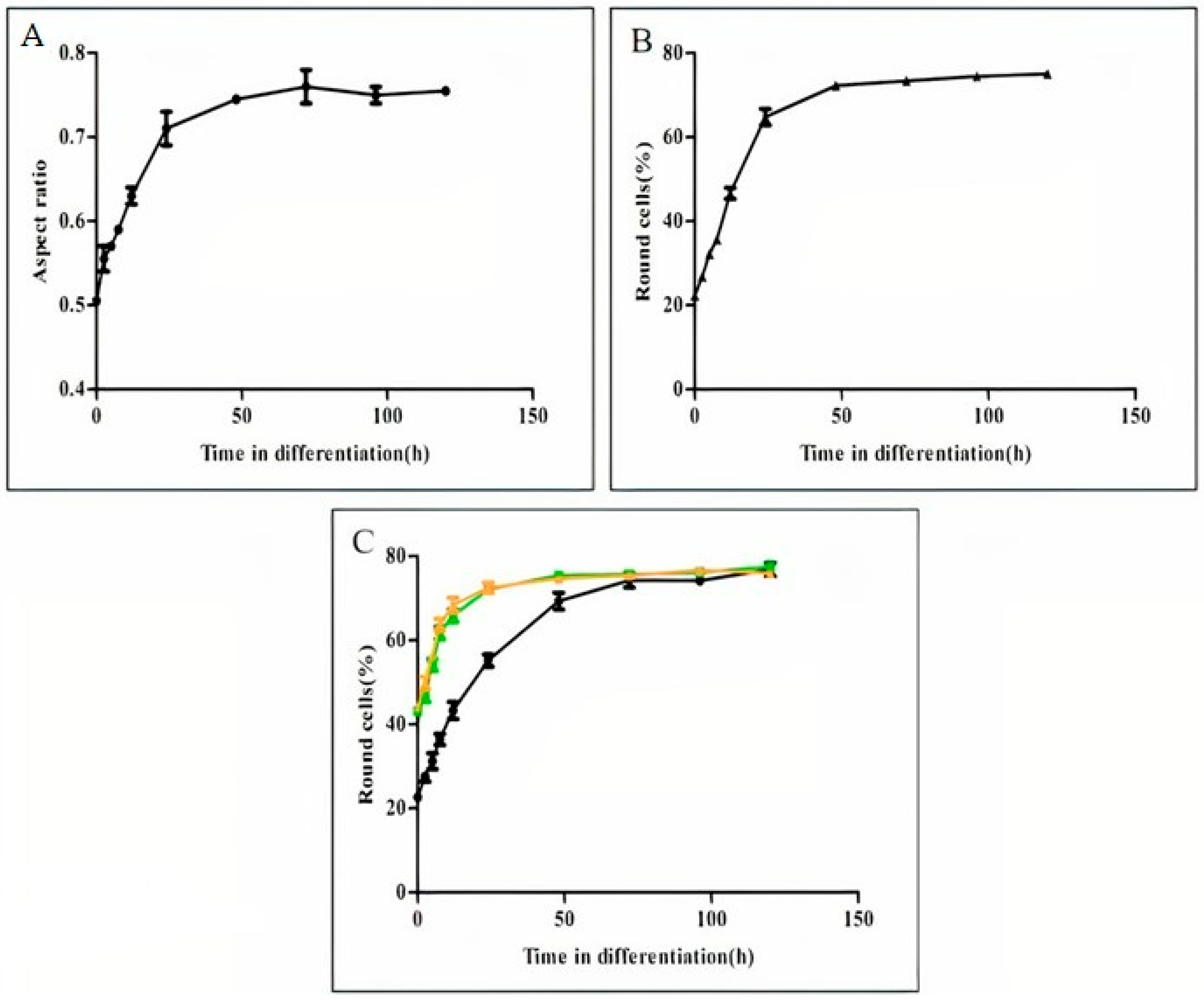
3.1. Differentiation-Derived Parasite Rounding and Elongating Process Differences
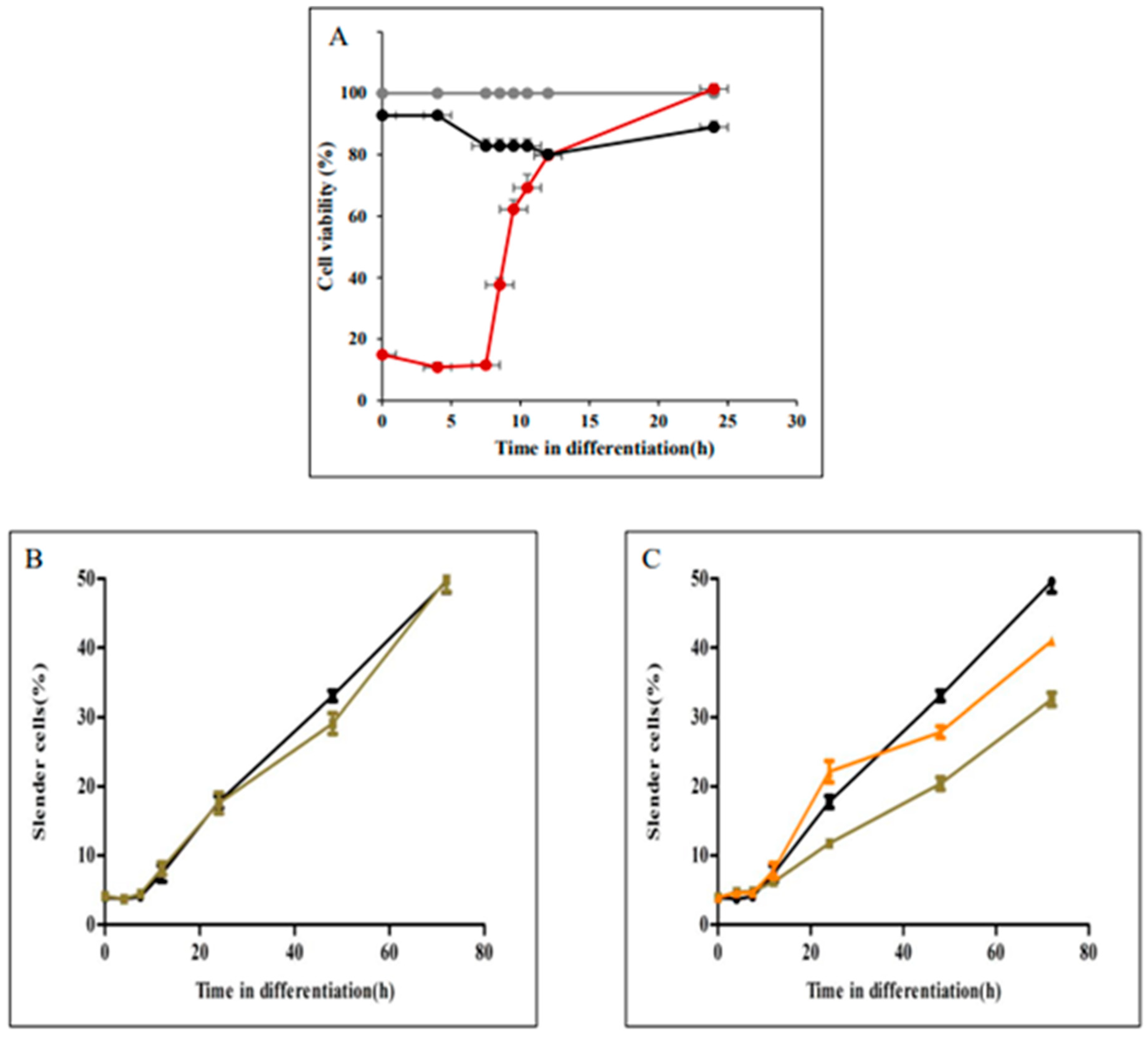
3.2. Microtubule Remodelling Is Required for Parasite Elongation, but Not for Rounding
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alvar, J.; Velez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M.; WHO Leishmaniasis Control Team. Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Molyneux, D.H.; Fenwick, A.; Ottesen, E.; Sachs, S.E.; Sachs, J.D. Incorporating a Rapid-Impact Package for Neglected Tropical Diseases with Programs for HIV/AIDS, Tuberculosis, and Malaria. PLoS Med. 2006, 3, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Handman, E.; Bullen, D.V. Interaction of Leishmania with the Host Macrophage. Trends Parasitol. 2002, 18, 332–334. [Google Scholar] [CrossRef]
- Rogers, M.E.; Chance, M.L.; Bates, P.A. The Role of Promastigote Secretory Gel in the Origin and Transmission of the Infective Stage of Leishmania mexicana by the Sandfly Lutzomyia Longipalpis. Parasitology 2002, 124, 495–507. [Google Scholar] [CrossRef]
- Podinovskaia, M.; Descoteaux, A. Leishmania and the Macrophage: A Multifaceted Interaction. Futur. Microbiol. 2015, 10, 111–129. [Google Scholar] [CrossRef]
- Domínguez, M.; Moreno, I.; López-Trascasa, M.; Toraño, A. Complement Interaction with Trypanosomatid Promastigotes in Normal Human Serum. J. Exp. Med. 2002, 195, 451–459. [Google Scholar] [CrossRef]
- Suga, M.; Kii, H.; Ueda, N.; Liu, Y.J.; Nakano, T.; Dan, T.; Uozumi, T.; Kiyota, Y.; Furue, M.K. A Morphology-Based Assay Platform for Neuroepithelial-like Cells Differentiated from Human Pluripotent Stem Cells. Int. J. Dev. Biol. 2018, 62, 613–621. [Google Scholar] [CrossRef]
- Sinclair, A.N.; de Graffenried, C.L. More than Microtubules: The Structure and Function of the Subpellicular Array in Trypanosomatids. Trends Parasitol. 2019, 35, 760. [Google Scholar] [CrossRef]
- Vedrenne, C.; Giroud, C.; Robinson, D.R.; Besteiro, S.; Bosc, C.; Bringaud, F.; Baltz, T. Two Related Subpellicular Cytoskeleton-Associated Proteins in Trypanosoma brucei Stabilize Microtubules. Mol. Biol. Cell 2002, 13, 1058–1070. [Google Scholar] [CrossRef]
- Gull, K. The Cytoskeleton of Trypanosomatid Parasites. Annu. Rev. Microbiol. 1999, 53, 629–655. [Google Scholar] [CrossRef]
- Sunter, J.; Gull, K. Shape, Form, Function and Leishmania Pathogenicity: From Textbook Descriptions to Biological Understanding. Open Biol. 2017, 7, 170165. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, R.J.; Gluenz, E.; Gull, K. The Limits on Trypanosomatid Morphological Diversity. PLoS ONE 2013, 8, e79581. [Google Scholar] [CrossRef] [PubMed]
- Barak, E.; Amin-Spector, S.; Gerliak, E.; Goyard, S.; Holland, N.; Zilberstein, D. Differentiation of Leishmania donovani in Host-Free System: Analysis of Signal Perception and Response. Mol. Biochem. Parasitol. 2005, 141, 99–108. [Google Scholar] [CrossRef]
- Zilberstein, D. In Vitro Culture for Differentiation Simulation of Leishmania spp. Methods Mol. Biol. 2020, 2116, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Bachmaier, S.; Witztum, R.; Tsigankov, P.; Koren, R.; Boshart, M.; Zilberstein, D. Protein Kinase A Signaling during Bidirectional Axenic Differentiation in Leishmania. Int. J. Parasitol. 2016, 46, 75–82. [Google Scholar] [CrossRef]
- Fischer-Weinberger, R.; Bachmaier, S.; Dandugudumula, R.; Phan, I.A.; Almoznino, M.; Githur, G.B.; Polatoglou, E.; Tsigankov, P.; Nitzan Koren, R.; Myler, P.J.; et al. A Divergent Protein Kinase A in the Human Pathogen Leishmania Is Associated with Developmental Morphogenesis. bioRxiv 2021. [Google Scholar] [CrossRef]
- Headland, S.E.; Jones, H.R.; D’Sa, A.S.V.; Perretti, M.; Norling, L.V. Cutting-Edge Analysis of Extracellular Microparticles Using ImageStream(X) Imaging Flow Cytometry. Sci. Rep. 2014, 4, 5237. [Google Scholar] [CrossRef]
- Johansson, J.; Karlsson, A.; Bylund, J.; Welin, A. Phagocyte Interactions with Mycobacterium Tuberculosis--Simultaneous Analysis of Phagocytosis, Phagosome Maturation and Intracellular Replication by Imaging Flow Cytometry. J. Immunol. Methods 2015, 427, 73–84. [Google Scholar] [CrossRef]
- Singh, A.; Saha, T.; Begemann, I.; Ricker, A.; Nüsse, H.; Thorn-Seshold, O.; Klingauf, J.; Galic, M.; Matis, M. Polarized Microtubule Dynamics Directs Cell Mechanics and Coordinates Forces during Epithelial Morphogenesis. Nat. Cell Biol. 2018, 20, 1126–1133. [Google Scholar] [CrossRef]
- Heisenberg, C.P.; Bellaïche, Y. Forces in Tissue Morphogenesis and Patterning. Cell 2013, 153, 948. [Google Scholar] [CrossRef]
- Sahasrabuddhe, A.A.; Bajpai, V.K.; Gupta, C.M. A Novel Form of Actin in Leishmania: Molecular Characterisation, Subcellular Localisation and Association with Subpellicular Microtubules. Mol. Biochem. Parasitol. 2004, 134, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Weaver, B.A. How Taxol/Paclitaxel Kills Cancer Cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.A.; Wilson, L. Microtubules as a Target for Anticancer Drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Castle, B.T.; McCubbin, S.; Prahl, L.S.; Bernens, J.N.; Sept, D.; Odde, D.J. Mechanisms of Kinetic Stabilization by the Drugs Paclitaxel and vinblastine. Mol. Biol. Cell 2017, 28, 1238–1257. [Google Scholar] [CrossRef]
- Doherty, T.M.; Sher, A.; Vogel, S.N. Paclitaxel (Taxol)-Induced Killing of Leishmania major in Murine Macrophages. Infect. Immun. 1998, 66, 4553–4556. [Google Scholar] [CrossRef] [PubMed]
- Dagger, F.; Valdivieso, E.; Marcano, A.K.; Ayesta, C. Regulatory Volume Decrease in Leishmania mexicana: Effect of Anti-Microtubule Drugs. Mem. Inst. Oswaldo Cruz 2013, 108, 84–90. [Google Scholar] [CrossRef]
- Pan, A.A.; Pan, S.C. Leishmania mexicana : Comparative Fine Structure of Amastigotes and Promastigotes in vitro. Exp. Parasitol. 1986, 62, 254–265. [Google Scholar] [CrossRef]
- Darling, T.N.; Burrows, C.M.; Blum, J.J. Rapid Shape Change and Release of Ninhydrin-Positive Substances by Leishmania major Promastigotes in Response to Hypo-Osmotic Stress. J. Protozool. 1990, 37, 493–499. [Google Scholar] [CrossRef]
- Zilberstein, D.; Shapira, M. The Role of PH and Temperature in the Development of Leishmania Parasites. Annu. Rev. Microbiol. 1994, 48, 449–470. [Google Scholar] [CrossRef]
- Joshi, M.; Dwyer, D.M.; Nakhasi, H.L. Cloning and Characterization of Expressed Genes from in Vitro- Grown “amastigotes” of Leishmania donovani. Mol. Biochem. Parasitol. 1993, 58, 345–354. [Google Scholar] [CrossRef]
- Tsigankov, P.; Gherardini, P.F.; Helmer-Citterich, M.; Spath, G.F.; Zilberstein, D. Phosphoproteomic Analysis of Differentiating Leishmania Parasites Reveals a Unique Stage-Specific Phosphorylation Motif. J. Proteome Res. 2013, 12, 3405–3412. [Google Scholar] [CrossRef] [PubMed]



| Life Stage | Shape | Mean Aspect Ratio | Portion in Population (%) |
|---|---|---|---|
| Promastigote | All | 0.45 | 100 |
| Elongated | 0.37 | 58.3 | |
| Ovoid | 0.55 | 24 | |
| Round | 0.75 | 17.2 | |
| Amastigote | All | 0.75 | 100 |
| Elongate | 0.37 | 4.3 | |
| Ovoid | 0.57 | 14.4 | |
| Round | 0.81 | 78.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dandugudumula, R.; Fischer-Weinberger, R.; Zilberstein, D. Morphogenesis Dynamics in Leishmania Differentiation. Pathogens 2022, 11, 952. https://doi.org/10.3390/pathogens11090952
Dandugudumula R, Fischer-Weinberger R, Zilberstein D. Morphogenesis Dynamics in Leishmania Differentiation. Pathogens. 2022; 11(9):952. https://doi.org/10.3390/pathogens11090952
Chicago/Turabian StyleDandugudumula, Ramu, Renana Fischer-Weinberger, and Dan Zilberstein. 2022. "Morphogenesis Dynamics in Leishmania Differentiation" Pathogens 11, no. 9: 952. https://doi.org/10.3390/pathogens11090952





