Molecular Characterization of Anopheles algeriensis Theobald, 1903 (Diptera: Culicidae) Populations from Europe
Abstract
:1. Introduction
2. Results
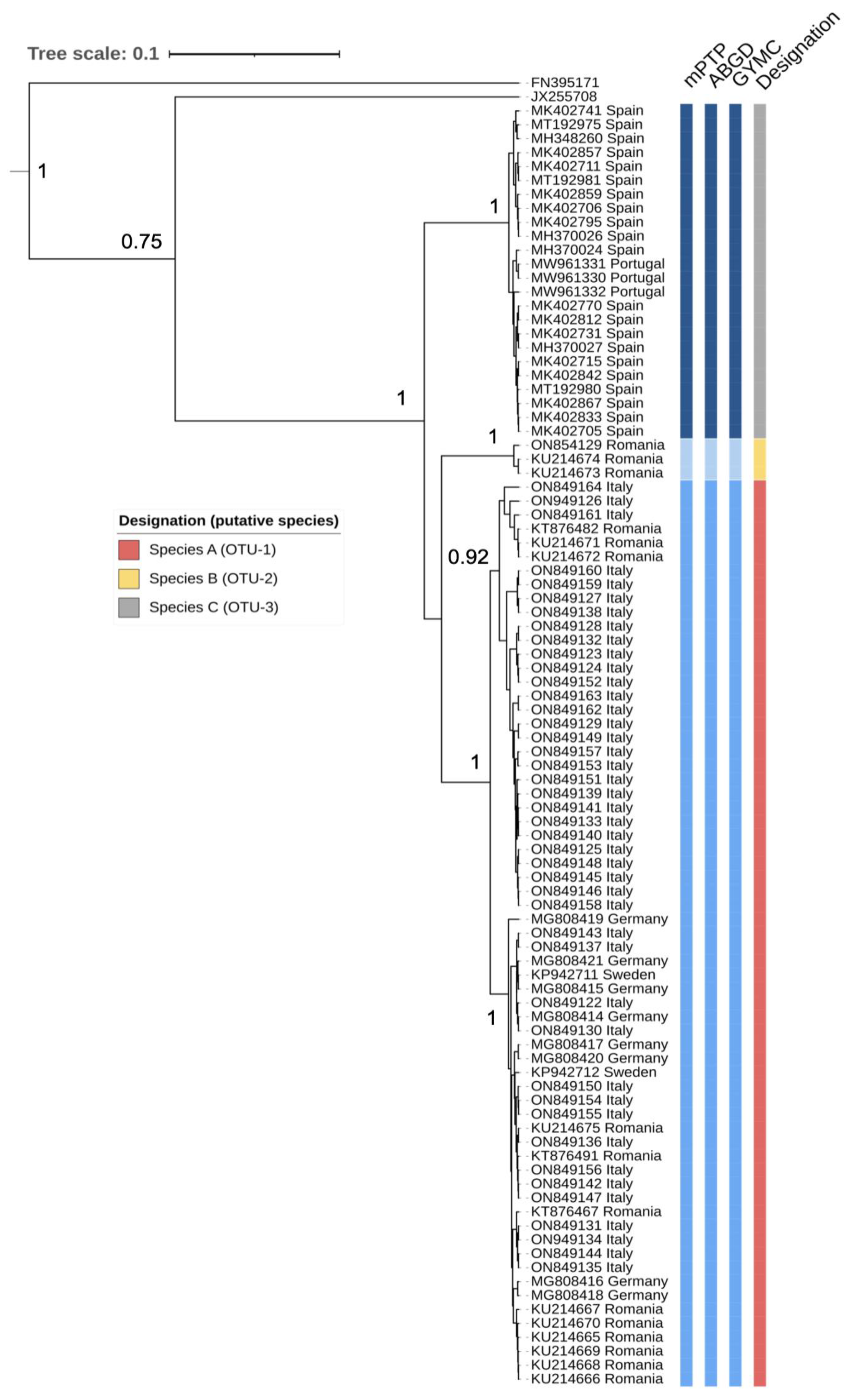
2.1. Phylogenetic Analysis and Species Delimitation Based on COI Sequences
2.2. Phylogenetic Analysis and Species Delimitation Based on ITS2 Sequences
3. Discussion
4. Materials and Methods
4.1. Mosquito Collection and Processing
4.2. Polymerase Chain Reaction (PCR) and Sequencing
4.3. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gubler, D.J. The Global Threat of Emergent/Re-emergent Vector-Borne Diseases. In Vector Biology, Ecology and Control; Atkinson, P.W., Ed.; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar] [CrossRef]
- WHO. World Malaria Report 2021. Available online: https://www.who.int/publications/i/item/9789240040496 (accessed on 10 June 2022).
- European Centre for Disease Prevention and Control. Multiple Reports of Locally-Acquired Malaria Infections in the EU—20 September 2017; ECDC: Stockholm, Sweden, 2017. [Google Scholar]
- NPHO. Epidemiological Surveillance Report Malaria in Greece; Malaria Report; NPHO: Athens, Greece, 2021. [Google Scholar]
- Boccolini, D.; Menegon, M.; Di Luca, M.; Toma, L.; Severini, F.; Marucci, G.; D’Amato, S.; Caraglia, A.; Maraglino, F.P.; Rezza, G.; et al. Non-imported malaria in Italy: Paradigmatic approaches and public health implications following an unusual cluster of cases in 2017. BMC Public Health 2020, 20, 857. [Google Scholar] [CrossRef]
- Brugueras, S.; Fernández-Martínez, B.; Martínez-de la Puente, J.; Figuerola, J.; Porro, T.M.; Rius, C.; Larrauri, A.; Gómez-Barroso, D. Environmental drivers, climate change and emergent diseases transmitted by mosquitoes and their vectors in southern Europe: A systematic review. Environ. Res. 2020, 191, 110038. [Google Scholar] [CrossRef] [PubMed]
- Piperaki, E.T.; Daikos, G.L. Malaria in Europe: Emerging threat or minor nuisance? Clin. Microbiol. Infect. 2016, 22, 487–493. [Google Scholar] [CrossRef]
- Ramsdale, C.; Snow, K. Distribution of Anopheles species in Europe. Eur. Mosq. Bull. 2000, 7, 1–26. [Google Scholar]
- Ponçon, N.; Toty, C.; L’Ambert, G.; Le Goff, G.; Brengues, C.; Schaffner, F.; Fontenille, D. Biology and dynamics of potential malaria vectors in southern France. Malar. J. 2007, 6, 18. [Google Scholar] [CrossRef]
- Török, E.; Tomazatos, A.; Cadar, D.; Horváth, C.; Keresztes, L.; Jansen, S.; Becker, N.; Kaiser, A.; Popescu, O.; Schmidt-Chanasit, J.; et al. Pilot longitudinal mosquito surveillance study in the Danube Delta Biosphere Reserve and the first reports of Anopheles algeriensis Theobald, 1903 and Aedes hungaricus Mihályi, 1955 for Romania. Parasit. Vectors 2016, 9, 196. [Google Scholar] [CrossRef]
- Scholte, E.J.; Hartog, W.J.; Reusken, C. A report of Anopheles algeriensis (Diptera: Culicidae) from The Netherlands. Entomol. Berichten 2011, 71, 39–42. [Google Scholar]
- Krüger, A.; Tannich, E. Rediscovery of Anopheles algeriensis Theob. (diptera: Culicidae) in Germany after half a century. J. Eur. Mosq. Control. Assoc. 2013, 31, 14–16. [Google Scholar]
- Tippelt, L.; Walther, D.; Scheuch, D.E.; Schäfer, M.; Kampen, H. Further reports of Anopheles algeriensis Theobald, 1903 (Diptera: Culicidae) in Germany, with evidence of local mass development. Parasitol. Res. 2018, 117, 2689–2696. [Google Scholar] [CrossRef]
- Ramsdale, C.D. Anopheles Mosquitoes and Imported Malaria in Libya. Mosq. System. 1990, 22, 34–40. [Google Scholar]
- Trari, B.; Dakki, M.; Harbach, R.E. An updated checklist of the Culicidae (Diptera) of Morocco, with notes on species of historical and current medical importance. J. Vector Ecol. 2017, 42, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Gaud, J.; Faure, J.; Maurice, A. Biogéographie des espèces anophéliennes au Maroc. Bull. Inst. Hygiène du Maroc 1949, 9, 145–164. [Google Scholar]
- Rioux, J.A.; Sinegre, G.; Croset, H.; Gabineau, A. Anopheles (A.) algeriensis Theobald, 1903, en Languedoc méditerranéen. Ann. Parasitol. 1966, 1, 91–100. [Google Scholar] [CrossRef]
- Raffaele, G. Note sull’eradicazione della malaria in Italia. Riv. Malariol. 1964, 43, 1–27. [Google Scholar]
- Romi, R.; Pontuale, G.; Sabatinelli, G. Le zanzare Italiane. Generalità e identificazione degli stadi preimaginali (Diptera, Culicidae). Fragm. Entomol. 1997, 29, 213–372. [Google Scholar]
- Raele, D.A.; Severini, F.; Boccolini, D.; Menegon, M.; Toma, L.; Vasco, I.; Franco, E.; Miccolis, P.; Desiante, F.; Nola, V.; et al. Entomological surveillance in former malaria-endemic areas of southern Italy. Pathogens 2021, 10, 1521. [Google Scholar] [CrossRef] [PubMed]
- Sergent, E.; Sergent, E. Les Insectes piqueurs inoculateurs de maladies infectieuses dans l’Afrique du Nord. In Comptes Rendus du Congrès de Sociétés Savantes (Sciences); Imprimerie Nationale: Paris, France, 1905. [Google Scholar]
- Guy, Y. Les rapports entre l’anophélisme et le paludisme. Bull. Soc. Sci. Nat. Phys. Maroc 1959, 39, 83–90. [Google Scholar]
- Ratnasingham, S.; Hebert, P.D.N. BOLD: The Barcode of Life Data System. Mol. Ecol. Notes 2007, 7, 355–364. Available online: www.barcodinglife.org. [CrossRef]
- Karimian, F.; Oshaghi, M.A.; Sedaghat, M.M.; Waterhouse, R.M.; Vatandoost, H.; Hanafi-Bojd, A.A.; Ravasan, N.M.; Chavshin, A.R. Phylogenetic analysis of the oriental-Palearctic-Afrotropical members of Anopheles (Culicidae: Diptera) based on nuclear rDNA and mitochondrial DNA characteristics. J. Infect. Dis. 2014, 67, 361–367. [Google Scholar] [CrossRef]
- Delgado-Serra, S.S.; Viader, M.; Ruiz-Arrondo, I.; Miranda, M.A.; Barceló, C.; Bueno-Marí, R.; Hernández-Triana, L.M.; Miquel, M.; Lester, K.; Jurado-Rivera, J.A.; et al. Molecular characterization of mosquito diversity in the Balearic Islands. J. Med. Entomol. 2021, 58, 608–615. [Google Scholar] [CrossRef]
- Marinucci, M.; Romi, R.; Mancini, P.; Di Luca, M.; Severini, C. Phylogenetic relationships of seven palearctic members of the Maculipennis Complex inferred from ITS2 sequence analysis. Insect Mol. Biol. 1999, 8, 469–480. [Google Scholar] [CrossRef]
- Coleman, A.W. ITS2 is a double-edged tool for eukaryote evolutionary comparison. Trends Genet. 2003, 19, 370–375. [Google Scholar] [CrossRef]
- Marrelli, M.T.; Sallum, M.A.; Marinotti, O. The second internal transcribed spacer of nuclear ribosomal DNA as a tool for Latin American anopheline taxonomy—A critical review. Mem. Inst. Oswaldo Cruz 2006, 101, 817–832. [Google Scholar] [CrossRef]
- Krzywinski, J.; Wilkerson, R.C.; Besansky, N.J. Evolution of mitochondrial and ribosomal gene sequences in anophelinae (Diptera: Culicidae): Implications for phylogeny reconstruction. Mol. Phylogenet. Evol. 2001, 18, 479–487. [Google Scholar] [CrossRef]
- Miaoulis, M.; Giantsis, I.A.; Schaffner, F.; Chaskopoulou, A. Re-examination of the taxonomic status of Anopheles hyrcanus and An. pseudopictus using a multilocus genetic approach. J. Vect. Ecol. 2018, 43, 179–183. [Google Scholar] [CrossRef]
- Severini, F.; Toma, L.; Di Luca, M.; Romi, R. Italian mosquitoes: General information and identification of adults (Diptera, Culicidae)/Le zanzare italiane: Generalità e identificazione degli adulti (Diptera, Culicidae). Fragm. Entomol. 2009, 41, 213–372. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Suchard, M.A.; Lemey, P.; Baele, G.; Ayres, D.L.; Drummond, A.J.; Rambaut, A. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol. 2018, 4, vey016. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior Summarization in Bayesian Phylogenetics Using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Kapli, P.; Lutteropp, S.; Zhang, J.; Kobert, K.; Pavlidis, P.; Stamatakis, A.; Flouri, T. Multi-rate Poisson tree processes for single-locus species delimitation under maximum likelihood and Markov chain Monte Carlo. Bioinformatics 2017, 33, 1630–1638. [Google Scholar] [CrossRef] [PubMed]
- Pons, J.; Barraclough, T.G.; Gomez-Zurita, J.; Cardoso, A.; Duran, D.P.; Hazell, S.; Kamoun, S.; Sumlin, W.D.; Vogler, A.P. Sequence-based species delimitation for the DNA taxonomy of undescribed insects. Syst. Biol. 2006, 55, 595–609. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, T.; Barraclough, T.G. Delimiting species using single-locus data and the Generalized Mixed Yule Coalescent approach: A revised method and evaluation on simulated data sets. Syst. biol. 2013, 62, 707–724. [Google Scholar] [CrossRef] [PubMed]
- Puillandre, N.; Lambert, A.; Brouillet, S.; Achaz, G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol. Ecol. 2012, 21, 1864–1877. [Google Scholar] [CrossRef]
- Kimura, M.A. Simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Leigh, W.; Bryant, D. POPART: Full-feature software for haplotype networkconstruction. Met. Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menegon, M.; Tomazatos, A.; Severini, F.; Raele, D.A.; Lilja, T.; Werner, D.; Boccolini, D.; Toma, L.; Vasco, I.; Lühken, R.; et al. Molecular Characterization of Anopheles algeriensis Theobald, 1903 (Diptera: Culicidae) Populations from Europe. Pathogens 2022, 11, 990. https://doi.org/10.3390/pathogens11090990
Menegon M, Tomazatos A, Severini F, Raele DA, Lilja T, Werner D, Boccolini D, Toma L, Vasco I, Lühken R, et al. Molecular Characterization of Anopheles algeriensis Theobald, 1903 (Diptera: Culicidae) Populations from Europe. Pathogens. 2022; 11(9):990. https://doi.org/10.3390/pathogens11090990
Chicago/Turabian StyleMenegon, Michela, Alexandru Tomazatos, Francesco Severini, Donato Antonio Raele, Tobias Lilja, Doreen Werner, Daniela Boccolini, Luciano Toma, Ilaria Vasco, Renke Lühken, and et al. 2022. "Molecular Characterization of Anopheles algeriensis Theobald, 1903 (Diptera: Culicidae) Populations from Europe" Pathogens 11, no. 9: 990. https://doi.org/10.3390/pathogens11090990







